Abstract
Hearing loss, including genetic hearing loss, is one of the most common forms of sensory deficits in humans with limited options of treatment. Adeno-associated virus (AAV)-mediated gene transfer has been shown to recover auditory functions effectively in mouse models of genetic deafness when delivered at neonatal stages. However, the mouse cochlea is still developing at those time points, whereas in humans, the newborn inner ears are already fully mature. For effective gene therapy to treat genetic deafness, it is necessary to determine whether AAV-mediated therapy can be equally effective in the fully mature mouse inner ear without causing damage to the inner ear. This study tested several AAV serotypes by canalostomy in adult mice. It is shown that most AAVs transduce the sensory inner hair cells efficiently, but are less efficient at transducing outer hair cells. A subset of AAVs also transduces non-sensory cochlear cell types. Neither the surgical procedure of canalostomy nor the AAV serotypes damage hair cells or impair normal hearing. The studies indicate that canalostomy can be a viable route for safe and efficient gene delivery, and they expand the repertoire of AAVs to target diverse cell types in the adult inner ear.
Keywords: : AAV, inner ear, hair cells, adult mouse, hearing
Introduction
Hearing loss is one of the most common forms of sensory deficits, affecting 360 million people (5% of the world's population), including 32 million children (www.who.int/en/). Hereditary hearing loss occurs at a frequency of 1 in 500 newborns, accounting for 70% of congenital sensorineural hearing loss.1,2 In recent years, major advances have been made in the identification of genes in which mutations cause genetic hearing loss. More than 100 deafness genes have been identified so far (http://hereditaryhearingloss.org/). In contrast, there is no medical treatment for severe hearing loss beyond cochlea implants. There is thus an urgent need to develop treatments for hearing loss due to the discovery of increasing number of deafness genes. Such treatments may include the supplement of missing genes, correction of gene mutations, drug treatment, and prenatal and pre-implantation diagnosis of genetic hearing loss.3
Gene therapy has emerged as a new platform of treatment for hereditary diseases,4–9 including genetic hearing loss. Viral vector-mediated gene therapy is most commonly used to supplement normal copies of genes whose functions have been lost. For the inner ear, various viral vectors have been studied for gene delivery in vivo, including adenovirus,10,11 adeno-associated virus (AAV),12–22 helper-dependent adenovirus,23 bovine adeno-associated virus,15 and herpes simplex virus.24 Among them, AAV-mediated gene therapy has demonstrated recovery of auditory functions in mouse models of genetic deafness with good efficacy, safety, and long-term effects, particularly by targeting the auditory sensory cells, hair cells (HCs).12,13,20,22
Different AAV serotypes have been successfully delivered into the neonatal mouse inner ear to target a wide range of cell types, including HCs, supporting cells (SCs), stria vascularis, and auditory neurons,25–28 providing opportunities to treat genetic hearing loss due to defective genes in different cell types. Routes of AAV delivery into the neonatal inner ear include cochleostomy or through the round window membrane (RWM), which direct the delivery into scala media or scala tympani, respectively.29 While similar approaches have been studied for adult inner-ear delivery, the results were less encouraging due to damage to inner-ear cells, particularly HCs, and consequent hearing loss. Application of gene therapy to treat genetic deafness requires the delivery into the mature inner ear, as human newborn inner ears are fully developed. Further, the inner-ear cell types targeted by AAVs could be different in neonatal and adult stages. It is therefore essential to establish the tropism of AAV vectors in the adult mammalian inner ear, as well as an efficient delivery route that does not damage inner-ear cells and preserves normal hearing. A recent study of AAV delivery via the posterior semicircular canal (PSCC) provided a safe and efficient delivery method for the adult mouse inner ear,28 supporting that the PSCC could be explored for the delivery of diverse AAV vectors.
Previously, 12 AAV vectors were screened in the neonatal and adult mouse inner ear by cochleostomy, and significant damages were shown to be induced in the adult inner ear.25 To identify effective and safe transduction of AAV vectors into the adult mouse inner ear, eight AAV serotypes and one adenovirus were selected for adult delivery by canalostomy. High-efficiency transduction of HCs was shown, especially inner hair cells (IHCs), by most of the AAVs, whereas outer hair cells (OHCs) are less permissive to gene transfer with most AAVs. Further, other adult inner-ear cell types infected by a subset of AAVs were identified. Importantly, canalostomy does not damage HCs or impair normal hearing. The study demonstrates that multiple AAV vectors can be safely and efficiently delivered via canalostomy into the adult mouse inner ear while preserving normal hearing.
Materials and Methods
Mice
All procedures met the National Institutes of Health guidelines for the care and use of laboratory animals, and were approved by the Massachusetts Eye and Ear Infirmary IACUC committee. Ten-week C57BL/6J male mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Eight-week CD1 mice were purchased from Charles River Laboratories (Wilmington, MA). The mice were randomly assigned to the different experimental groups, with at least three mice in each group.
Production of virus
AAVs of different serotypes, including AAV1, 2, 6.2, 8, 9, rh.39, and rh.43, were produced at Horae Gene Therapy Center of University of Massachusetts Medical School (Worcester, MA). All AAVs have a green fluorescent protein (GFP) reporter under the control of a chicken beta-actin promoter.25,30 The titers of each AAV were 1.8 × 1013, 1 × 1013, 8 × 1012, 9 × 1012, 1 × 1013, 1 × 1013, and 6 × 1012 genome copies (GCs)/mL, respectively. AAV2/Anc80L65.CMV.EGFP.WPRE (AAV2/Anc80L65)27 was produced at the Gene Transfer Vector Core within the Grousbeck Gene Therapy Center of Harvard Medical School (Boston, MA; http://vector.meei.harvard.edu/) with a titer of 5.6 × 1012 GCs/mL. Ad5-CMV-EGFP was purchased from Baylor College of Medicine (Houston, TX) with a titer of 4 × 1012 plaque-forming units (pfu)/mL.
Microinjection system
A fine polyimide tube (inner diameter 0.0039 inch, outer diameter 0.0050 inch; MicroLumen, Tampa, FL) was connected to a glass micropipette (WPI, Sarasota, FL) and held by a Nanoliter 2000 micromanipulator (WPI). The release speed was 179 nL/min, controlled by a MICRO4 microinjection controller (WPI). The total virus volume was 1 or 2 μL without dilution for each injection.
Canalostomy
Mice were anesthetized using an intraperitoneal injection of xylazine (10 mg/kg) and ketamine (100 mg/kg). The right post-auricular region was shaved and disinfected with 10% povidone-iodine. Under the operating microscope, a 10 mm post-auricular incision was made, and the right pinna and sternocleidomastoid muscle were extracted to expose the PSCC, located in the margin of temporal bone. A small hole was drilled in the PSCC with a Bonn micro probe (Fine Science Tools, Foster City, CA) and then left open for a couple of minutes until there was no obvious perilymph leakage. The tip of the polyimide tube was inserted into the PSCC toward the ampulla, and tissue adhesive (3M Vetbond, St. Paul, MN) was used to seal the hole. The tightness of the seal was visually confirmed by the absence of perilymph leakage. After injection, the tubing was cut, and approximately 5 mm of tubing was left connected to the PSCC. The residual tubing was sealed by tissue adhesive, and then the skin was closed with 5-0 Ethilon suture (Ethicon, Inc., Somerville, NJ). The total surgery duration was approximately 20 min, including a 6 min injection period.28
Auditory testing
Two weeks after injection, auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE) recordings were conducted, as described previously,25,31 at 32°C in a soundproof chamber. Mice were anesthetized by intraperitoneal injection of xylazine (10 mg/kg) and ketamine (100 mg/kg). Acoustic stimuli were delivered through a custom acoustic assembly consisting of two miniature dynamic electrostatic earphones (CDMG15008-03A, CUI) to generate primary tones and a miniature microphone (FG-23329-PO7, Knowles) to record ear-canal sound pressure near the eardrum. Custom LabVIEW software controlling National Instruments 24-bit soundcards (6052E) generated all ABR/DPOAE stimuli and recorded all responses.
To measure ABRs, needle electrodes were inserted at the vertex, the ventral edge of the pinna, and a ground reference near the tail. ABR potentials were evoked with 5 ms tone pips (0.5 ms rise–fall with a cos2 onset, delivered at 35/s). The response was amplified (10,000 × ), filtered (100 Hz–3 kHz) pass-band, digitized, and averaged (1,024 responses) at each SPL. The sound level was raised in 5 dB steps from 30 dB (below typical threshold) up to 90 dB SPL at frequencies from 5.66 to 45.25 kHz (in half-octave steps). Upon visual inspection of stacked waveforms, “threshold” was defined as the lowest SPL level at which any wave could be detected. Threshold was defined as 95 dB SPL if no wave exhibited at 90 dB SPL.
To measure DPOAEs, the cubic distortion product was recorded in response to primaries f1 and f2, and the primary tones were set so that the frequency ratio (f2/f1) was 1.2 and so that f2 level was 10 dB SPL below f1 level. For each f2/f1 primary pair, primaries were swept in 5 dB steps from 20 dB SPL to 80 dB SPL (for f2). At each level, the amplitude of the DPOAE at 2f1–f2 was extracted from the averaged spectra, along with the noise floor. Threshold was computed by interpolation as the f2 level required to produce a DPOAE at 5 dB SPL.
Immunohistochemistry
All injected and non-injected cochleae were harvested after animals were sacrificed by CO2 inhalation, immediately after ABR/DPOAE measurement. Temporal bones were fixed in 4% paraformaldehyde at 4°C overnight and then decalcified in 120 mM of EDTA for at least 5 days. The cochleae were dissected in pieces from the decalcified tissue for whole-mount immunofluorescence. Tissues were infiltrated with 0.3% Triton X-100 and blocked with 8% donkey serum for 1 h before primary antibodies. They were incubated with 1:500 rabbit anti-MYO7A (#25-6790; Proteus BioSciences, Ramona, CA), 1:750 chicken anti-GFP (ab13970; Abcam, Cambridge, United Kingdom), and 1:350 goat anti-SOX2 (sc-17319; Santa Cruz, Dallas, TX) overnight at room temperature, and then secondary antibodies for 1 h after three rinses with phosphate-buffered saline. All Alexa Fluor secondary antibodies were from Invitrogen (Carlsbad, CA): goat anti-chicken Alexa Fluor 594 (A-11042), donkey anti-goat Alexa Fluor 647 (A-21447), and donkey anti-rabbit Alexa Fluor 647 (A-31573) were used at 1:500 dilution. DAPI (5 mg/mL, D3571; Life Technologies, Carlsbad, CA) was added in secondary antibodies at 1:500 dilution. Specimens were mounted in ProLong Gold Antifade Mountant medium (P36930; Life Technologies).
Cell counting quantification
Confocal images were taken with a Leica TCS SP5 microscope using a 20 × or 63 × glycerin-immersion lens. For IHC and OHC counting, z-stacks were acquired by maximum intensity projections of z-stacks for each segment by ImageJ, and composite images showing the whole cochlea were constructed in Adobe Photoshop. A frequency map was constructed for each cochlea by measuring the spiral extent of all the dissected cochlear pieces and converting cochlear location to frequency using a plug-in of ImageJ (www.masseyeandear.org/research/ent/eaton-peabody/epl-histology-resources/imagej-plugin-for-cochlear-frequency-mapping-in-whole-mounts/). MYO7A-positive IHCs and OHCs and non-HC cells (by position and morphology) were counted in a region spanning 200 μm at different frequencies. All MYO7A-negative cells from the limbus to the edge of outer sulcus are considered non-HC cells. Those non-HC cells in the sensory epithelia are SCs. They include inner border cells (IBCs), inner phalangeal cells (IPhCs), inner and outer pillar cells (IPCs and OPCs), Deiters' cells (DCs), and Hensen's cells (HeCs).
Statistical analysis
Statistical analyses were performed using Prism v6.0 (GraphPad Software, San Diego, CA). Analysis of variance (ANOVA) with Bonferroni corrections was used for multiple comparisons followed by pair-wise comparisons for ABRs and DPOAEs, and Student's t-test for quantification of HC survival and virus transduction efficiencies.
Results
AAVs transduce cochlear HCs in adult mice
It has previously been shown that AAV delivery into the adult mammalian cochlea via cochleostomy induces significant HC death, particularly in OHCs.14,25 Compared to cochleostomy, canalostomy has been shown to be a safer route for gene delivery, with no HC death after injection.28 The main aims of this study were to use canalostomy to deliver different AAV serotypes into the adult cochlea, and to evaluate the transduction of AAVs in cochlear cell types and the potential impact on normal hearing.
Eight AAV vectors of different serotypes were used (AAV1, 2, 6.2, 8, 9, rh.39, rh.43, Anc80L65). These were injected into the 10-week-old adult mouse cochlea by canalostomy, and auditory function was studied two weeks post injection followed by evaluation of AAV transduction in the cochlea. Immunohistochemistry was performed with an anti-GFP antibody to detect GFP, in addition to endogenous AAV-mediated GFP expression. HCs were labeled by HC marker MYO7A. Each adult cochlea was dissected into pieces for imaging, and infected cells and HC survival were evaluated according to the mouse cochlea frequency map.32
In the auditory system, IHCs function by converting mechanical stimuli of fluid vibrations of the cochlea induced by sounds into electrical signals, whereas OHCs are responsible for amplification of the vibrations in the cochlea that are transduced by IHCs.33 By canalostomy, injected AAVs traveled from apex to base in a gradient, which may result in different infection rates along the length of the cochlea.
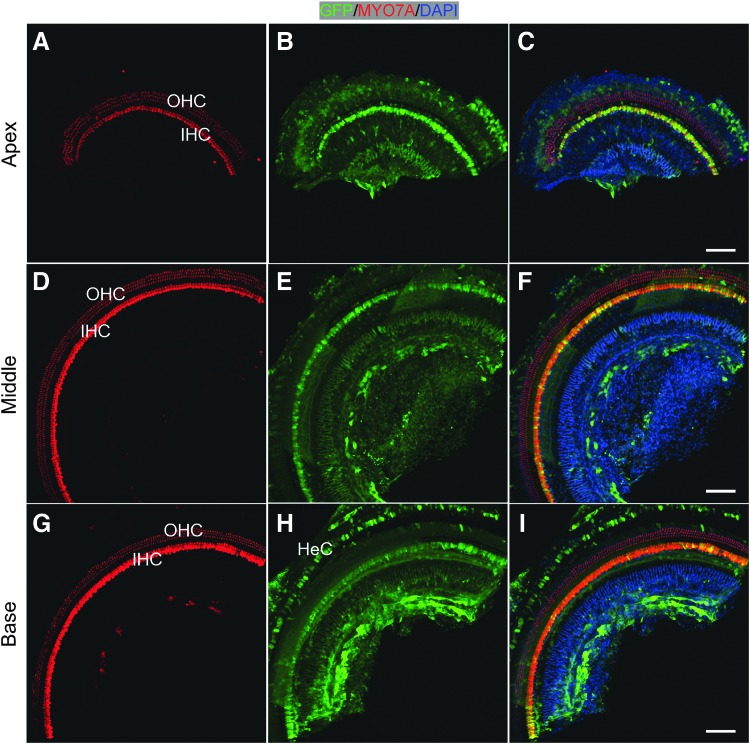
We first injected AAVrh.43 into 10-week-old adult mouse cochlea, and analysis was performed 2 weeks later. Overall, one row of IHCs and three rows of OHCs were arrayed regularly across the entire length of the cochlea in the injected ear, shown in the representative regions of the apex (corresponding to the frequencies 5.66–8 kHz), middle (corresponding to the frequencies 16–22.64 kHz), and base (corresponding to the frequencies 32–45.25 kHz), an indication that no major HC death was induced by adult cochlea injection or AAV infection (Fig. 1). There was uniform IHC infection by AAVrh.43 across the cochlea, with more than 25 IHCs per 200 μm (representing 93.2–99.2% of all IHCs) becoming GFP+ (Table 1). The highest GFP intensity was found in the apex, corresponding to the low-frequency region, while the lowest GFP intensity was in the base, corresponding to the high-frequency region, suggesting that either the number of AAV particles entering IHCs diminished as the AAV traveled from apex to base, or the regional IHCs with different characteristics reacted differently to AAV infection (Fig. 1). OHCs were largely devoid of GFP signal. In addition, AAVrh.43 consistently infected HeCs in the base, a SC subtype within the sensory epithelium (Fig. 1G–I).
Figure 1.
Adeno-associated virus (AAV) rh.43 transduced inner hair cells (IHCs) uniformly throughout the cochlea in adult mice. Hair cells (HCs) were labeled with MYO7A (red). Two weeks after injection via the posterior semicircular canal (PSCC), whole-mount immunofluorescence showed that virtually all the IHCs were green fluorescent protein (GFP) positive from apex (A–C), to middle (D–F), to base (G–I), with more intense GFP signals in the apical turn. AAVrh.43 also infected Hensen's cells (HeCs). Some non-sensory cells medial to IHCs were also infected. No HC death was observed throughout the cochlea. Scale bars: 100 μm.
Table 1.
Efficiency of HC infection by AAV injection through canalostomy
| Number/percentage of GFP+ HCs by frequencies (kHz) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serotype | Cell type | 5.66 | 8 | 11.32 | 16 | 22.64 | 32 | 45.25 |
| AAV1 | IHC | 2.8 ± 0.4 | 2.1 ± 0.5 | 1.2 ± 0.3 | 3.2 ± 1.3 | 1.8 ± 0.2 | 1.9 ± 0.2 | 1.3 ± 0.3 |
| 10.4 ± 1.3% | 7.9 ± 2.0% | 4.4 ± 1.0% | 11.8 ± 4.7% | 6.6 ± 0.9% | 7.0 ± 0.6% | 4.9 ± 1.2% | ||
| OHC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAV2 | IHC | 24.2 ± 4.9 | 25.8 ± 2.2 | 26.7 ± 0.5 | 23.4 ± 0.5 | 25.5 ± 1.5 | 23.1 ± 4.6 | 13.1 ± 10.0 |
| 89.6 ± 18.0% | 95.4 ± 8.0% | 98.9 ± 1.9% | 86.5 ± 13.9% | 94.4 ± 5.7% | 85.5 ± 17.0% | 48.5 ± 36.9% | ||
| OHC | 16.6 ± 13.0 | 9.8 ± 10.0 | 8.7 ± 7.9 | 5.8 ± 2.4 | 6.8 ± 6.2 | 1.3 ± 1.3 | 0 | |
| 20.5 ± 16.0% | 12.1 ± 12.3% | 10.8 ± 9.7% | 7.1 ± 3.0% | 8.4 ± 7.7% | 1.6 ± 1.6% | 0.1 ± 0.2% | ||
| AAV2/Anc80L65 | IHC | 26.4 ± 1.0 | 26.6 ± 0.7 | 26.0 ± 0.9 | 26.6 ± 0.7 | 21.9 ± 8.5 | 24.1 ± 5.0 | 22.7 ± 4.1 |
| 97.8 ± 3.8% | 98.4 ± 2.7% | 96.3 ± 3.2% | 98.4 ± 2.7% | 81.1 ± 31.3% | 89.3 ± 18.5% | 84.1 ± 15.1% | ||
| OHC | 57.4 ± 23.5 | 54.4 ± 23.1 | 54.8 ± 25.1 | 32.2 ± 25.7 | 12.3 ± 10.9 | 8.4 ± 14.7 | 2.0 ± 3.5 | |
| 70.9 ± 29.8% | 67.2 ± 28.5% | 67.7 ± 31.0% | 39.7 ± 31.7% | 15.2 ± 13.4% | 10.4 ± 18.1% | 2.5 ± 4.3% | ||
| AAV6.2 | IHC | 2.6 ± 0.4 | 3.2 ± 0.5 | 1.2 ± 0.3 | 0.8 ± 0.2 | 1.1 ± 0.2 | 0.7 ± 0.2 | 0.9 ± 0.2 |
| 9.6 ± 1.3% | 11.9 ± 1.7% | 4.4 ± 1.0% | 3.1 ± 0.8% | 4.0 ± 0.9% | 2.6 ± 0.6% | 3.3 ± 0.7% | ||
| OHC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAV8 | IHC | 16.8 ± 2.1 | 16.5 ± 5.1 | 19.4 ± 4.6 | 21.7 ± 3.4 | 24.9 ± 5.0 | 19.6 ± 7.4 | 17.8 ± 13.4 |
| 62.2 ± 7.7% | 61.0 ± 18.9% | 71.7 ± 16.9% | 80.3 ± 12.7% | 92.4 ± 18.6% | 72.6 ± 27.5% | 65.9 ± 49.7% | ||
| OHC | 1.5 ± 1.6 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5.5 ± 6.1% | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AAV9 | IHC | 16.8 ± 2.2 | 15.9 ± 2.1 | 16.4 ± 3.2 | 16.7 ± 5.1 | 24.1 ± 5.0 | 13.3 ± 3.2 | 15.4 ± 8.3 |
| 62.2% ± 8.0% | 58.8% ± 7.6% | 60.6% ± 11.8% | 61.8% ± 19.0% | 89.4 ± 18.6% | 49.3 ± 11.9% | 56.9 ± 30.7% | ||
| OHC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAVrh.39 | IHC | 15.0 ± 4.2 | 11.4 ± 3.2 | 20.3 ± 3.2 | 9.8 ± 1.3 | 17.8 ± 2.0 | 16.5 ± 2.1 | 9.9 ± 4.6 |
| 55.6 ± 15.7% | 42.3 ± 12.0% | 75.0 ± 11.8% | 36.2 ± 4.7% | 65.9 ± 7.5% | 61.1 ± 7.9% | 36.6 ± 17.2% | ||
| OHC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAVrh.43 | IHC | 26.8 ± 0.4 | 25.2 ± 0.8 | 25.5 ± 2.2 | 25.0 ± 1.7 | 25.4 ± 1.8 | 25.5 ± 1.1 | 25.8 ± 1.5 |
| 99.2 ± 1.4% | 93.2 ± 3.1% | 94.4 ± 8.2% | 92.7 ± 6.1% | 94.2 ± 6.4% | 94.3 ± 3.9% | 95.6 ± 5.6% | ||
| OHC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
The infected cells are indicated by the number per 200 μm as well as by the percentage of all respective HCs.
HC, hair cells; AAV, adeno-associated virus; GFP, green fluorescent protein; IHC, inner hair cells; OHC, outer hair cells.
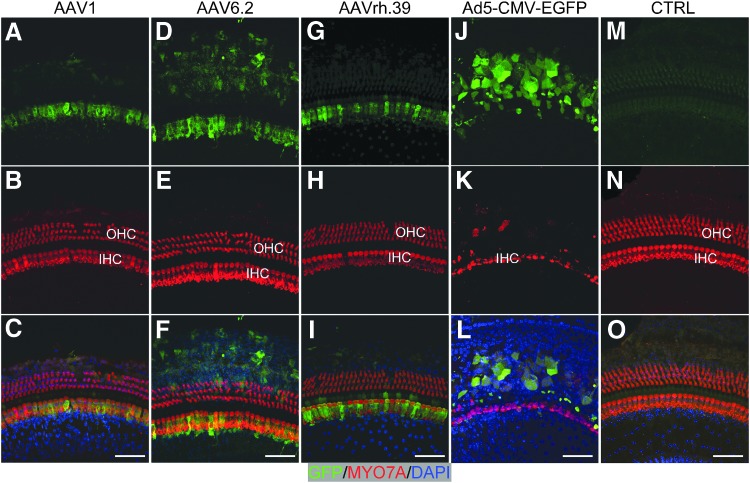
Subsequently, seven AAV vectors were studied. Among them, five AAVs (AAV2, 2/Anc80L65, 8, 9, and rh.39) infected IHCs with different efficiencies. High-efficiency transduction was detected with AAV2, with 25.5 ± 1.5–26.7 ± 0.5 GFP+ IHCs per 200 μm (∼95% of IHCs) in the cochlear regions that correspond to the sounds of 8, 11.32, and 22.64 kHz (Fig. 2A–C). The transduction efficiency was slightly lower in other cochlear regions, shown by fewer GFP+ IHCs (24.2 ± 4.9 [5.66 kHz], 23.4 ± 0.5 [16 kHz], 23.1 ± 4.6 [32 kHz], and 13.1 ± 10.0 [45.25 kHz] per 200 μm, respectively; Table 1). AAV2/Anc80L65, a predicted ancestor of the AAV serotypes 1, 2, 8, and 9 by evolutionary lineage, has been shown to infect IHCs and OHCs efficiently in neonatal cochlea34 through RWM and canalostomy. In the adult cochlea, AAV2/Anc80L65 transduced more than 26 IHCs per 200 μm (96% of all IHCs) in low-frequency regions (5.66–16 kHz), and 21.9–24.1 IHCs per 200 μm (81–89%) in high-frequency regions (22.64–45.25 kHz). AAV8 and AAV9 transduced IHCs in all cochlear regions, but at lower rates: AAV8 transduced 21.7–24.9 IHCs per 200 μm (80–92%) in the regions corresponding to 16–22.64 kHz (Fig. 2G–I), and 16.5–19.4 IHCs per 200 μm (61–73%) for the remaining of cochlea. AAV9 transduced 13.3–16.8 IHCs per 200 μm (49–62%) in all cochlear regions, with the exception of 24.1 ± 5.2 transduced IHCs per 200 μm (89.4 ± 18.6%) at the region corresponding to 22.64 kHz (Fig. 2J–L; Table 1). AAVrh.39 transduced 15.0–20.3 IHCs per 200 μm at 5.66, 11.32, 22.64, and 32 kHz, with lower efficiency at other frequencies (Fig. 3 and Table 1). AAV1 and AAV6.2 infected only IHCs with low efficiency and a low level of GFP expression (Fig. 3 and Table 1).
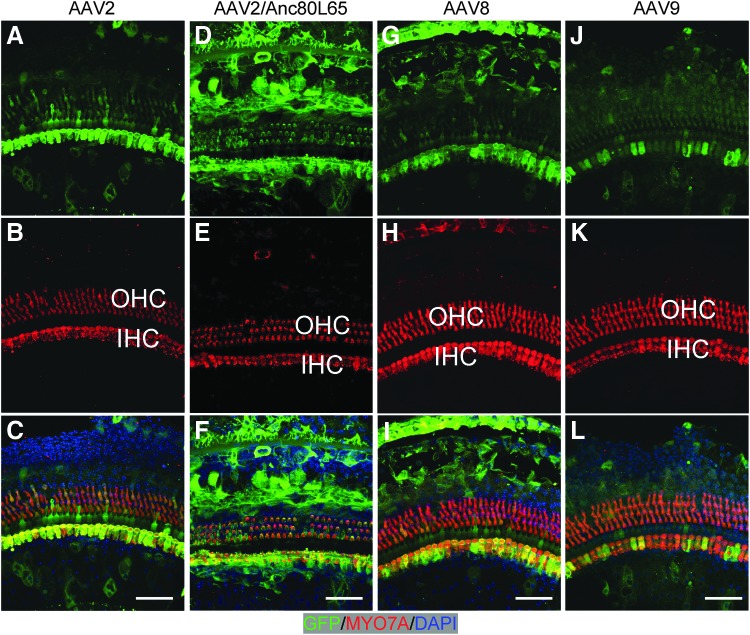
Figure 2.
AAV vectors transduced diverse inner-ear cell types in adult cochleae. Two weeks after injection via canalostomy, whole-mount immunofluorescence showed AAV infection in the sensory and non-sensory cells from the apical turn of the cochlea. Hair cells were labeled with MYO7A (red). (A–C) AAV2 infected most IHCs and some outer hair cells (OHCs). (D–F) AAV2/Anc80L65 infected most IHCs as well as most OHCs. (G–I) AAV8 infected most IHCs, with weak expression in some OHCs. (J–L) AAV9 infection was limited to some IHCs. Scale bars: 50 μm.
Figure 3.
AAV vectors transduced inner-ear cells in adult cochleae. Two weeks post injection through canalostomy, whole-mount immunofluorescence showed AAV infection in the sensory and non-sensory cells from the apical turn of the cochlea. Hair cells were labeled with MYO7A (red). AAV1 (A–C), AAV6.2 (D–F), and AAVrh.39 (G–I) infected mostly IHCs, with moderate expression level. OHCs were not transduced. (J–K) Adult cochlea infected by Ad5-CMV-GFP using the same procedure. Ad5-CMV-GFP injection killed most OHCs without infection of IHCs. Some non-HCs in the sensory region were GFP positive although their cell types were unknown. (M–O) An example of an uninjected cochlea in which no GFP was detected, whereas the contralateral cochlea was injected with AAVrh.43 that infected IHCs. Scale bars: 50 μm.
Generally, AAVs transduced adult OHCs less efficiently than IHCs. AAV2 and 2/Anc80L65 transduced some OHCs, with a low level of GFP expression in the adult cochlea. After AAV2 injection, 5.8–16.6 GFP+ OHCs per 200 μm (7–21%) were identified from the region corresponding to 5.66–22.64 kHz. AAV2/Anc80L65 infected more OHCs than AAV2, with 32.2–57.4 GFP+ OHCs per 200 μm (39–71%) in low and middle frequencies, and showed a gradient of decrease from apex to base turn (Fig. 2D and Table 1). Minor OHC death was observed by the number of missing OHCs in AAV2/Anc80L65-infected cochlea (1.31 ± 0.9%; Fig. 2D–F). AAV8 infected only 1.5 ± 1.7 OHCs per 200 μm at 5.66 kHz (Fig. 2G–I and Table 1). AAV1, 6.2, 9, rh.39, and rh.43 did not infect OHCs (Figs. 1, 2J–L, and 3A–I, and Table 1).
AAVs infect SCs and other non-sensory cells in adult mice
The mammalian cochlea consists of multiple non-sensory cell subtypes, including SCs and auditory neurons, all of which play essential functions in hearing. SCs maintain homeostasis of the ionic environment in the endolymph and perilymph, and modulate extracellular matrixes.35 SCs such as pillar cells and DCs provide a structural scaffold to enable mechanical stimulation of sensory HCs. The most commonly mutated gene resulting in human deafness, GJB2, is essential in gap junctions and is primarily expressed in SCs of the inner ear.36
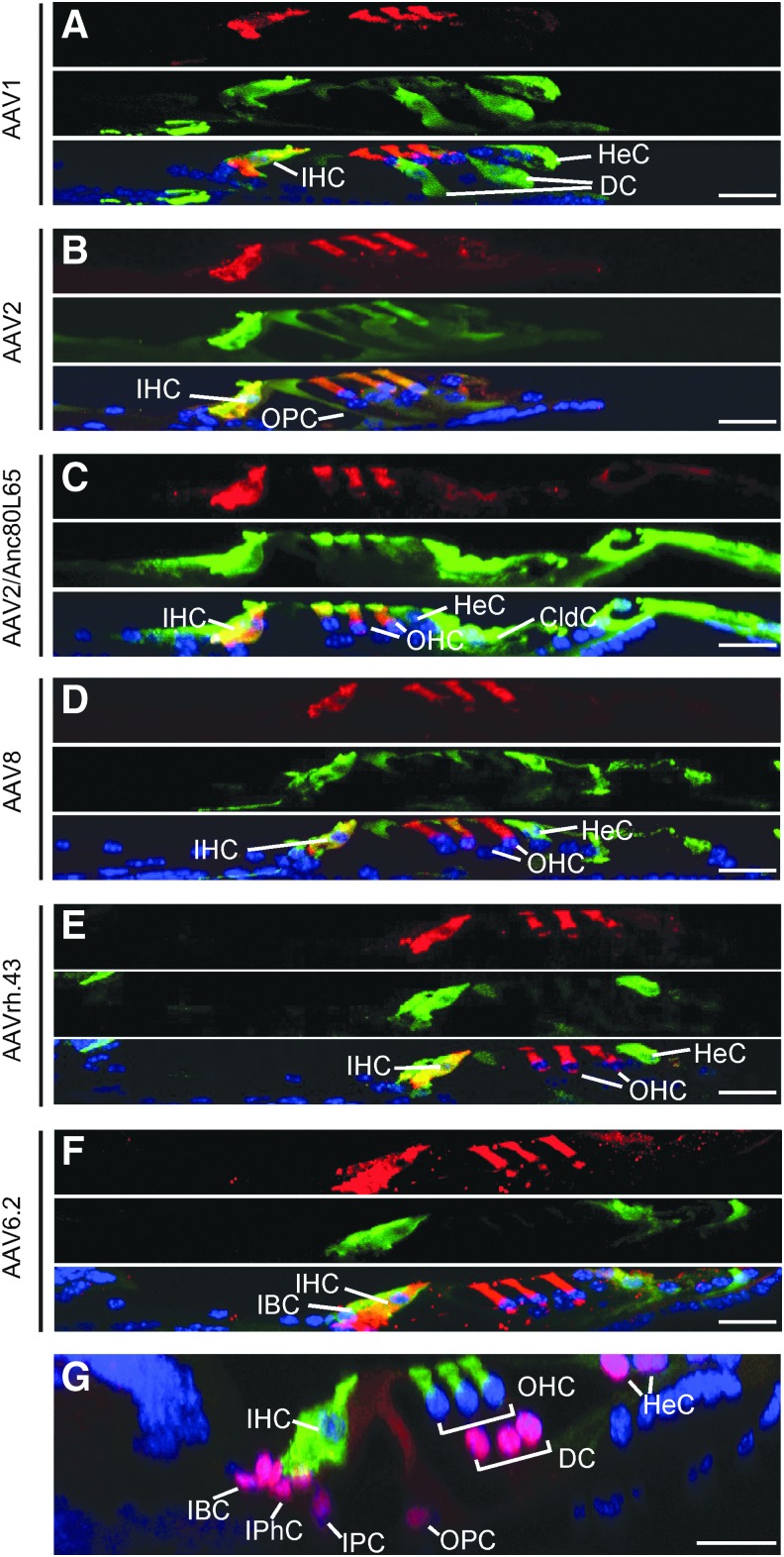
In the sensory epithelium, AAV1, 6.2, and rh.43 transduced SCs more efficiently than other viruses. AAV1 transduced 35.1–42.7 SCs per 200 μm at 5.66 and 8 kHz, and 8.0–19.1 SCs per 200 μm, including DCs and HeCs at middle and high frequencies. AAV6.2 transduced 42.3–67.3 SCs per 200 μm, including IBCs from apex to base, except at 5.66 kHz (30.7 ± 5.1) and at 45.25 kHz (31.0 ± 29.5). AAVrh.43 transduced 17.4–23.8 SCs per 200 μm, including HeCs throughout the whole cochlea (Fig. 4 and Table 2).
Figure 4.
Inner-ear cell subtypes infected by AAV vectors in adult mice. Cross-sections (y–z) of the confocal images of whole-mount immunofluorescence of the middle turn cochlea 2 weeks after AAV delivery by canalostomy. (A) AAV1-infected IHCs, Deiters' cells (DC), and HeCs. (B) AAV2-infected IHCs, OHCs, and outer pillar cells (OPC). (C) AAV2/Anc80L65-infected IHCs, OHCs, HeCs, and Claudius cells (CldC). (D) AAV8-infected IHCs, HeCs, and CldC. (E) AAVrh.43-transduced IHCs and HeCs. (F) AAV6.2-infected IHCs and inner border cells (IBC). (A–F) MYO7A—red; GFP—green, and DAPI—blue. (G) An example of cross-section of an uninjected adult cochlea in which HCs were labeled with a marker MYO7A (green) and SCs labeled with Sox2 (red). Scale bars: 20 μm. IPhC, inner phalangeal cells; IPC, inner pillar cells.
Table 2.
Efficiency of non-HC infection by AAV injection through canalostomy
| AAV1 | Sensory | 42.7 ± 22.1 | 35.1 ± 12.0 | 19.1 ± 4.2 | 13.3 ± 2.2 | 19.8 ± 3.9 | 11.9 ± 1.6 | 8.0 ± 11.8 |
| Non-sensory | 25.7 ± 25.2 | 11.3 ± 11.7 | 5.2 ± 7.3 | 5.3 ± 5.9 | 4.8 ± 4.7 | 3.9 ± 3.4 | 0 | |
| AAV2 | sensory | 6.0 ± 5.5 | 11.1 ± 10.5 | 4.5 ± 3.0 | 1.4 ± 1.3 | 0.4 ± 0.7 | 0 | 0.4 ± 0.5 |
| Non-sensory | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAV2/Anc80L65 | Sensory | 13.9 ± 6.8 | 14.6 ± 6.2 | 8.9 ± 7.9 | 7.7 ± 7.3 | 5.4 ± 4.7 | 5.3 ± 6.8 | 4.0 ± 4.7 |
| Non-sensory | 33.0 ± 16.2 | 37.5 ± 13.5 | 30.6 ± 12.6 | 33.0 ± 12.5 | 25.5 ± 8.3 | 19.8 ± 16.5 | 25.9 ± 21.5 | |
| AAV6.2 | Sensory | 30.7 ± 5.1 | 67.3 ± 28.2 | 42.3 ± 10.6 | 53.0 ± 15.0 | 51.3 ± 7.8 | 60.0 ± 31.8 | 31.0 ± 29.5 |
| Non-sensory | 9.3 ± 4.3 | 13.9 ± 8.3 | 0 | 0 | 0 | 0 | 0 | |
| AAV8 | Sensory | 12.0 ± 6.3 | 10.2 ± 8.2 | 22.1 ± 11.4 | 14.0 ± 7.3 | 17.3 ± 12.1 | 6.8 ± 7.6 | 14.5 ± 10.0 |
| Non-sensory | 17.9 ± 2.6 | 16.8 ± 5.2 | 20.8 ± 5.6 | 27.8 ± 4.5 | 26.0 ± 2.0 | 19.6 ± 7.4 | 20.3 ± 9.1 | |
| AAV9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAVrh.39 | Sensory | 16.8 ± 12.3 | 11.6 ± 7.7 | 7.9 ± 1.3 | 1.7 ± 0.3 | 3.2 ± 0.9 | 3.0 ± 1.5 | 2.4 ± 1.0 |
| Non-sensory | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| AAVrh.43 | Sensory | 23.8 ± 1.3 | 22.0 ± 2.0 | 26.7 ± 0.3 | 17.4 ± 7.7 | 22.5 ± 6.1 | 21.4 ± 3.0 | 23.7 ± 5.8 |
| Non-sensory | 42.1 ± 5.9 | 31.0 ± 13.0 | 35.4 ± 13.1 | 21.4 ± 19.0 | 22.0 ± 27.5 | 12.1 ± 15.5 | 10.0 ± 8.6 |
The infected cells are indicated by the number per 200 μm.
AAV2, 2/Anc80L65, 8, and rh.39 transduced some SCs with lower efficiencies. AAV2 transduced 4.5–11 OPCs per 200 μm at lower frequencies (5.66–11.32 kHz). AAV2/Anc80L65 infected 4.0–14.6 SCs per 200 μm, including HeCs across all frequencies. AAV8 transduced 10.2–22.1 SCs per 200 μm, including HeCs at all frequencies, except 32 kHz (6.8 ± 7.6). AAVrh.39 transduced 7.9–16.8 SCs per 200 μm, including pillar cells and IBCs at 5.66–11.32 kHz, and 1.7–3.2 SCs per 200 μm at other frequencies (Table 2). AAV9 rarely transduced non-HCs in sensory epithelia.
AAV2/Anc80L65 and 8 also transduced non-sensory cells, including Claudius cells outside the sensory epithelial region. AAV2/Anc80L65 transduced more cells in low and middle frequencies, while AAV8 had a higher transduction rate from middle to high frequencies (Table 2).
It is known that in the neonatal cochlea, AAV injected in one ear can migrate to the contralateral side.37 To determine if AAVs delivered by canalostomy in adults have similar features, the uninjected ear was examined for signs of infection 2 weeks after AAV injection to the contralateral ear. In all AAV-injected animals, no GFP-positive cells were observed by antibody labeling, even in the AAV2- and AAV2/Anc80L65-injected animals with high GFP expression level in the IHCs (Fig. 3M–O). Therefore, AAV does not migrate from the injected side to the uninjected contralateral side in the first 2 weeks post injection in adults via canalostomy.
AAV adult delivery does not impair cochlear functions or HCs
A total of 29 10-week-old C57BL/6J male mice were injected with AAVs. All injected mice survived. Surgical wound infection, tympanitis, and vestibular dysfunction were not observed 2 weeks after canalostomy, an indication that it was overall a safe and reliable surgical procedure. To study how each AAV affects hearing, possibly due to surgical procedure, AAV toxicity, or expression of GFP, we performed ABR tests that measure the sound-evoked neural output of the cochlea, as well as DPOAE tests that measure the amplification mediated by the OHCs.31
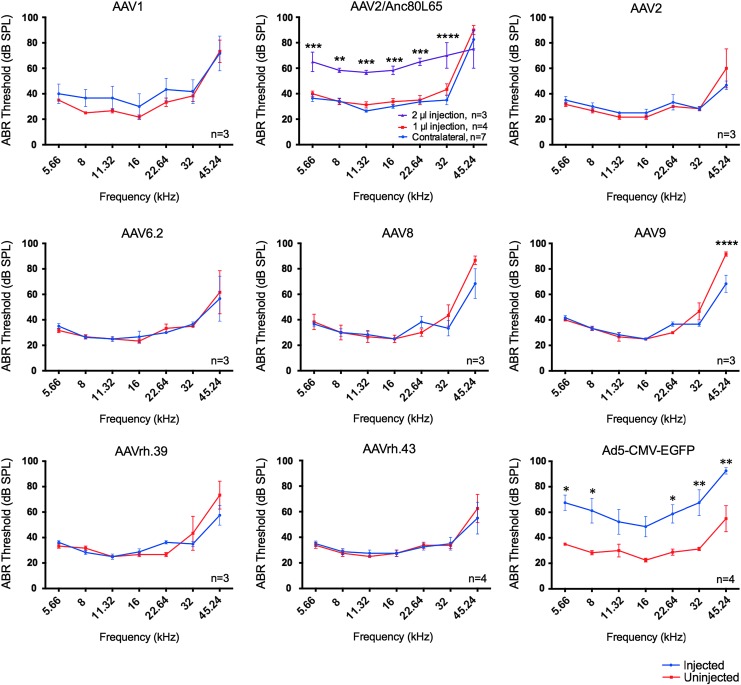
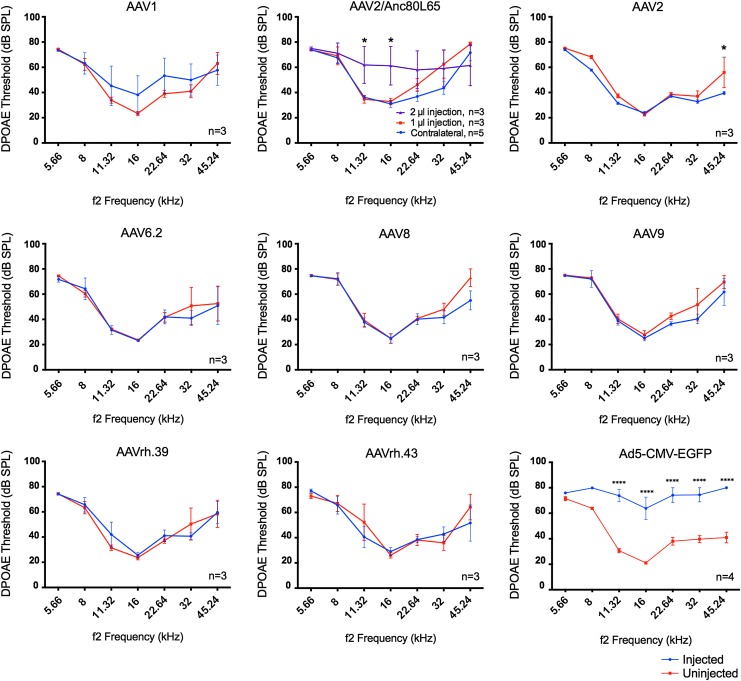
Between injected and uninjected inner ears, ABR thresholds were remarkably similar among different AAVs, illustrating preservation of normal hearing (Fig. 5). Only AAV9-infected inner ears showed significantly reduced ABR thresholds of 23 dB at 45.24 kHz (Fig. 5), an indication of better auditory function in the injected ears that may be mediated by GFP expression. DPOAE thresholds were largely maintained after AAV injections, an indication of preservation of OHC functions (Fig. 6). A significant reduction of 16 dB in DPOAE threshold at 45.24 kHz was observed after AAV2 injection, again an indication of better OHC function by GFP expression. Based on the data, it is concluded that adult injection of AAVs by canalostomy does not significantly affect normal hearing.
Figure 5.
AAV transduction in adults does not impair normal hearing. Among all the AAVs tested at 1 μL, auditory brainstem response (ABR) tests showed no threshold shifts in the AAV-injected adult inner ears compared to the uninjected control ears 2 weeks after canalostomy. ABR thresholds were significantly reduced (i.e., better auditory function) only in AAV9-injected cochleae at 45.24 kHz. When injected with 2 μL, AAV2/Anc80L65-injected cochleae showed significant ABR threshold elevation across all frequencies compared to the 1 μL injection. In contrast, a 1 μL injection of Ad5-CMV-GFP by canalostomy caused significant threshold elevation across all frequencies. Analysis of variance (ANOVA) test: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Figure 6.
AAV transduction by canalostomy in adult does not impair OHC function shown by distortion product otoacoustic emissions (DPOAE). Among all the AAVs tested, there were no DPOAE threshold shifts in the AAV-injected adult inner ears compared to the uninjected control ears 2 weeks after canalostomy. DPOAE thresholds were significantly reduced (i.e., better auditory function) only in AAV2-injected cochleae at 45.24 kHz. Moderately significant threshold reduction was only observed in AAV2-injected cochleae at 45.24 kHz, again an indication of better OHC function by injection. In contrast, injection of Ad5-CMV-GFP by canalostomy caused significant threshold shifts from 11.32 to 45.24 kHz. ANOVA test: *p < 0.05; ****p < 0.0001.
One microliter of AAVs was injected into the adult inner ear without significant hearing impairment. There could be a limit in the volume of virus injected without impairing normal hearing. To determine the upper limit of injection volume, 2 μL of AAV2/Anc80L65 was injected into the adult mouse cochlea. Hearing tests 2 weeks later showed significantly elevated ABR thresholds of 24–35 dB across the frequencies from 5.66 to 32 kHz (Fig. 5; AAV2/Anc80L65) and significantly elevated DPOAE thresholds of 16–30 dB from 11.32 to 32 kHz (Fig. 6; AAV2/Anc80L65). Thus, an injection volume of 1 μL is optimal for adult viral inner-ear delivery, and 2 μL will likely impair normal hearing significantly.
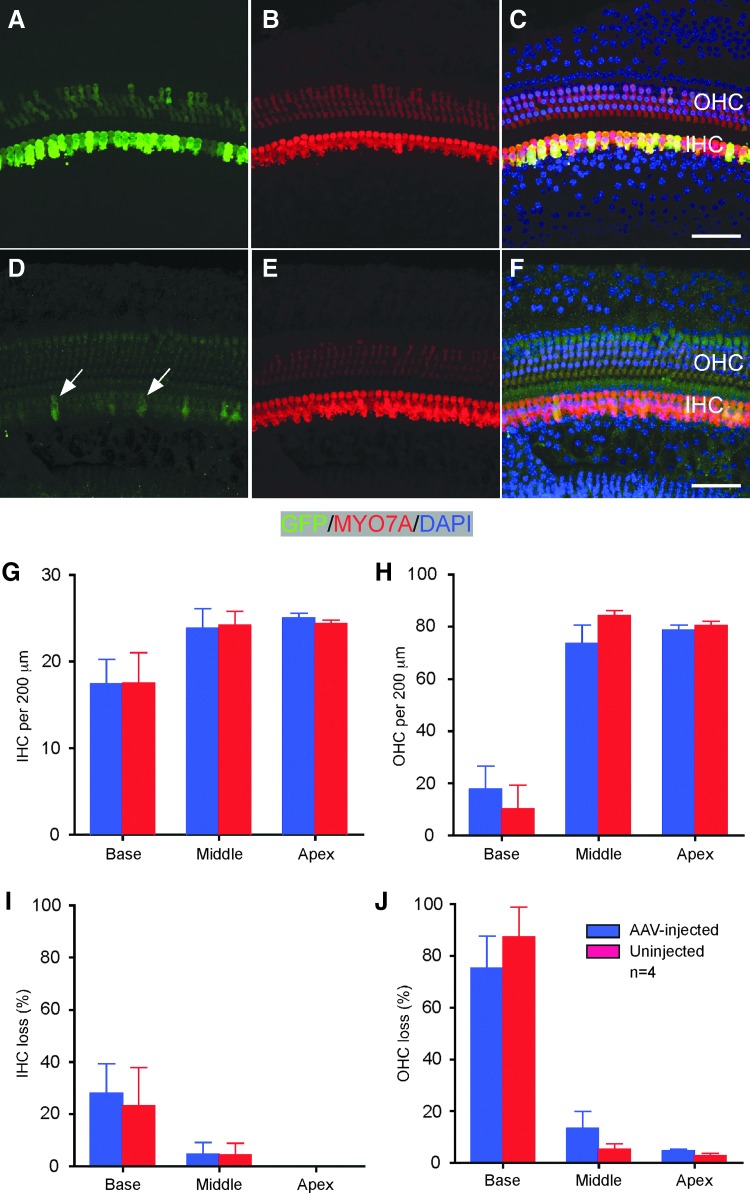
No significant HC loss was observed in AAV-injected cochleae 2 weeks after canalostomy. To determine further if HCs survive long term and whether the level of transgene expression is maintained, CD1 mice were studied 51 days after a 1 μL injection of AAV2/Anc80L65-GFP through canalostomy. No significant difference was found in the number of HCs between injected and uninjected ears (Fig. 7). In the injected cochlea, the expression of GFP remained at a level comparable to that 2 weeks after injection, prominent in all IHCs and weak in some OHCs, demonstrating sustained expression of genes mediated by AAV delivery. Interestingly, in the uninjected contralateral cochlea, sporadic IHCs were detected as having weak GFP signals (Fig. 7D), an indication that AAV could migrate from injected to uninjected side a long time after the initial injection. It is thus concluded that the AAV delivery method does not impair HCs, and the expression of transgenes can be maintained long term.
Figure 7.
AAV transduction by canalostomy in adults does not impair HC survival and maintains transgene expression long term. (A–F) Fifty-one days after AAV2/Anc80L65-GFP injection, no significant HC loss was detected in the middle turn of the injected (A–C) compared to the uninjected ear (D–F). Arrows indicate sporadic and weak GFP+ IHCs in the uninjected ear, likely due to migration of viral particles from the injected ear. Green, GFP; red, MYO7A. (G–J) there is no significant difference in total number of HCs (G and H) or HC loss (I and J) between injected and uninjected ears. n = 4. Scale bars: 50 μm.
Adenovirus damages HCs and impairs cochlea functions
Adenovirus has been delivered to adult mammalian inner-ear SCs.38 It has been previously shown that Ad5-CMV-EGFP could be effectively delivered to neonatal IHCs and OHCs without damaging HCs.25 To study if Ad5-CMV-EGFP infects adult cochlea, injection by canalostomy was performed in 10-week-old C57BL/6J mice, followed by ABR and DPOAE tests to assess auditory functions 2 weeks after injection. After injection of 1 μL of Ad5-CMV-EGFP, two out of four injected mice exhibited severe head tilting, but not circling behavior, suggesting vestibular dysfunctions. An average of >30 dB elevation in ABR thresholds was found across all frequencies in the injected ears compared to the uninjected ears with normal hearing (Fig. 5). Similarly, an average DPOAE threshold elevation of 36 dB was detected in most frequencies in the injected ears (Fig. 6). The results strongly suggest that Ad5-CMV-EGFP adult injection by canalostomy is detrimental to normal hearing and OHC function.
Further, immunolabeling was performed to study cell types infected by Ad5-CMV-EGFP by canalostomy. Near-complete OHC loss and some IHC loss were observed in the injected inner ears. No MYO7A/GFP double-positive IHCs (Fig. 3J–L) were detected, indicating Ad5-CMV-EGFP induces OHC death and is unable to infect IHCs in adult cochlea.
Discussion
This study compared different AAV serotypes for their specificities targeting the adult mouse cochlea by canalostomy, and determined that the approach is efficient for gene delivery primarily into the auditory HCs without impairing normal hearing.
AAV-based gene therapy has been successfully used to treat diseases in animal models, and it is currently being tested in clinical trials.39–42 The elaborate structure and exquisite functions of the inner ear require coordinated action of diverse inner-ear cell types, including the sensory HCs, SCs, neurons, and stria vascularis, and gene defects in any of these cell types can result in hearing loss.33,43 The availability of multiple AAV serotypes offers the opportunity to target different inner-ear cell types. AAV vectors have been extensively evaluated in the neonatal mouse inner ear, and are shown to infect a wide range of cochlear cell types. By cochleostomy, neonatal HCs, SCs, auditory neurons, as well as the stria vascularis were infected with different AAVs with varying expression levels.14,25 Hearing was maintained in adult mice after cochleostomy-mediated delivery at the neonatal stage,14,25 but was significantly impaired by injection at the adult stage.14 Delivery of AAVs to inner-ear cell types at the neonatal stage has resulted in successful hearing rescue in genetic hearing loss mouse models of autosomal recessive and dominant non-syndromic hearing loss, including Vglut3,20 Kcnq1,26 and Tmc1,13 and of syndromic hearing loss models such as Usher syndrome.12
While most of the hearing rescue studies are focused on the delivery in the neonatal animals, successful gene therapy by AAV requires efficient delivery and hearing rescue in mature inner ears. Furthermore, due to high degrees of heterogeneities of genetic hearing loss, it is important to evaluate the cell types that can be targeted by AAV in addition to HCs in the mature inner ear. In human newborns, the structure of the inner ear, differentiation status of cell types, and auditory functions are nearly identical to adults.44 This is in contrast to the neonatal mouse inner ear that still undergoes development without recordable auditory functions.45 Study of AAV delivery in the adult mouse inner ear with terminally differentiated cells and mature auditory functions is thus a necessary step toward its potential applications in humans.
The procedures that lead to successful neonatal delivery such as cochleostomy or RWM injection may not be adequate for the mature inner ear, as cochleostomy damages existing OHCs and induces hearing loss,25 while the RWM approach was less efficient in transducing inner-ear cells in addition to causing hearing loss.25,46 Canalostomy through PSCC has been shown to be effective for the delivery into the vestibular system, but is less efficient for the auditory organ.47,48 By canalostomy, the virus can presumably access the endolymphatic compartment through helicotrema and directly infect cochlea cells immersed in endolymph. The surgical procedure via PSCC avoids opening of the middle ear and exposing the facial nerve, therefore minimizing potential injury to the nerve and associated blood vessels. A recent study showed that AAV2/Anc80L65 transduced all the IHCs throughout the cochlea and the majority of OHCs at the apex of the cochlea in adult mice by canalostomy.28 While the study suggested the potential by canalostomy for AAV-mediated gene delivery into the adult mammalian inner ear, systematic screening of different AAV serotypes and the optimization of the procedure are likely required for efficient adult inner-ear delivery.
By evaluating eight AAVs in the adult mouse inner ear through canalostomy, it is shown that the procedure is safe, as hearing is preserved in the injected inner ears, similar to the uninjected control inner ears. It is further shown that AAV1, 6.2, 9, rh.39, and rh.43 infect mostly IHCs. AAV2, 2/Anc80L65, and 8 infect IHCs with high efficiency and OHCs with generally lower efficiencies. Anc80L65, consistent with the reports in newborn mice via RWM injection and prior reports in adult mice with canalostomy, demonstrated overall the best combined IHC and OHC transduction efficiencies. Different types of SCs and non-sensory cells were also infected by AAV subtypes.
There have been more than 100 causative genes implicated in hearing loss, many of which affect HC function.33,49 Delivery by AAV into mature HCs would be ideal to target deafness genes of HC origin in order to ameliorate the disease phenotypes. An important observation from this study is that more AAVs subtypes are able to infect diverse SCs when injected in neonatal cochleae than in adults,25 an indication that the developmental stages change the tropism of AAV transduction. The results emphasize the importance of evaluating AAV vectors at appropriate developmental stages that are directly relevant to the human inner ear.
The studies also illustrate the consistency and difference between AAV injections in adult and neonatal inner ears. It has been shown previously that by cochleostomy, AAV1, 2, 8, 9, and rh.43 infected neonatal and adult IHCs with different efficiencies, and all of the AAVs damaged adult OHCs.25 The current study demonstrated that these AAVs also infect adult IHCs through canalostomy while preserving all OHCs. Thus, OHC death seen in adult injection by cochleostomy is likely the result of the surgical procedure rather than intrinsic properties of AAVs on adult OHCs. AAV2, 8, 9, and rh43 infect 3–21% IHCs in neonatal cochleae and 13.2–61.6% IHCs in adult cochleae through cochleostomy.25 However, AAV2 infects 48.5–99.2% IHCs after injection into adult cochleae through canalostomy (Fig. 2A–C). AAV2 infects neonatal OHCs with an efficiency of 3% in the apex to 39% in the base,25 whereas it infects only ∼2% adult OHCs in the current study. AAV2/Anc80L65 transduces up to ∼90% of OHCs when injected at neonatal stages through RWM injection,27 but infects 60–85% OHCs at 5.66–11.32 kHz and 20–30% at other frequencies by canalostomy in adults.28 The difference in infection efficiencies between mature and developing OHCs is likely due to the change of expression of virus entry receptors on the HCs or the alteration of other barriers to transduction during development.
Gene delivery by both AAV and adenovirus may restore hearing in genetic deafness mouse models by gene replacement or supplementation, although AAV is considered safer and provides long-term expression of the transgene. In the present studies, AAVs infected cell types in the adult mouse inner ear without damage to cells or impairment to hearing. In contrast, the adenoviral vector tested resulted in major OHC death in adult cochleae. Thus, AAV is better tolerated than the adenoviral vector in both HC survival and maintenance of normal hearing after injection. Due to limited inner-ear space, the volume of AAV that can be safely administered has to be determined. Injection of 1 μL of AAV by canalostomy did not cause hearing loss in any of the 29 injected ears or vestibular dysfunction in mice. The results support that 1 μL of AAV can be safely administered. However, after 2 μL AAV injection, a significant elevation of ABR and DPOAE thresholds was detected across all frequencies. All the AAVs we used have similar titers, of which AAV2/Anc80L65 has the lowest. The number of viral particles in the injection of 2 μL of AAV2/Anc80L65 is not significantly higher than 1 μL of other AAVs. This suggests that there is an upper limit of the volume of AAV (between 1 and 2 μL) that can be safely administered. However, the limit can also be significantly affected by the variation in actual functional titers of different AAVs, and may have to be determined separately for each individual AAV.
In the current study, 1 μL of adenovirus injection in the adult inner ear severely attenuated auditory functions, indicating a high degree toxicity of adenovirus in adult mouse inner ears. It has been reported that delivery of 0.5 and 1 μL of adenovirus did not cause major damage to the inner ear or hearing threshold shifts in mice.48 The discrepancy may be due to the injection procedure, different responses to adenovirus by different mouse strains, or the processes used to produce adenovirus. Our adenovirus study also showed worse ABR/DPOAE threshold shifts than previous studies in mouse48 and rat,47 possibly because five-fold higher concentration of virus was used. (The titer of adenovirus in this study was 4 × 1012 pfu/mL compared to 0.8 × 1012 pfu/mL in Kawamoto's study). Adenovirus is known to elicit immune response,11 which combined with its toxicity and short-term expression of transgenes makes adenovirus less ideal for long-term expression of transgenes for gene therapy to treat genetic deafness.
This study identifies multiple AAV vectors capable of infecting HCs, as well as some SCs and non-sensory cells. To develop treatment for diverse forms of genetic deafness, AAV vectors that efficiently transduce other adult inner-ear cell types, such as stria vascularis and auditory neurons, need to be discovered. Thus, there is a need to continue to identify new AAV subtypes.
This study demonstrates that AAV vectors are useful tools for gene therapy to treat genetic deafness in the mature mouse inner ear. The approach may establish an efficient and safe route for inner-ear delivery in adults, without hearing impairment. Combined with the information on the cochlear cell types targeted, this represents an important step toward developing treatment for different types of genetic hearing loss.
Acknowledgments
This work was supported by R01 DC006908 (to Z.Y.C.) and the Key Project of National Natural Science Foundation of China (81230021; to W.-J.K.). We are also grateful for support from the David-Shulsky Foundation (to Z.Y.C.), a Frederick and Ines Yeatts Hair Cell Regeneration grant (to Y.T., M.H., Y.S., A.R., and H.W.), the Curing Kids Fund (to M.H. and Z.Y.C.), the Broad Institute (to Z.Y.C.), the Scholarships from Chinese Scholarship Council (to Y.T. and H.W.), and Foundation Fighting Blindness, Giving/Grousbeck, and Lonza Houston (to L.H.V.). We thank Jun Suzuki for his assistance with the procedure.
Author Disclosure
Z.Y.C. is a member of scientific advisory board of Rescue Hearing, Inc. G.G. is a founder of Voyager Therapeutics and holds equity in the company. G.G. is an inventor on patents with potential royalties licensed to Voyager Therapeutics and other biopharmaceutical companies. L.H.V. is a listed inventor on patents for AAV9, AAV6.2, and Anc80L65, which have been licensed to various pharmaceutical and biotechnology companies, including Lonza Houston that supports research in the Vandenberghe lab. L.H.V. is co-founder and stock holder of GenSight Biologics, a neurosensory gene therapy company, and consults and lectures for companies with gene therapy interest including in some in the hearing and balance space. No competing financial interests exist for the remaining authors.
References
- 1.Morton NE. Genetic epidemiology of hearing impairment. Ann N Y Acad Sci 1991;630:16–31 [DOI] [PubMed] [Google Scholar]
- 2.Van Camp G, Willems PJ, Smith RJ. Nonsyndromic hearing impairment: unparalleled heterogeneity. Am J Hum Genet 1997;60:758–764 [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong W, Wang D, Gao Y, et al. Reproductive management through integration of PGD and MPS-based noninvasive prenatal screening/diagnosis for a family with GJB2-associated hearing impairment. Sci China Life Sci 2015;58:829–838 [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro PA. Gene transfer for neovascular age-related macular degeneration. Hum Gene Ther 2011;22:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colella P, Auricchio A. Gene therapy of inherited retinopathies: a long and successful road from viral vectors to patients. Hum Gene Ther 2012;23:796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corydon TJ. Antiangiogenic eye gene therapy. Hum Gene Ther 2015;26:525–537 [DOI] [PubMed] [Google Scholar]
- 7.Dalkara D, Goureau O, Marazova K, Sahel JA. Let there be light: gene and cell therapy for blindness. Hum Gene Ther 2016;27:134–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotin RM. Prospects for the use of adeno-associated virus as a vector for human gene therapy. Hum Gene Ther 1994;5:793–801 [DOI] [PubMed] [Google Scholar]
- 9.Williams DA, Smith FO. Progress in the use of gene transfer methods to treat genetic blood diseases. Hum Gene Ther 2000;11:2059–2066 [DOI] [PubMed] [Google Scholar]
- 10.Husseman J, Raphael Y. Gene therapy in the inner ear using adenovirus vectors. Adv Otorhinolaryngol 2009;66:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu Y, Tao Y, Li W, et al. Adenovirus vectors target several cell subtypes of mammalian inner ear in vivo. Neural Plast 2016;2016:9409846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan B, Askew C, Galvin A, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol 2017;35:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Askew C, Rochat C, Pan B, et al. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med 2015;7:295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpatrick LA, Li Q, Yang J, et al. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther 2011;18:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata SB, Di Pasquale G, Cortez SR, et al. Gene transfer using bovine adeno-associated virus in the guinea pig cochlea. Gene Ther 2009;16:990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Okada T, Nomoto T, et al. Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med 2007;39:170–175 [DOI] [PubMed] [Google Scholar]
- 17.Iizuka T, Kanzaki S, Mochizuki H, et al. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum Gene Ther 2008;19:384–390 [DOI] [PubMed] [Google Scholar]
- 18.Bedrosian JC, Gratton MA, Brigande JV, et al. In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther 2006;14:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Okada T, Sheykholeslami K, et al. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther 2005;12:725–733 [DOI] [PubMed] [Google Scholar]
- 20.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012;75:283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalwani AK, Walsh BJ, Reilly PG, et al. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther 1996;3:588–592 [PubMed] [Google Scholar]
- 22.Isgrig K, Shteamer JW, Belyantseva IA, et al. Gene therapy restores balance and auditory functions in a mouse model of Usher syndrome. Mol Ther 2017;25:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenzel GI, Xia A, Funk E, et al. Helper-dependent adenovirus-mediated gene transfer into the adult mouse cochlea. Otol Neurotol 2007;28:1100–1108 [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Frisina RD, Bowers WJ, et al. HSV amplicon-mediated neurotrophin-3 expression protects murine spiral ganglion neurons from cisplatin-induced damage. Mol Ther 2001;3:958–963 [DOI] [PubMed] [Google Scholar]
- 25.Shu Y, Tao Y, Wang Z, et al. Identification of adeno-associated viral vectors that target neonatal and adult mammalian inner ear cell subtypes. Hum Gene Ther 2016;27:687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Q, Wang J, Li Q, et al. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange–Nielsen deafness syndrome. EMBO Mol Med 2015;7:1077–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landegger LD, Pan B, Askew C, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol 2017;35:280–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki J, Hashimoto K, Xiao R, et al. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep 2017;7:45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukui H, Raphael Y. Gene therapy for the inner ear. Hear Res 2013;297:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao G, Alvira MR, Somanathan S, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci U S A 2003;100:6081–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang M, Kantardzhieva A, Scheffer D, et al. Hair cell overexpression of Islet1 reduces age-related and noise-induced hearing loss. J Neurosci 2013;33:15086–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller M, von Hunerbein K, Hoidis S, et al. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 2005;202:63–73 [DOI] [PubMed] [Google Scholar]
- 33.Geleoc GS, Holt JR. Sound strategies for hearing restoration. Science 2014;344:1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinn E, Pacouret S, Khaychuk V, et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep 2015;12:1056–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monzack EL, Cunningham LL. Lead roles for supporting actors: critical functions of inner ear supporting cells. Hear Res 2013;303:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuchi T, Kimura RS, Paul DL, et al. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 1995;191:101–118 [DOI] [PubMed] [Google Scholar]
- 37.Kho ST, Pettis RM, Mhatre AN, et al. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol Ther 2000;2:368–373 [DOI] [PubMed] [Google Scholar]
- 38.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 2005;11:271–276 [DOI] [PubMed] [Google Scholar]
- 39.Ashtari M, Cyckowski LL, Monroe JF, et al. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 2011;121:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on Phase 1 trial. Lancet 2016;388:661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a Phase 1 dose-escalation trial. Lancet 2009;374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller U, Barr-Gillespie PG. New treatment options for hearing loss. Nat Rev Drug Discov 2015;14:346–365 [DOI] [PubMed] [Google Scholar]
- 44.Haith MM. Sensory and perceptual processes in early infancy. J Pediatr 1986;109:158–171 [DOI] [PubMed] [Google Scholar]
- 45.Morsli H, Choo D, Ryan A, et al. Development of the mouse inner ear and origin of its sensory organs. J Neurosci 1998;18:3327–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res 1999;136:124–130 [DOI] [PubMed] [Google Scholar]
- 47.Gassner D, Durham D, Pfannenstiel SC, et al. Canalostomy as a surgical approach for cochlear gene therapy in the rat. Anat Rec (Hoboken) 2012;295:1830–1836 [DOI] [PubMed] [Google Scholar]
- 48.Kawamoto K, Oh SH, Kanzaki S, et al. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther 2001;4:575–585 [DOI] [PubMed] [Google Scholar]
- 49.Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med 2006;354:2151–2164 [DOI] [PubMed] [Google Scholar]