Abstract
Ex vivo regional gene therapy strategies using animal mesenchymal stem cells genetically modified to overexpress osteoinductive growth factors have been successfully used in a variety of animal models to induce both heterotopic and orthotopic bone formation. However, in order to adapt regional gene therapy for clinical applications, it is essential to assess the osteogenic capacity of transduced human cells and choose the cell type that demonstrates the best clinical potential. Bone-marrow stem cells (BMSC) and adipose-derived stem cells (ASC) were selected in this study for in vitro evaluation, before and after transduction with a lentiviral two-step transcriptional amplification system (TSTA) overexpressing bone morphogenetic protein 2 (BMP-2; LV-TSTA-BMP-2) or green fluorescent protein (GFP; LV-TSTA-GFP). Cell growth, transduction efficiency, BMP-2 production, and osteogenic capacity were assessed. The study demonstrated that BMSC were characterized by a slower cell growth compared to ASC. Fluorescence-activated cell sorting analysis of GFP-transduced cells confirmed successful transduction with the vector and revealed an overall higher but not statistically significant transduction efficiency in ASC versus BMSC (90.2 ± 4.06% vs. 80.4 ± 8.51%, respectively; p = 0.146). Enzyme-linked immunosorbent assay confirmed abundant BMP-2 production by both cell types transduced with LV-TSTA-BMP-2, with BMP-2 production being significantly higher in ASC versus BMSC (239.5 ± 116.55 ng vs. 70.86 ± 24.7 ng; p = 0.001). Quantitative analysis of extracellular deposition of calcium (Alizarin red) and alkaline phosphatase activity showed that BMP-2-transduced cells had a higher osteogenic differentiation capacity compared to non-transduced cells. When comparing the two cell types, ASC/LV-TSTA-BMP-2 demonstrated a significantly higher mineralization potential compared to BMSC/LV-TSTA-BMP-2 7 days post transduction (p = 0.014). In conclusion, this study demonstrates that transduction with LV-TSTA-BMP-2 can significantly enhance the osteogenic potential of both human BMSC and ASC. BMP-2-treated ASC exhibited higher BMP-2 production and greater osteogenic differentiation capacity compared to BMP-2-treated BMSC. These results, along with the fact that liposuction is an easy procedure with lower donor-site morbidity compared to BM aspiration, indicate that adipose tissue might be a preferable source of MSCs to develop a regional gene therapy approach to treat difficult bone-repair scenarios.
Keywords: : gene therapy, bone repair, BMP-2, lentivirus, adipose-derived stem cells, bone-marrow stem cells
Introduction
Current treatment strategies for difficult bone-loss scenarios in settings such as trauma, tumor, and joint replacement surgeries have limitations with regards to efficiency, morbidity, and cost.1–4 Tissue engineering attempts to provide an alternative treatment strategy for such problems. Regional ex vivo gene therapy is an attractive tissue-engineering option, as it allows the delivery of both osteoprogenitor cells and an osteoinductive growth factor to a specific anatomic site where the transduced cells can induce bone formation.3,5
Ex vivo regional gene therapy strategies that deliver bone morphogenetic protein (BMP) have induced both heterotopic and orthotopic bone formation in animal studies. The advantage of an ex vivo strategy is that the investigator can select the cell type to be transduced. Rodent and porcine bone-marrow cells6–11; rodent skeletal muscle–derived cells12–14; rat, porcine, and human adipose-derived stem cells (ASC)15–18; rabbit periosteal cells19,20; and rodent skin fibroblasts21,22 have been used successfully as cellular delivery vehicles to enhance bone repair. However, in order to adapt regional gene therapy for clinical applications, it is essential to assess the osteogenic capacity of transduced human cells and choose the cell type that demonstrates the best clinical potential.
The ideal human cell type for gene therapy applied to bone-repair scenarios should be easy to obtain from the patient, carry low donor-site morbidity, be available in large numbers, and expand rapidly and efficiently in cell culture. It should also be associated with a high transduction efficiency, leading to prolonged, high-level production of bone morphogenetic protein 2 (BMP-2), when transduced with the proper viral vector. Moreover, it may be beneficial to use cells that have the innate ability to express an osteogenic phenotype in the proper environment, thus acting as both a vehicle for BMP-2 delivery (paracrine effect) and also directly participate in the bone repair (autocrine effect).
Researchers have long investigated human mesenchymal stem cells (MSCs) from various sources, alone or combined with a growth factor, for their ability to induce bone formation and healing.18,23–27 During the past few years, adult MSCs have been isolated from different sources.28 Bone marrow (BM)-derived stem cells first described by Friedenstein et al.29 have been considered the gold standard for cell therapies. However, other sources of similar cell populations are being investigated, since BM-derived MSC isolation requires a painful and invasive procedure. Muscle-derived stem cells and periosteal cells may have clinical potential, but it is more difficult to harvest these cells and expand them in culture. Recently, ASC have received more attention as an alternative source for MSCs, since processed lipoaspirates are easier to obtain from different anatomic sites, with a minimally invasive procedure carrying a low risk of complications and minor donor-site morbidity.30 However, there is still controversy as to whether ASC have the same, inferior, or superior osteogenic potential compared to bone-marrow mesenchymal stem cells (BMSC).31–34
Thus, this study evaluated BMSC and ASC. Although both adenoviral- and lentiviral vector (LV)-based gene therapy strategies have demonstrated potential to enhance bone repair in animal models, a lentivirus carrying the cDNA for BMP-2 was selected as the viral vector of choice in this study. Previous studies in the authors' laboratory have clearly shown that lentivirally mediated BMP-2 delivery is associated with a prolonged, stable production of BMP-2 and superior quality of bone repair compared to adenoviral gene therapy.35–37
The goals of this study were to (1) test the ability of a LV carrying the cDNA for BMP-2 to transduce human cells successfully, leading to abundant BMP-2 production in vitro; (2) assess the osteogenic capacity of BMP-2 transduced human cells versus non-transduced cells; and (3) compare the osteogenic potential of BMP-2 transduced BMSC versus BMP-2 transduced ASC and identify the cell type that demonstrates the best potential for further study.
Material and Methods
Isolation of human cells
The Institutional Review Board reviewed and approved the protocol (coded specimens study) before the commencement of any experiments involving human tissue and cells. Normally, both the lipoaspirates and BM from the femoral intramedullary canal are discarded after the surgery. These discarded lipoaspirates and bone marrow were collected for use in this study. All of the specimens were completely de-identified; no patient information, other than the patient's sex and age, were recorded. Patients with significant comorbidities, a known history of chronic hepatitis B virus or hepatitis C virus infection, or taking immunosuppressive or disease modifying agents were excluded.
Human BM
Human BM was obtained from 13 healthy patients (nine males), with a mean age of 54.9 ± 11 years, undergoing primary total hip replacement surgery for osteoarthritis at the authors' institution. During primary total hip arthroplasty (THA), the BM is cleared from the intramedullary canal so that the femoral implant can be placed into the femur. A surrogate technique was developed to collect this BM, which is usually discarded during a THA. The BM was transferred into sterile 50 mL tubes and diluted with phosphate-buffered saline (PBS) in a 1:1 ratio. Mononuclear cells were isolated by density gradient centrifugation using Histopaque 1077 (Sigma–Aldrich, St. Louis, MO). The cells were re-suspended in Dulbecco's modified Eagle's medium (DMEM; Corning Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Omega Scientific, Tarzana, CA).
Human adipose tissue
Human adipose tissue was obtained from 13 healthy patients (one male), with a mean age of 42.9 ± 10 years, undergoing abdominal, buttock, and/or thigh liposuction as elective surgery. Lipoaspirates were harvested by standard liposuction techniques, after infusion of the subcutaneous fat with a solution of diluted lidocaine and epinephrine to minimize pain and intraoperative blood loss (tumescent liposuction).38 The raw lipoaspirate was processed according to a previously established protocol39,40 to obtain the stromal vascular fraction (SVF). In brief, 500 cc of the lipoaspirate was transferred into a sterile glass beaker and washed extensively with Dulbecco's PBS (DPBS; Caisson Laboratories, North Logan, UT) to remove epinephrine/lidocaine, debris, and red blood cells. The washed adipose fraction was then digested with 0.1% collagenase (type I; Sigma–Aldrich) at 37°C with gentle swirling every 5–10 min. The cells were further processed, filtered, and incubated with ACK Lysing Buffer (Lonza, Basel, CH) to remove the red blood cells. After two washes with PBS to eliminate the ACK lysing buffer, the resultant cell pellet (SVF) was re-suspended in DMEM +10% FBS supplemented with antibiotics/antimycotics.
Cell culture
Both cell types were grown in culture for three passages before transduction. In brief, cells were counted with a hemocytometer using trypan blue, plated in DMEM +10% FBS, and maintained in a humidified atmosphere and 5% CO2 at 37°C. SVF was plated at a concentration of 5 × 106 cells per 10 cm plate, whereas BM cells were plated at 10-fold higher densities (50 × 106 cells per 10 cm plate) because of the higher number of contaminating hematopoietic cells.33,41 The culture medium was replaced every 3–4 days, and contaminating red blood cells and other non-adherent cells were removed. When confluent, the adherent cells were passaged at a density of 0.8–1.0 × 106 cells per plate.
In order to determine the cell proliferation rate for both cell types, generation time (in days) and number of cells per passage were documented. Generation time was defined as days in culture from initial plating (passage 0 cells) to passage 3. Cell yield was determined by counting the number of cells per cell type, rising from three 10 cm plates of passage 0 cells (BM: three plates of 50 × 106 mononuclear cells; adipose tissue: three plates of 5 × 106 SVF cells). This process was replicated five times (five samples per cell type).
Transduction with LV
In order to obtain enhanced gene expression, a standard LV (LV-RhMLV-BMP-2) was modified to a two-step transcriptional amplification (TSTA) system, as described before.8,42,43 Transduction with LV-TSTA-BMP-2 was used to assess the cells' osteogenic potential and osteogenic-related gene expression profiles, whereas transduction with LV-TSTA-GFP was done to determine transduction efficiency. The LVs for GFP and BMP-2 transduction were identical except for the inserted genes.
The TSTA system requires two different LVs: the transactivator vector encoding the RhMLV promoter and the GAL4-VP16 transactivator cDNA (LV-RhMLV-GAL4-VP16), and the transgene expression vector encoding the G5 promoter and the BMP-2 or eGFP cDNA (LV-G5-BMP-2 or LV-G5-GFP), under the control of the GAL4 responsive promoter. All LVs were generated by transfecting 293T cells (American Type Culture Collection, Manassas, VA), under conditions described on a previously established protocol.8,43
The titers of LV-TSTA vectors were determined by quantifying p24 protein contents in vector solution by enzyme-linked immunosorbent assay (ELISA; Quantikine, R&D Systems, Minneapolis, MN).
Passage 3 cells were plated into a 24-well plate at a concentration of 1 × 106 cells per well in 1 mL of DMEM +10% FBS containing 8 μg/mL of polybrene. The cells were co-transduced overnight with LV-GAL4-VP16 and LV-G5-BMP-2 or LV-G5-GFP at a multiplicity of infection (MOI) of 25 for each vector based on a previously published study that showed higher transduction efficiency with minimal viral toxicity-related cell death at a MOI of 25.8 Twenty-four hours after the viral transduction, the cells were centrifuged (200 g for 5 min) and washed with PBS three times to remove extracellular viruses. Viral transductions were carried out at 37°C, 5% CO2.
Immunophenotypic characterization
Cell surface markers were analyzed by multicolor flow cytometric (fluorescence-activated cell sorting [FACS]) analysis to evaluate whether the culture-expanded cells harvested from adipose tissue and BM had the cell marker profile of MSCs based on the criteria described by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT).44 For both tissues, FACS analysis was performed in both non-transduced and LV-TSTA-BMP-2 transduced cells 7 days post transduction. Cell preparation and FACS analysis were done following standard BD Stemflow protocols. In brief, a subset of passage 3 cells was attained for immunophenotypic analysis. Half of the cells were analyzed immediately, whereas the other half were transduced with LV-TSTA-BMP-2 and then left in culture for 7 days before FACS analysis. After trypsinization, the cells were washed and then re-suspended in BD Pharmingen Stain Buffer (BD Biosciences, Franklin Lakes, NJ) at a concentration of 5 × 106 cells/mL. One hundred microliters of the cell suspension was transferred into a separate tube and stained with the following antibodies: CD73, CD90, CD105, CD44, and hMSC Negative Cocktail (CD34, CD11b, CD19, CD45, HLA-DR). Unstained and single-color controls were also prepared. The tubes were incubated on ice in the dark for 30 min, washed twice, and re-suspended in 400 μL of BD Pharmingen Stain Buffer(BD Biosciences). Analysis was performed using a BD LSR II flow cytometer (BD Biosciences). After setting up the proper compensation, forward scatter and side scatter areas were used to exclude cell debris and doublets. The subsequent data were analyzed with the Flowjo software (FlowJo LLC, Ashland, OR).
Transduction efficiency
Transduction efficiency was determined by flow cytometry. Cells from three different patients (per tissue type) were cultured in 10 cm plates (1 × 106 cells) until passage 3 and then transduced with the LV-TSTA-GFP vector. The cells were washed 24 h later to remove any extracellular viruses and then plated again for a further 24 h. The cells were then trypsinized and re-suspended in PBS. Non-transduced target cells were used as control. FACS was performed with a BD LSR II flow cytometer (BD Biosciences) and subsequent analysis with Flowjo software (FlowJo LLC).
Quantification of BMP-2 production in vitro
Cells were collected and re-suspended in 1 mL of fresh medium after the end of the overnight transduction period. The cells were then plated and cultured for an additional 24 h. The conditioned media of transduced ASC and BMSC were harvested after the 24 h incubation and analyzed using ELISA (Quantikine; R&D Systems) for BMP-2 identification and quantitation. A total of 10 samples per cell type were used for this aspect of the study. Each sample was run in triplicate. The BMP-2 production was standardized by cell number and reported as nanogram of BMP-2 per day by 1 × 106 cells.
Osteogenic differentiation potential
Transduction with the LV-TSTA-BMP vector was used to assess the cells' osteogenic potential. Non-transduced cells were used as control. Osteogenic differentiation was induced by culturing 1 × 106 ASC and BMSC with DMEM containing 10% FBS, antibiotics/antimycotics, 0.1 μM of dexamethasone, 50 μg/mL of L-ascorbic acid, and 10 mM of β-glycerophosphate in a six-well plate. Five samples per cell type were used in total. Cells were seeded in duplicate for each experiment. Osteogenic differentiation of ASC and BMSC was assessed 7 days after exposure to LV-TSTA-BMP, using histochemical staining for Alizarin red mineralization and alkaline phosphatase (ALP) activity and subsequent quantitative analysis with images analysis software and/or spectrophotometry. Alkaline phosphatase staining of the cells was performed using a commercial kit, as per the manufacturer's instructions (ALP staining kit II; Stemgent, Cambridge, MA). For mineralization staining by Alizarin red S, the cells were fixed with 10% formaldehyde for 10 min, washed with PBS, and stained with 2% Alizarin red S solution for 30 min. Following staining, each well was imaged using a Nikon's AZ100 Multizoom microscope (Nikon Instruments, Inc., Melville, NY) and analyzed with Bioquant analysis software (Bioquant Image Analysis, Nashville, TN). The average percentage of staining per field was determined by examining the stained wells at 4 × magnification. Five representative rectangular areas (center, 12, 3, 6, and 9 o'clock positions) were selected at each culture well in a uniform manner. The mean of the five areas was taken and used for statistical analysis.
Spectrophotometry for Alizarin red
Quantification of Alizarin red S was performed by a colorimetric assay, as previously described.45 Stained cells in a six-well plate were incubated with 800 μL of 10% acetic acid at room temperature for 30 min with gentle shaking. After incubation, the now loosely attached stained layer was transferred to a 1.5 mL centrifuge tube and heated at 85°C for 10 min, then cooled on ice for 5 min. The cell slurry was then centrifuged at 20,000 g for 15 min. Five hundred microliters of the supernatant was removed into a new 1.5 mL tube and mixed with 200 μL of ammonium hydroxide. One hundred and fifty microliter aliquots were transferred to a 96-well plate (triplicates) and read at 405 nm by a plate reader.
Statistical analysis
Statistical analysis was done with IBM SPSS Statistics for Windows v22 (IBM Corp., Armonk, NY), with the significance level set at 0.05. Data are reported as mean and standard deviation. An independent samples t-test was done to compare cell yield and proliferation rate between the two cell types, association between sex and cell yield/mL of tissue, as well as BMP-2 production and transduction efficiency between transduced BMSC and ASC. One-way analysis of variance and post hoc analysis with Tukey's range test were used for Alizarin red and ALP staining comparisons. Correlation testing for age versus cell yield and age versus BMP-2 production was done with simple linear regression.
Results
Assessment of cell proliferation
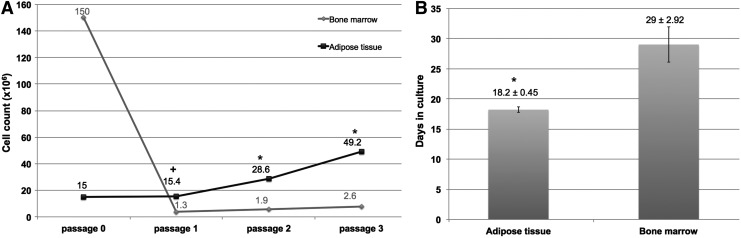
The average volume of harvested tissue was 77.45 ± 57.52 mL for BM and 500 ± 0 mL for adipose tissue. Human BM analysis revealed no association between patient sex and cell yield/mL of harvested tissue (p = 0.873), or between patient age and the number of isolated mononuclear cells/mL of BM (R = 0.226, p = 0.505; Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/hum). Moreover there was no association between patient age and cell yield/mL of tissue for human lipoaspirate (R = 0.107, p = 0.728; Supplementary Table S2). It was not possible to assess for an association between sex and cell yield because there was only one male patient. The number of isolated nucleated cells/mL of tissue was significantly higher for BM compared to adipose tissue (3.53 × 106 ± 1.83 × 106 vs. 0.18 × 106 ± 0.07 × 106; p < 0.001; Supplementary Tables S1 and S2). However, cells isolated from adipose tissue demonstrated a higher proliferation rate compared to BM cells. Generation time from initial plating (passage 0 cells) to passage 3 was significantly shorter in ASC compared to BMSC (18.2 vs. 29 days, respectively; p < 0.001). Moreover, although the initial number of BM nucleated cells plated for culture-expansion was sixfold higher than that of SVF cells (120 × 106 vs. 20 × 106), cell yield was higher in adipose tissue cultures compared to BM cultures at every passage; passage 1 (15.42 × 106 ± 4.22 × 106 vs. 1.28 × 106 ± 0.33 × 106, respectively; p = 0.002), passage 2 (28.62 × 106 ± 4.71 × 106 vs. 1.92 × 106 ± 0.54 × 106, respectively; p < 0.001), and passage 3 (49.2 × 106 ± 4.44 × 106 vs. 2.62 × 106 ± 0.96 × 106, respectively; p < 0.001; Fig. 1).
Figure 1.
Proliferation capacity of bone-marrow stem cells (BMSC) and adipose-derived stem cells (ASC). (A) Number of cells per cell type per passage, rising from three 10 cm plates of passage 0 cells (bone marrow [BM]: 50 × 106 mononuclear cells/plate; adipose tissue: 5 × 106 stromal vascular fraction [SVF] cells/plate). Cells isolated from lipoaspirates had a higher average cell count at every passage (P1–P3) compared to BM cells. (B) Generation time (in days) from initial plating (passage 0 cells) to passage 3. Results are presented as average days in culture ± standard deviation. Generation time was significantly shorter in ASC versus BMSC. *p < 0.001 vs. BMSC; +p = 0.002 vs. BMSC.
CD marker profile of cells
Immunophenotypic analysis of non-transduced BMSC and ASC showed high expression of the typical MSC markers CD44, CD73, CD90, and CD105. However, expression of CD90 and CD105 was higher in ASC compared to BMSC (CD90: 83.6% vs. 59%; CD105: 92.8% vs. 42.5%, respectively). Both cell types displayed minimal (<1%) expression of hematopoietic markers (CD45, CD11b, CD19, and HLA-DR; Table 1). This combination of CD markers is consistent with the MSC phenotype based on the criteria established by the ISCT on the minimal set of specific surface antigen expression to characterize a cell population as MSC44; 75.6% of the ASC co-expressed CD105, CD73, and CD90 and were negative for the hematopoietic lineage markers. In contrast, only 20.8% of the BMSC co-expressed the proper markers to be characterized as true MSCs (Supplementary Fig. S1).
Table 1.
Cell surface markers expressed by human BMSC and ASC before and after transduction with LV-TSTA-BMP-2, as revealed by flow cytometry
| Bone marrow | Adipose tissue | |||
|---|---|---|---|---|
| Non-transduced BMSC | BMSC/LV-TSTA-BMP-2 | Non-transduced ASC | ASC/LV-TSTA-BMP-2 | |
| CD90 | 59% | 28.01% | 83.6% | 16.5% |
| CD105 | 42.5% | 6.27% | 92.8% | 18% |
| CD73 | 99.5% | 99.7% | 99.1% | 96.4% |
| CD44 | 97.7% | 79.1% | 99.2% | 74.6% |
| HSC markers | 0.30% | 0.46% | 0.54% | 1.31% |
BMSC, bone-marrow stem cells; ASC, adipose-derived stem cells.
Transduction of the cells with a LV containing the cDNA for BMP-2 changed the expression profile of most of the MSC markers. The expression of both CD90 and CD105 significantly decreased in BMP-2 transduced BMSC and ASC compared to their non-transduced counterparts. CD44 expression was reduced to a lesser extent. Expression of hematopoietic lineage markers remained significantly low, even after the BMP-2 transduction (Table 1).
Transduction efficiency
FACS analysis of LV-TSTA-GFP transduced cells 48 h post transduction confirmed that both BMSC and ASC were successfully transduced with the GFP gene. There was a trend toward higher transduction efficiency in ASC, but the difference between the two cell types was not statistically significant (90.2 ± 4.06% in ASC vs. 80.4 ± 8.51% in BMSC; p = 0.146; Fig. 2).
Figure 2.
Flow cytometric analysis of green fluorescent protein (GFP) expression in non-transduced and LV-TSTA-GFP transduced BMSC and ASC. GFP expression was detected in 80.4% of the transduced BMSC and 90.2% of the transduced ASC 48 h post transduction.
In vitro BMP-2 production by LV-TSTA-BMP-2 transduced cells
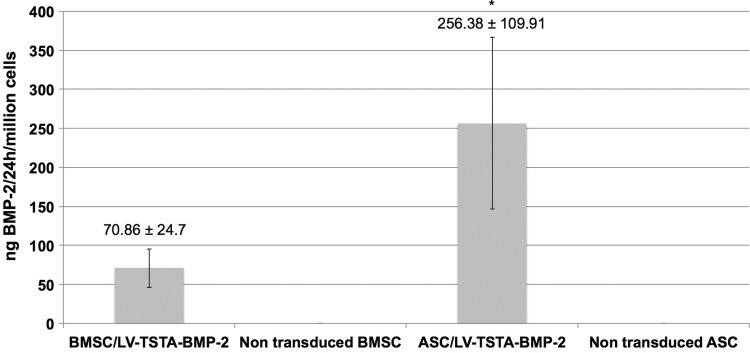
ELISA confirmed successful transduction with LV-TSTA-BMP-2 and abundant BMP-2 production over a 24 h period from both BMSC and ASC. At a MOI of 25, 1 × 106 BMSC produced 70.86 ± 24.70 ng of BMP-2 in a 24 h period, whereas ASC produced 239.5 ± 116.55 ng. Patient age and sex did not seem to correlate with BMP-2 production for BM samples (p = 0.764 and R = 0.046, p = 0.9, respectively). Moreover, no association between patient age and BMP-2 production was noted for adipose tissue samples (R = 0.099, p = 0.786; Supplementary Fig. S2). It was not possible to evaluate the relationship between sex and BMP-2 production in liposuction patients, since there was only one male patient. When comparing BMP-2 production between the two cell types, it was clear that BMP-2 production by transduced ASC was significantly higher compared to BMSC (p = 0.001). Non-transduced human cells did not produce any BMP-2 (0.00 ng; Fig. 3).
Figure 3.
In vitro bone morphogenetic protein 2 (BMP-2) production (ng) over a 24 h period by one million LV-TSTA-BMP-2 transduced human cells (BMSC and ASC). Non-transduced cells were used as negative control. Results are presented as mean ± standard deviation. *p < 0.001 vs. BMSC/LV-TSTA-BMP-2.
In vitro osteogenic potential of non-transduced and BMP-2 transduced human cells
The effects of BMP-2 on the osteogenic differentiation of human BM and adipose tissue were examined. Quantitative evaluation of extracellular deposition of calcium (Alizarin red) and extracellular ALP activity demonstrated that BMP-2 transduced BMSC and ASC had a higher osteogenic differentiation capacity compared to non-transduced cells.
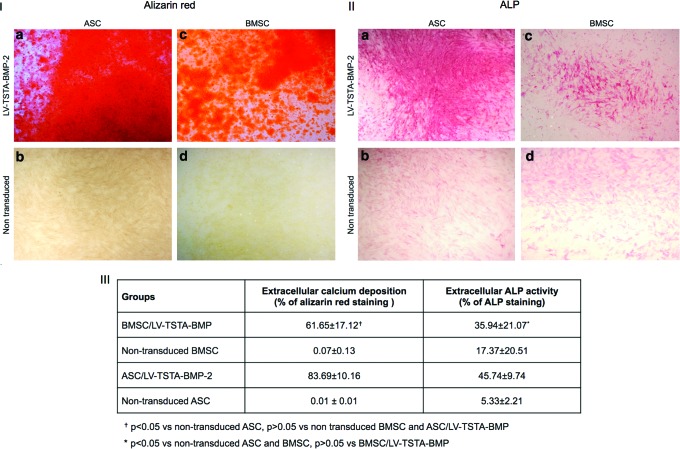
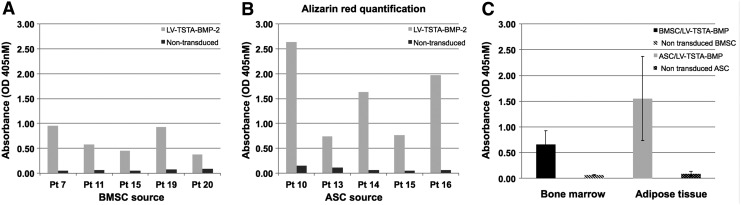
Alizarin red staining demonstrated abundant extracellular deposition of calcium by BMP-2 transduced BMSC and ASC. In contrast, minimal or no calcified extracellular matrix was seen in non-transduced cell cultures (Fig. 4I). Quantitative analysis of Alizarin red–stained cell cultures with Bioquant demonstrated significantly higher mineralization in ASC/LV-TSTA-BMP-2 compared to BMSC/LV-TSTA-BMP-2 at 7 days post transduction (p = 0.014). No statistically significant difference was shown between non-transduced ASC and BMSC (Fig. 4III). These results were confirmed by spectrophotometric quantification of the Alizarin red–stained monolayer (Fig. 5).
Figure 4.
(I) Representative images (2 × magnification) of Alizarin red stained non-transduced and BMP-2 transduced human cells. Alizarin red staining demonstrated abundant extracellular deposition of calcium in BMP-2 treated ASC (a) and BMSC (c). Minimal extracellular mineralization potential was noted in non-transduced ASC (b) and BMSC (d). (II) Representative images (2 × magnification) of ALP staining (pink) to assess extracellular alkaline phosphatase (ALP) activity. Extracellular ALP activity was generally greater in BMP-2 transduced cells (a and c) compared to non-transduced cells (b and d). (III) Quantitative analysis of Alizarin red and ALP assays with images analysis software. BMSC/LV-TSTA-BMP-2 exhibited a higher calcium deposition compared to non-transduced ASC and BMSC (p < 0.001) and a higher ALP activity compared to non-transduced ASC (p = 0.030). ASC/LV-TSTA-BMP-2 demonstrated a significantly higher percentage of ALP and Alizarin red staining compared to non-transduced ASC (p = 0.004 and p < 0.001, respectively) and BMSC (p = 0.047 and p < 0.001). ASC/LV-TSTA-BMP-2 presented a greater mineralization potential versus BMSC/LV-TSTA-BMP-2 (p = 0.014). Color images available online at www.liebertpub.com/hum
Figure 5.
Extracellular deposition of calcium from passage 3 non-transduced and BMP-2 transduced BMSC (A) and ASC (B) cultured in osteogenic medium for 7 days. Alizarin red quantification was done with spectrophotometry for five patient samples (Pt) per cell type. (A) BMP-2 transduced BMSC cell cultures had a higher percentage of Alizarin red staining than non-transduced BMSC for all of the BM donors. (B) ASC/LV-TSTA-BMP-2 showed increased osteogenic differentiation potential compared to non-transduced ASC. (C) Overall, Alizarin red staining was higher in ASC/LV-TSTA-BMP-2 compared to BMSC/LV-TSTA-BMP-2 (p = 0.022).
Extracellular ALP activity was generally greater in BMP-2 transduced cells compared to non-transduced cells (Fig. 4II). However, this difference in ALP staining was statistically significant only when comparing BMSC/LV-TSTA-BMP-2 versus non-transduced ASC (p = 0.030) and ASC/LV-TSTA-BMP-2 versus non-transduced ASC (p = 0.004) and non-transduced BMSC (p = 0.047). Although there was a higher percentage of ALP-positive cells in ASC/TSTA-BMP-2 compared to BMSC/LV-TSTA-BMP-2 cell cultures, the difference was not statistically significant (p = 0.753; Fig. 4III).
Discussion
Fracture nonunion and inadequate bone repair in settings such as trauma, tumor, joint replacement, and limb reconstructive surgeries remain among the most challenging problems in orthopedic surgery. Autologous bone graft is the gold standard in such cases, but it is associated with limited graft availability and donor site morbidity.2 Alternative therapies include bone allografts, demineralized bone matrixes, delivery of growth factors, such as recombinant bone morphogenetic protein 2 (rhBMP-2), and cell therapies. RhBMP-2 is a potent osteoinductive agent that is approved by the Food and Drug Administration for clinical use for anterior lumbar spinal fusions and treatment of open tibia fractures.46 However, rhBMP-2 is associated with inconsistent clinical results and several complications due to the supraphysiologic doses of BMP needed to promote adequate bone repair and the inefficient delivery systems presently in use.47–49 Over the past few years, cell-based therapies have emerged as a potential treatment option to promote bone repair. Numerous researchers have verified the osteogenic capacity of MSCs and explored the option of using adult stem cells from BM and adipose tissue as a therapeutic option for the management of bone-repair problems. Human BMSC and ASC have been reported to lead to ectopic bone formation50–52 and exhibit bone regeneration potential in calvarian bone defects in rodents and larger animals upon implantation.53–57 However, implantation of human MSC alone in animal critical-sized long bone defects leads to limited or no bone formation.18,58,59 Clinical studies have also evaluated BM cells' potential to lead to effective bone regeneration. Implantation of autologous BM aspirate, alone or combined with cast immobilization or internal/external fixation, has shown promising results for the treatment of tibial diaphyseal nonunions.60–62 However, there are concerns regarding the efficacy of autologous BM in large bone defects or displaced fractures.62 Therefore, there is an unmet clinical need for the successful management of large bone defects or defects associated with compromised biological environments.
In such cases, a combination of stem cells and an osteoinductive signal might be needed to achieve adequate bone formation. Regional gene therapy allows for the delivery of osteoprogenitor cells producing osteoinductive factors to a specific anatomic site where they induce bone formation. In contrast to recombinant proteins, gene therapy has been shown to exhibit a sustained, low-level BMP-2 production, leading to a longer half-life of the osteoinductive signal and thus increased recruitment and differentiation of osteoprogenitor cells at the site of injury.37 Moreover, previous studies from the authors' laboratory have shown that gene therapy using rodent cells can be used successfully to enhance bone healing in critical-sized bone defects, with the quality of bone produced being equal or superior to rhBMP-2.6,8,9,63,64
In vivo gene therapy has also demonstrated the efficacy of BMP-2, −4, and −6 in rat, rabbit, and equine models of bone repair.65 These approaches have predominantly utilized local injection of adenoviral66,67 or retroviral vectors68 or implantation of gene-activated matrixes (GAMs).69,70 An innovative modification of the GAM technology was proposed by Ito et al.71 In this approach, allografts or synthetic scaffolds coated with recombinant adeno-associated viruses overexpressing BMP-2 induced a regenerative response that led to enhanced osteogenesis in both femoral and calvarial critical-sized defects in mice.72,73 However, in vivo gene therapy has some potential disadvantages, including an overall lower transduction efficiency, difficulty targeting the cell population of interest, and possible risks stemming from the direct inoculation of viruses. Thus, an ex vivo regional gene therapy approach was pursued.
The ultimate goal is to develop ex vivo regional gene therapy as one aspect of a comprehensive tissue-engineering strategy that can consistently heal challenging bone defects while reducing the overall cost. In order to move gene therapy from the bench to bedside, it is essential to assess the osteogenic capacity of transduced autologous human cells. Human BMSC and ASC were selected for in vitro evaluation in this study, since they are the most commonly studied, best characterized, and clinically relevant.
This study establishes the feasibility of transducing human MSCs, derived from BM and adipose tissue, with a two-step transcriptional amplification LV overexpressing BMP-2 to enhance their osteogenic potential. Transduction with LV-TSTA-BMP-2 was associated with abundant BMP-2 production from both cell types. A great deal of variability was observed in the BMP-2 production by human cells obtained from the 26 patients enrolled in the study. These differences could have been indicative of the overall health of the cells, transduction efficiency, or innate variability between cell populations obtained from different patients. No difference with respect to viability and BMP-2 production was observed between BMSC harvested from male or female patients. Furthermore, there was no clear correlation between the amount of BMP-2 produced and the patients' age for both BM and adipose tissue. Therefore, a tissue-engineering strategy using gene therapy with autologous BMSC or ASC could potentially apply to a wide spectrum of patients, regardless of their age, without compromising BMP-2 production and the cells' osteogenic potential.
BMP-2 significantly increased the MSCs' in vitro osteogenic potential; LV-TSTA-BMP-2 exposed ASC and BMSC had an overall higher extracellular calcium deposition and ALP activity compared to the non-transduced control cells cultured in osteogenic media. These results confirm the hypothesis that BMP-2, as a product of gene therapy, can lead to rapid commitment of human MSCs toward the osteogenic phenotype. This could be an indication that in an in vivo bone-repair scenario, these BMP-2 transduced human cells could possibly act not only as vehicles to deliver BMP-2 at the injury site, but also as contributors in the bone-healing process by differentiating into osteoblasts themselves. This combination of a paracrine and an autocrine effect could enhance bone healing. The role of the transduced cells in the bone-repair process is still somewhat unclear. Using mouse BM cells (MBMC) transduced with a LV expressing red fluorescent protein (RFP) and BMP-2 (LV-RFPch-BMP-2) in a mouse femoral defect model, Pensak et al.64 demonstrated that the transduced donor cells were able to stimulate host cells to migrate around the bone defect and differentiate into osteoprogenitor cells, but did not seem to participate in the bone healing themselves as differentiated osteoblasts. However, the authors did not use a pure population of MSC but passage 1 unpurified adherent MBMC that were not characterized in any way before implantation. Thus, it is unclear what percentage of this heterogeneous mixture of cells were true multipotent MSCs. The observation that implanted donor cells do not actively participate in bone healing in vivo might be a result of the fact that the cells were not true MSCs, and therefore they did not have the innate ability to differentiate into osteoblasts. On the contrary, other studies in mouse radial defects demonstrated the ability of BMP-2 transduced MSCs not only to stimulate host cells by delivering BMP-2 at the injury site, but also to differentiate into osteocyte-like cells themselves and become incorporated into the newly formed bone.74,75 Further in vivo studies with human BMSC and ASC are needed to establish clearly the role of transduced MSCs in bone repair.
To the authors' knowledge, this is the first report of LV-mediated BMP-2 production by human BMSC and ASC to induce osteogenic differentiation in vitro. Previous studies in the authors' laboratory have demonstrated the efficacy of BMP-2 adenoviral (Ad) gene therapy using human MSC in vitro and in vivo.18,23,24,76–78 However, due to the limitations associated with the use of Ad gene therapy35,79 and the clear superiority of LV gene therapy with regards to target gene expression,36,37 this study focused on developing gene therapy using human cells with the more potent LV vector. In order to enhance BMP-2 production further, a standard LV vector (LV-RhMLV-BMP-2) was modified to a TSTA system that requires co-transduction with two different lentiviruses to lead to production of the protein of interest.8 Both cell types were successfully transduced with the LV-TSTA system as determined by FACS analysis. At a MOI of 25, the transduction efficiency was >90% for the ASC and 80% for the BMSC. The transduction efficiency achieved in this study with the LV-TSTA-GFP vector was significantly higher for both cell types compared to the rates observed with Ad gene therapy in previous studies (55% for ASC and 35% for BMSC with an Ad-Lac-Z vector77). Moreover, in this study, higher BMP-2 production was noted from one million LV-TSTA-BMP-2 transduced human BMSC and ASC 24 h post transduction (70.86 ng and 239.5 ng, respectively) compared to Ad-BMP-2 transduced cells (previously reported as 25.5 ng and 32.7 ng, respectively77). Finally, the results were superior with regards to transduction efficiency and BMP-2 production as compared to multiple non-viral gene delivery strategies with both BMSC and ASC. For example, transfection efficiency was 68.2 ± 4.1% for eGFP–nucleofected BMSC,80 18.4 ± 5.9% for liposome-mediated eGFP transfected BMSC,81 50% for ASC transfected with GFP-microporation, and <1% in ASC GFP-transfected using the calcium-phosphate precipitation method, standard electroporation, and cationic polymer techniques.82 BMP-2 production was also lower, with BMP-2–nucleofected BMSC producing 5.6–11.5 ng/106 cells/24 h and BMP-2-ASC transfected by microporation producing approximately 7 ng/107 cells of BMP-2 over a period of 3 days.83
The potential osteogenic capacity of ASC versus BMSC has not been definitely determined in the literature.31,32 In this study, although both BMSC and ASC were transduced successfully with the LV-TSTA-BMP-2 system, leading to abundant BMP-2 production and rapid induction of osteogenic differentiation, ASC seemed to be more potent. ASC had a higher transduction efficiency and produced almost three times more BMP-2 than BMSC when transduced with the TSTA-BMP-2 vector. It was also noted that although non-transduced ASC and BMSC had similar osteogenic potential, BMP-2-treated ASC produced a more robust osteogenic response compared to BMSC/LV-TSTA-BMP-2, as determined by the higher extracellular ALP activity and extracellular matrix mineralization. Moreover, cells isolated from adipose tissue were characterized by a more rapid cell growth and higher cell yield at all passages compared to BM. Finally, the majority (75.6%) of cultured adipose-derived cells co-expressed the proper CD markers to be characterized as true MSCs, in contrast to BM cells (20.8%). Attaining large numbers of stem cells at early passages, (i.e., only 2 weeks in tissue culture) would eliminate the need for costly and lengthy cell culture expansion, which would risk possible contamination and gene mutations and prolong the period of time between initial autologous tissue harvest and re-implantation back into the patient's bone defect.
There are several limitations to this study. First, the two human cell types were assessed only in vitro, and thus it is not known how these cells behave in vivo. Further experiments, in an in vivo environment, are needed to appraise better the differences in the osteogenic differentiation potential and bone induction capacity of human cells transduced with a LV-TSTA-BMP-2 vector before firm conclusions can be drawn. Another limitation is the fact that both BM and adipose tissue were harvested only from healthy patients. Thus, although the correlation between sex and age and BMP-2 production was studied, no other clinical factors were assessed with regards to BMP-2 production or osteogenic potential. It would be interesting to explore in future studies the possible differences in BMP-2 production from transduced ASC and BMSC in groups of patients with different concomitant diseases. These differences will be particularly important in defining patient eligibility criteria for the use of ex vivo regional gene therapy in bone repair problems.
In conclusion, this study demonstrates that transduction with a LV-TSTA-BMP-2 vector can significantly enhance the osteogenic potential of both human BMSC and ASC. LV-TSTA-BMP-2-treated ASC exhibited higher BMP-2 production and greater osteogenic differentiation capacity compared to BMP-2-treated BMSC. These results, along with the fact that liposuction is an easy procedure with a lower donor-site morbidity compared to BM aspiration, indicate that adipose tissue might be a preferable source of MSC to develop an ex vivo regional gene therapy approach to treat difficult bone-loss scenarios. However, further experiments are needed to assess the in vivo bone induction capacity of various human cells transduced with the LV-TSTA-BMP-2 vector.
Supplementary Material
Acknowledgments
This work was supported by a research grant from the National Institute of Health to J.R.L. (R01AR057076). Special thanks to Keck Hospital staff and Dr. Yoho's office personnel who provided rapid response to our inquiries and assisted us in obtaining the samples. We would also like to thank Bernadette Masinsin of the Flow Cytometry Core, Eli and Edythe Broad CIRM Center for Regenerative Medicine and Stem Cell Research at USC for the technical assistance in FACS analysis.
Author Disclosure
J.R.L. has received royalties from Depuy, Inc., has served as a paid consultant for Kuros, Inc., and is a shareholder in Hip Innovation Technologies, Inc. The remaining authors have no conflicts to report.
References
- 1.Brinker MR. Non-unions: evaluation and treatment. In: Browner BD, Levine AM, Jupiter JB, et al., eds. Skeletal Trauma: Basic Science, Management and Reconstruction. 4th ed. Philadelphia, PA: W.B. Saunders, 2009:615–707 [Google Scholar]
- 2.St John TA, Vaccaro AR, Sah AP, et al. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop 2003;32:18–23 [PubMed] [Google Scholar]
- 3.Virk MS, Lieberman JR. Biologic adjuvants for fracture healing. Arthritis Res Ther 2012;14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471–491 [DOI] [PubMed] [Google Scholar]
- 5.Pensak MJ, Lieberman JR. Gene therapy for bone regeneration. Curr Pharm Des 2013;19:3466–3473 [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 1999;81:905–917 [DOI] [PubMed] [Google Scholar]
- 7.Lieberman JR, Le LQ, Wu L, et al. Regional gene therapy with a BMP2 producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res 1998;16:330–339 [DOI] [PubMed] [Google Scholar]
- 8.Virk MS, Sugiyama O, Park SH, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther 2011;19:960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu WK, Sugiyama O, Park SH, et al. Lentiviral mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone 2007;40:931–938 [DOI] [PubMed] [Google Scholar]
- 10.Chang SC, Chuang HL, Chen YR, et al. Ex vivo gene therapy in autologous bone marrow stromal stem cells for tissue-engineered maxillofacial bone regeneration. Gene Ther 2003;10:2013–2019 [DOI] [PubMed] [Google Scholar]
- 11.Chang SC, Lin TM, Chung HY, et al. Large-scale bicortical skull bone regeneration using ex vivo replication-defective adenoviral-mediated bone morphogenetic protein-2 gene-transferred bone marrow stromal cells and composite biomaterials. Neurosurgery 2009;65:75–81; discussion 81–83. [DOI] [PubMed] [Google Scholar]
- 12.Shen HC, Peng H, Usas A, et al. Structural and functional healing of critical-size segmental bone defects by transduced muscle-derived cells expressing BMP4. J Gene Med 2004;6:984–991 [DOI] [PubMed] [Google Scholar]
- 13.Wright V, Peng H, Usas A, et al. BMP4-expressing muscle derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther 2002;6:169–178 [DOI] [PubMed] [Google Scholar]
- 14.Shen HC, Peng H, Usas A, et al. Ex vivo gene therapy-induced endochondral bone formation: comparison of muscle-derived stem cells and different subpopulations of primary muscle-derived cells. Bone 2004;34:982–992 [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Ma QJ, Dang GT, et al. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived adult stem cells expressing BMP-7. Cytotherapy 2005;7:273–281 [DOI] [PubMed] [Google Scholar]
- 16.Lin L, Fu X, Zhang X, et al. Rat adipose-derived stromal cells expressing BMP4 induce ectopic bone formation in vitro and in vivo. Acta Pharmacol Sin 2006;27:1608–1615 [DOI] [PubMed] [Google Scholar]
- 17.Sheyn D, Pelled G, Zilberman Y, et al. Nonvirally engineered porcine adipose tissue-derived stem cells: use in posterior spinal fusion. Stem Cells 2008;26:1056–1064 [DOI] [PubMed] [Google Scholar]
- 18.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng 2005;11:120–129 [DOI] [PubMed] [Google Scholar]
- 19.Breitbart AS, Grande DA, Mason JM, et al. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg 1999;42:488–495 [PubMed] [Google Scholar]
- 20.Sun M, Tan W, Wang K, et al. Effects of allogenous periosteal-derived cells transfected with adenovirus-mediated BMP-2 on repairing defects of the mandible in rabbits. J Oral Maxillofac Surg 2013;71:1789–1799 [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Zou Y, Yuan Z, et al. Autografts and xenografts of skin fibroblasts delivering BMP-2 effectively promote orthotopic and ectopic osteogenesis. Anat Rec (Hoboken) 2009;292:777–786 [DOI] [PubMed] [Google Scholar]
- 22.Hirata K, Tsukazaki T, Kadowaki A, et al. Transplantation of skin fibroblasts expressing BMP-2 promotes bone repair more effectively than those expressing Runx2. Bone 2003;32:502–512 [DOI] [PubMed] [Google Scholar]
- 23.Peterson B, Iglesias R, Zhang J, et al. Genetically modified human derived bone marrow cells for posterolateral lumbar spine fusion in athymic rats. Spine 2005;30:283–289 [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki M, Zuk PA, Zou J, et al. Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine 2008; 33:863–869 [DOI] [PubMed] [Google Scholar]
- 25.Hibi H, Yamada Y, Ueda M, et al. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg 2006;35:551–555 [DOI] [PubMed] [Google Scholar]
- 26.Mesimäki K, Lindroos B, Törnwall J, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg 2009;38:201–209 [DOI] [PubMed] [Google Scholar]
- 27.Shayesteh YS, Khojasteh A, Soleimani M, et al. Sinus augmentation using human mesenchymal stem cells loaded into a β-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106:203–209 [DOI] [PubMed] [Google Scholar]
- 28.Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011;14:9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;14:230–247 [PubMed] [Google Scholar]
- 30.Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J 2012;2:154–162 [PMC free article] [PubMed] [Google Scholar]
- 31.Liao HT, Chen CT. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells 2014;6:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012;21:2724–2752 [DOI] [PubMed] [Google Scholar]
- 33.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003;174:101–109 [DOI] [PubMed] [Google Scholar]
- 34.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage 2005;13:845–853 [DOI] [PubMed] [Google Scholar]
- 35.Virk MS, Conduah A, Park SH, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone 2008;42:921–931 [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama O, An DS, Kung SP, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther 2005;11:390–398 [DOI] [PubMed] [Google Scholar]
- 37.Feeley BT, Conduah AH, Sugiyama O, et al. In vivo molecular imaging of adenoviral versus lentiviral gene therapy in two bone formation models. J Orthop Res 2006;24:1709–1721 [DOI] [PubMed] [Google Scholar]
- 38.Wang WZ, Fang XH, Williams SJ, et al. Lidocaine-induced ASC apoptosis (tumescent vs. local anesthesia). Aesthetic Plast Surg 2014;38:1017–1023 [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Heydarkhan-Hagvall S, Hedrick M, et al. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp 2013;e50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211–228 [DOI] [PubMed] [Google Scholar]
- 41.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone 1992;13:69–80 [DOI] [PubMed] [Google Scholar]
- 42.Iyer M, Wu L, Carey M, et al. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci U S A 2001;98:14595–14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alaee F, Bartholomae C, Sugiyama O, et al. Biodistribution of LV-TSTA transduced rat bone marrow cells used for “ex-vivo” regional gene therapy for bone repair. Curr Gene Ther 2015;15:481–491 [DOI] [PubMed] [Google Scholar]
- 44.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317 [DOI] [PubMed] [Google Scholar]
- 45.Gregory CA, Gunn WG, Peister A, et al. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 2004;329:77–84 [DOI] [PubMed] [Google Scholar]
- 46.Garrison KR, Donell S, Ryder J, et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess 2007;11:1–150 [DOI] [PubMed] [Google Scholar]
- 47.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 2014;14:552–559 [DOI] [PubMed] [Google Scholar]
- 48.Woo EJ. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin Orthop Relat Res 2013;471:1707–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carofino BC, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Joint Surg Am 2008;90 Suppl 1:99–110 [DOI] [PubMed] [Google Scholar]
- 50.Krebsbach PH, Kuznetsov SA, Satomura K, et al. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 1997;63:1059–1069 [DOI] [PubMed] [Google Scholar]
- 51.Ye X, Yin X, Yang D, et al. Ectopic bone regeneration by human bone marrow mononucleated cells, undifferentiated and osteogenically differentiated bone marrow mesenchymal stem cells in beta-tricalcium phosphate scaffolds. Tissue Eng Part C Methods 2012;18:545–556 [DOI] [PubMed] [Google Scholar]
- 52.Hicok KC, Du Laney TV, Zhou YS, et al. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng 2004;10:371–380 [DOI] [PubMed] [Google Scholar]
- 53.Zong C, Xue D, Yuan W, et al. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cell Mater 2010;20:109–120 [DOI] [PubMed] [Google Scholar]
- 54.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol 2004;22:560–567 [DOI] [PubMed] [Google Scholar]
- 55.Li X, Yao J, Wu L, et al. Osteogenic induction of adipose-derived stromal cells: not a requirement for bone formation in vivo. Artif Organs 2010;34:46–54 [DOI] [PubMed] [Google Scholar]
- 56.Levi B, James AW, Nelson ER, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 2010;5:e11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen Y, Jiang B, Cui J, et al. Superior osteogenic capacity of different mesenchymal stem cells for bone tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e324–332 [DOI] [PubMed] [Google Scholar]
- 58.Cuomo AV, Virk M, Petrigliano F, et al. Mesenchymal stem cell concentration and bone repair: potential pitfalls from bench to bedside. J Bone Joint Surg Am 2009;91:1073–1083 [DOI] [PubMed] [Google Scholar]
- 59.Burastero G, Scarfì S, Ferraris C, et al. The association of human mesenchymal stem cells with BMP-7 improves bone regeneration of critical-size segmental bone defects in athymic rats. Bone;47:117–126 [DOI] [PubMed] [Google Scholar]
- 60.Connolly JF, Guse R, Tiedeman J, et al. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res 1991;266:259–270 [PubMed] [Google Scholar]
- 61.Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand 1993;64:671–672 [DOI] [PubMed] [Google Scholar]
- 62.Hernigou P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 2005;87:1430–1437 [DOI] [PubMed] [Google Scholar]
- 63.Bougioukli S, Jain A, Sugiyama O, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone 2016;84:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pensak M, Hong S, Dukas A, et al. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther 2015;22:467–475 [DOI] [PubMed] [Google Scholar]
- 65.Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol 2015;11:234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishihara A, Shields KM, Litsky AS, et al. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or −6 in equine osteotomy and ostectomy models. J Orthop Res 2008;26:764–771 [DOI] [PubMed] [Google Scholar]
- 67.Betz OB, Betz VM, Nazarian A, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther 2007;14:1039–1044 [DOI] [PubMed] [Google Scholar]
- 68.Rundle CH, Miyakoshi N, Kasukawa Y, et al. In vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4. Bone 2003;32:591–601 [DOI] [PubMed] [Google Scholar]
- 69.Kolk A, Tischer T, Koch C, et al. A novel nonviral gene delivery tool of BMP-2 for the reconstitution of critical-size bone defects in rats. J Biomed Mater Res A 2016;104:2441–2455 [DOI] [PubMed] [Google Scholar]
- 70.Umebayashi M, Sumita Y, Kawai Y, et al. Gene-activated matrix comprised of atelocollagen and plasmid DNA encoding BMP4 or Runx2 promotes rat cranial bone augmentation. Biores Open Access 2015;4:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito H, Koefoed M, Tiyapatanaputi P, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med 2005;11:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yazici C, Takahata M, Reynolds DG, et al. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther 2011;19:1416–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dupont KM, Boerckel JD, Stevens HY, et al. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res 2012;347:575–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gamradt SC, Abe N, Bahamonde ME, et al. Tracking expression of virally mediated BMP-2 in gene therapy for bone repair. Clin Orthop Relat Res 2006;450:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gazit D, Turgeman G, Kelley P, et al. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gene Med 1999;1:121–133 [DOI] [PubMed] [Google Scholar]
- 76.Dragoo JL, Lieberman JR, Lee RS, et al. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg 2005;115:1665–1673 [DOI] [PubMed] [Google Scholar]
- 77.Dragoo JL, Choi JY, Lieberman JR, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res 2003;21:622–629 [DOI] [PubMed] [Google Scholar]
- 78.Hsu WK, Wang JC, Liu NQ, et al. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. J Bone Joint Surg Am 2008;90:1043–1052 [DOI] [PubMed] [Google Scholar]
- 79.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med 1996;334:1185–1187 [DOI] [PubMed] [Google Scholar]
- 80.Aslan H, Zilberman Y, Arbeli V, et al. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889 [DOI] [PubMed] [Google Scholar]
- 81.Wang J, Li J, Deng N, et al. Transfection of hBMP-2 into mesenchymal stem cells derived from human umbilical cord blood and bone marrow induces cell differentiation into chondrocytes. Minerva Med 2014;105:283–288 [PubMed] [Google Scholar]
- 82.Abdul Halim NS, Fakiruddin KS, Ali SA, et al. A comparative study of non-viral gene delivery techniques to human adipose-derived mesenchymal stem cell. Int J Mol Sci 2014;15:15044–15060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee SJ, Kang SW, Do HJ, et al. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 2010;31:5652–5659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.