Abstract
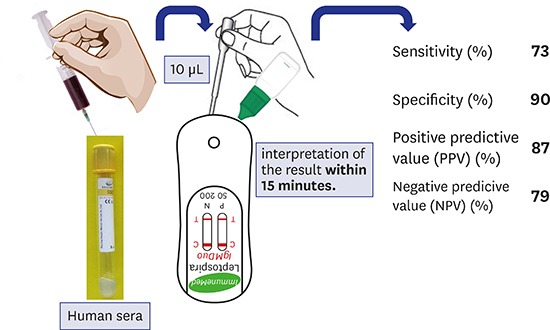
Leptospirosis is a febrile zoonotic disease. Routine diagnosis of leptospirosis is based on the detection of specific antibodies with serological tests. The aim of our study was to determine the usefulness of immunochromatographic assay (ICA), ImmuneMed Leptospira IgM Duo Rapid test kit from Korea, in rapid screening of acute leptospirosis in emergency cases with limited expertise. A total of 197 serum samples (93 positive, 104 negative) were selected randomly. The test has good diagnostic sensitivity 73% and specificity 90%. With positive predictive value of 87% and negative predictive value of 79%, this reassures patients have higher chance of correct diagnosis. This ICA is acceptable for screening of leptospirosis but confirmation with microscopic agglutination test should follow.
Keywords: Diagnostics, Leptospirosis, Immunochromatographic Test, Serological Tests, Serum IgM Antibodies
Graphical Abstract
Leptospirosis is a zoonotic disease caused by the spirochete Leptospira, member of the family Leptospiraceae. In the genus of Leptospira, nine pathogenic species have been recognized, with more than 300 serovars arranged in 25 serogroups.1 Recently, approximately 500,000 severe cases were reported annually worldwide with a significant rising trend causing public health concern.2 Based on the disease notification data collected in Malaysia, total age-standardized incidence rate of leptospirosis for 2012 and 2013 was 29.02 per 100,000 while total case fatality rate was 1.47%.3 Leptospirosis presents itself in a non-specific clinical manifestation ranging from mild flu-like symptoms that can mimic other infectious disease to severe conditions like pulmonary hemorrhage and acute kidney injury.4 Prompt diagnosis of leptospirosis can aid clinicians in choosing the appropriate antibiotics which is essential for better prognosis.
The few laboratory tests currently available in Malaysia are microscopic agglutination test (MAT) which is the gold standard method, detection of the organism DNA using polymerase chain reaction (PCR), isolation of organism through culture methods and detection of antibodies to the organism using enzyme-linked immunosorbent assay.5 Unfortunately, these tests require maintenance of a large panel of live pathogenic Leptospira stock cultures and specialized laboratory technical expertise, both of which are limited in local hospitals and are too time-consuming, leading to late diagnosis.6,7 The ideal diagnostic test should not only have high sensitivity and specificity during the acute phase, but also be economical, widely available and give quick results.
In this study, we evaluated a solid phase commercial gold immunochromatographic assay (ICA), which was produced by the ImmuneMed Leptospira IgM Duo Rapid test kit from ImmuneMed Inc. (Chuncheon, Korea) for rapid, semi-quantitative detection of serum IgM against Leptospira antigen. The kit was evaluated for its potential usefulness in rapid screening of acute leptospirosis cases at local health facilities.
A total of 197 serum samples were selected randomly and used in this study. Ninety-three serum samples were confirmed positive and 104 serum samples confirmed negative for leptospirosis by MAT. All these samples were from inpatients admitted to various hospitals in Malaysia who suffered from acute febrile illness and clinically suspected to have leptospirosis.
The selected serum samples were examined by the MAT, using a panel of 20 serotypes and strains: the reference strains obtained from the World Health Organization (WHO) Collaborative Centre for Leptospirosis in Amsterdam were serovars Australis, Autumnalis, Bataviae, Ballum, Canicola, Grippotyphosa, Icterohaemorrhagiae, Javanica, Pomona, Pyrogenes, Tarassovi, Sejroe, and Patoc and 6 locally isolated strains. MAT was performed at the Reference Laboratory for Leptospirosis, Institute of Medical Research, Kuala Lumpur employing WHO standard protocol to detect total leptospiral antibody. Samples confirmed positive for leptospirosis are based on the global criterion; when there is a fourfold rise in titers detected between acute and convalescent sera or when a single serum specimen has a titre of > 1:400. Meanwhile, the confirmed negative samples had MAT titer < 1:50 (indicating that fever may be due to other causes).8
A total number of 35 serum samples that tested positive serologically to other types of infectious diseases (dengue fever: 10, typhus: 5, syphilis with venereal disease research laboratory [VDRL] positive: 5, scrub typhus: 10 and melioidosis: 5) were chosen randomly and taken from the serology reference laboratory in the Institute for Medical Research to act as a test to determine the percentage of cross reactivity with the ICA. All 35 serum samples were tested against and confirmed by the lab. These samples were different samples and not included in the 197 samples of this study.
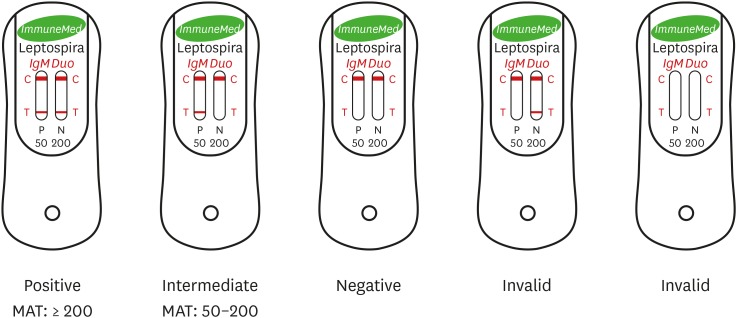
The ImmuneMed Leptospira IgM Duo Rapid test kit from Korea is claimed by the manufacturer to be suitable for detecting acute phase (IgM) antibody. All 197 samples were performed using the ICA according to the manufacturer's instructions. Results are interpreted if the red line appears on the testing device; positive, negative and intermediate results were determined by subjective visual interpretation as shown on Fig. 1.
Fig. 1. The representative pictures for commercialized ImmuneMed Leptospira IgM Duo Rapid test kit from Chuncheon, Korea, indicating negative and positive results for detecting leptospirosis. Red colorization of the test line (T) indicates the presence of human antibody against Leptospira and the red control line (C) represents the valid test result. Demonstration is positive in kit ‘a’ (both P and N windows; 50 and 200 cut-off titres), intermediate in kit ‘b’ (P window; 50 cut-off titre), negative in kit ‘c’ (only red colorization of [C] at both P and N windows), and invalid in kit ‘d’ & kit ‘e’ in ICA results.
ICA = immunochromatographic assay, MAT = microscopic agglutination test.
From the results obtained, diagnostic performance was calculated by comparing the ICA results with those of MAT for each patient. A two-by-two table was constructed (Table 1), in which the MAT result was cross-tabulated with the comparative ICA results to define the numbers of true-positive, false-positive, false-negative (inclusive of intermediate results), and true-negative results (inclusive of intermediate results). The ICA sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were determined against MAT and calculated to indicate standard diagnostic efficiency of the test.
Table 1. Sensitivity and specificity of the ICA by the ImmuneMed Leptospira IgM Duo Rapid test kit against MAT (gold standard).
| Test by ICA | MAT positive | MAT negative | Total | |
|---|---|---|---|---|
| ICA positive | 68 (true positive) | 10 (false positive) | 78 | |
| ICA negative (intermediate result included)a | 25 (false negative) | 94 (true negative) | 119 | |
| Total | 93 | 104 | 197 | |
| Diagnostic sensitivity (%) | 73 | |||
| Diagnostic specificity (%) | 90 | |||
| PPV (%) | 87 | |||
| NPV (%) | 79 | |||
ICA = immunochromatographic assay, MAT = microscopic agglutination test, PPV = positive predictive value, NPV = negative predictive value.
aInterpretation of ImmuneMed ICA intermediate result: inconclusive and not positive.
From the results in this study, it is evidenced that the test efficiency of the ICA is quite satisfactory. The sensitivity of the ICA depreciates slightly at 73% but the specificity reaches 90%, based on first and only submitted samples. Indeed, in spite of its high specificity, this rapid kit has slightly low sensitivity, especially during the acute phase of leptospirosis, when antibiotics are expected to have the greatest benefit. This can be explained by the fact that these samples are usually collected at an early stage of the disease when IgG antibodies are not yet present at detectable levels because IgM antibodies become detectable during the first week of illness.9 Therefore, it is more likely the test shows positive results in clinically suspected patients of leptospirosis after one week of illness due to the production of antibodies.
Seroconversion in cases of leptospirosis usually takes place between 6–10 days after the onset of symptoms so the MAT and other antibody detection based rapid diagnostic tests (RDTs) may have low sensitivity during early phase of the infection.9 The incubation phase of leptospirosis from exposure to onset of symptoms averages from 7 to 12 days, though it can be as short as 3 days or as long as a month. Based on a study, the variability in the duration of the incubation phase is evident in the 6–29 days' lag between exposure and onset of symptoms like fever (38°C–40°C), rigors, headache, retro-orbital pain, photophobia, conjunctival suffusion, nausea and vomiting, diarrhea and muscle pain localized to the calf and lumbar areas.10 A previously reported paper supported that the sensitivity and specificity of RDTs at different days' post onset (DPO) increased when results of a follow-up sample were included.11 Therefore, if suspicious samples collected early in the phase of infections test negative using MAT or RDT, it is advisable to repeat the ICA tests with second samples collected a few days later.
The PPV and the NPV are useful when clinicians are considering the value of a certain test. Ideally, clinicians are hoping for a test which can correctly identify all patients with the disease and also correctly identify all disease-free patients. Hence, with a low number of false negative and a few false positive, giving to the PPV and NPV in this study showing 87% and 79%, respectively, it is reassuring that patients will have a higher chance of a correct diagnosis.
Samples tested using ICA that were only positive at the 50-titer cut-off were considered as intermediate, the results were automatically regarded as negative detection for this assay (Supplementary Table 1). According to the product information for ImmuneMed Rapid kit, ‘intermediate’ results should be interpreted as not positive and inconclusive if the samples were collected from an endemic region and it is necessary to do the test again within 2–7 days. Because leptospirosis is endemic in Malaysia,12,13,14 the serum samples collected in this study are considered to be from an endemic region. In this study, there were a total of 25 ‘intermediate’ ICA results which shows there are inconclusive results so it is advisable that intermediate results should warrant evaluation of second samples for further confirmation to avoid wrong reporting.
Our study suggests that, in regions of Malaysia where MAT is not easily available, ICA could be the most reliable approach for laboratory confirmation of clinically suspected cases of leptospirosis. The test kit has several other advantages. It is rapid (15 minutes), and easy to handle without the need of sophisticated equipment. It can be performed in laboratories or in the field and is suitable as a point of care test. The test can be used in resource-poor settings in Malaysia, by investigators with only limited training. Cost-effectiveness is also the main decision factor to choose ICA for routine use in remote areas.
Specificity is also a problem when using antibody detection based rapid tests. Cross reacting antibodies occur with many other cases of acute febrile illnesses.4 A total of 35 various infectious disease serum samples (not included in the 197 samples) were taken from the serology reference laboratories of Institute for Medical Research Kuala Lumpur to be used to test the cross reactivities of ImmuneMed Leptospira IgM Duo (ICA) Rapid tests with other causes of acute febrile illnesses. The percentage of cross reactivity is displayed in Table 2. Among other infections tested in this study, cross reactivities occurred when ICA was performed on samples that were positive for dengue, VDRL as well as scrub typhus with the percentage cross reactivities of 10%, 20%, and 20%, respectively (Table 2). It is particularly difficult to determine which would be the actual diagnoses in such circumstances unless diagnosis can be confirmed with other methods like culture or PCR to detect the DNA of the respective bacteria or virus. The selection of a serodiagnostic assay is very much dependent on several factors, like for example, the clinical possibility of disease, the anticipated workload, and the availability of confirmatory testing in more specialized laboratories.15 Therefore, in view of the emerging infectious threat, prompt diagnosis of leptospirosis is essential for both patient care and efficient implementation of public health measures. It is most important to have an efficient and practical diagnostic test that is rapid, accessible and practical to clinicians as a screening test while waiting for confirmatory test results.16
Table 2. Cross reactivities of ImmuneMed Leptospira IgM Duo (ICA) Rapid tests with other causes of acute febrile illnesses.
| Total No. of serum samples tested positive serologically to other types of infectious diseases | ImmuneMed Leptospira IgM Duo Rapid test | Percentage of cross reactivity | |
|---|---|---|---|
| Positive | Negative | ||
| Dengue (n = 10) | 1 | 9 | 10 |
| Typhus (n = 5) | 0 | 5 | 0 |
| Syphilis (VDRL) (n = 5) | 1 | 4 | 20 |
| Scrub typhus (n = 10) | 2 | 8 | 20 |
| Melioidosis (n = 5) | 0 | 5 | 0 |
ICA = immunochromatographic assay, VDRL = venereal disease research laboratory.
In conclusion, the ImmuneMed Leptospira IgM Duo Rapid test kit (ICA manufactured by the ImmuneMed Inc.) is a practical screening test with reasonably good diagnostic sensitivity and specificity. This test may be sufficiently sensitive for screening tests and can reasonably avoid false positive reporting with the high PPV. To avoid false positive reporting, it is recommended that samples that only showed positive at the IgM 50 cut-off titre (intermediate) be repeated with a second sample from the same patient collected a few days later or at convalescent.
ACKNOWLEDGMENTS
We thank the Director General of Ministry of Health Malaysia for permission to publish this manuscript.
Footnotes
Funding: This study was supported by the Diagnostic Operational Budget 2014 from the Leptospirosis Laboratory of Bacteriology Unit, Institute for Medical Research Kuala Lumpur, under the Ministry of Health Malaysia.
Disclosure: The authors of this study have no works in relation with the company of ImmuneMed Inc., Chuncheon, Korea or its products. The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Amran F. Data curation: Amran F, Noor Halim NA. Formal analysis: Noor Halim NA. Investigation: Amran F. Methodology: Noor Halim NA. Writing - original draft: Ling LY. Writing - review & editing: Amran F.
SUPPLEMENTARY MATERIAL
Raw data of serum samples
References
- 1.Office International des Épizooties (OIE) OIE Terrestrial Manual 2014. Paris, France: OIE; 2014. Leptospirosis; p. Chapter 2.1.9. [Google Scholar]
- 2.Hartskeerl RA, Collares-Pereira M, Ellis WA. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clin Microbiol Infect. 2011;17:494–501. doi: 10.1111/j.1469-0691.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan WL, Soelar SA, Azri M, Hussin N, Cheah WK, Verasahib K, et al. Leptospirosis incidence and mortality in Malaysia. Southeast Asian J Trop Med Public Health. 2016;47:434–440. [PubMed] [Google Scholar]
- 4.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disease Control Division Department of Public Health Ministry of Health Malaysia. Guidelines for the Diagnosis, Management, Prevention and Control of Leptospirosis in Malaysia. 1st ed. Putrajaya: Ministry of Health Malaysia; 2011. [Google Scholar]
- 6.Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46(4):245–252. doi: 10.1016/j.jmii.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Niloofa R, Fernando N, de Silva NL, Karunanayake L, Wickramasinghe H, Dikmadugoda N, et al. Diagnosis of leptospirosis: comparison between microscopic agglutination test, IgM-ELISA and IgM rapid immunochromatography test. PLoS One. 2015;10(6):e0129236. doi: 10.1371/journal.pone.0129236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Human leptospirosis: guidance for diagnosis, surveillance and control. [Updated 2003]. [Accessed August 8, 2017]. http://www.who.int/iris/handle/10665/42667.
- 9.Goris MG, Leeflang MM, Boer KR, Goeijenbier M, van Gorp EC, Wagenaar JF, et al. Establishment of valid laboratory case definition for human leptospirosis. J Bacteriol Parasitol. 2012;3:132. [Google Scholar]
- 10.Morgan J, Bornstein SL, Karpati AM, Bruce M, Bolin CA, Austin CC, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34:1593–1599. doi: 10.1086/340615. [DOI] [PubMed] [Google Scholar]
- 11.Courdurie C, Le Govic Y, Bourhy P, Alexer D, Pailla K, Theodose R, et al. Evaluation of different serological assays for early diagnosis of leptospirosis in Martinique (French West Indies) PLoS Negl Trop Dis. 2017;11(6):e0005678. doi: 10.1371/journal.pntd.0005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Jalii IM, Bahaman AR. A review of human leptospirosis in Malaysia. Trop Biomed. 2004;21:113–119. [PubMed] [Google Scholar]
- 13.Thayaparan S, Robertson ID, Fairuz A, Suut L, Abdullah MT. Leptospirosis, an emerging zoonotic disease in Malaysia. Malays J Pathol. 2013;35:123–132. [PubMed] [Google Scholar]
- 14.Lim JK, Murugaiyah VA, Ramli A, Abdul Rahman H, Mohamed N, Shamsudin N, et al. A case study: leptospirosis in Malaysia. Webmedcentral. 2011;2(12):WMC002764 [Google Scholar]
- 15.Panwala T, Rajdev S, Mulla S. To evaluate the different rapid screening tests for diagnosis of leptospirosis. J Clin Diagn Res. 2015;9(2):DC21–DC24. doi: 10.7860/JCDR/2015/11188.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekhar WY, Soo EH, Gopalakrishnan V, Devi S. Leptospirosis in Kuala Lumpur and the comparative evaluation of two rapid commercial diagnostic kits against the MAT test for the detection of antibodies to leptospira interrogans. Singapore Med J. 2000;41:370–375. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data of serum samples