Abstract
Context:
Mercury is used extensively in the preparation of Siddha medicines, after purification. In this study, we present 32 patients of mercury toxicity following unauthorized Siddha medicine intake who mimicked neuromyotonia clinically. We analyzed the clinical features of these patients, the role of autoimmunity in etiopathology, and compared it with acquired neuromyotonia.
Subjects and Methods:
This is a retrospective study to analyze inpatients in a tertiary care center, admitted with mercury toxicity following Siddha medicine intake from August 2012 to October 2016. We analyzed the clinical features, laboratory data including mercury, arsenic and lead levels in blood, and serum voltage-gated potassium channels (VGKC)-CASPR2 Ab in selected patients.
Results:
Thirty-two patients who had high blood mercury levels following Siddha medicine intake were included in the study. All patients (100%) had severe intractable neuropathic pain predominantly involving lower limbs. Twenty-six (81.25%) patients had fasciculations and myokymia. Fifteen patients (46.86%) had autonomic dysfunction (postural hypotension and resting tachycardia). Nine (28.12%) patients had encephalopathic features such as dullness, apathy, drowsiness, or delirium. Anti-VGKC Ab was positive in 12 patients with myokymia. All the patients in the study consumed Siddha medicines obtained from unauthorized dealers.
Conclusions:
Mercury toxicity following Siddha medicine intake closely mimics acquired neuromyotonia; severe intolerable neuropathic pain is the hallmark feature; Positive VGKC-CASPR2 antibody in some patients must be due to triggered autoimmunity secondary to mercury toxicity due to Siddha medicine intake. The government should establish licensing system to prevent distribution of unauthorized Siddha medicines.
Keywords: Mercury toxicity, myokymia, neuromyotonia, Siddha medicine toxicity, voltage gated potassium channels-CASPR2 Ab
INTRODUCTION
Mercury is used extensively in the preparation of Siddha medicines[1,2] after purification. In the past 5 years, we have come across series of cases who presented with severe lower limb painful dysesthesia, myokymia, and fasciculations involving lower limbs or whole body, autonomic dysfunction, and encephalopathy. All these patients had subacute illness started along with or following ingestion of unauthorized Siddha medicines; blood mercury level was high in all of them and some patients had positive anti-voltage-gated potassium channels (VGKC) (CASPR2) antibodies. In this study, we present 32 patients of mercury toxicity following unauthorized Siddha medicine intake who mimicked neuromyotonia clinically.
SUBJECTS AND METHODS
The study was done in a tertiary care center from August 2012 to October 2016. This study is a retrospective analysis of all patients with mercury toxicity (with high blood mercury level) from the prospectively maintained data matrix of inpatients of our unit. Patients who were admitted and found to have high blood mercury level were included in the study. All the selected patients in our study were admitted in the hospital with subacute onset severe pain and dysesthesia involving both lower limbs, pain in upper limbs in some patients, autonomic dysfunction in the form of resting tachycardia, postural hypotension and episodes of excessive sweating, myokymia, and fasciculations; some patients had encephalopathic features such as confusion, disorientation, irrelevant talk, and apathy. All the patients had Siddha medicine intake either in the form of tablet, powder, or syrup form. Most of the patients developed symptoms during Siddha medicine intake and some patient developed symptoms with a short interval after stopping the medicine intake.
Detailed clinical examination and neurological assessment were done for all the patients. All patients underwent detailed blood investigations to rule out other causes of neuropathy. Complete blood count, fasting and postprandial blood sugar, urea, creatinine, sodium, potassium, liver function test, thyroid profile, HIV serology, Serum HBsAg, HCV antibody, vasculitic profile, electrocardiogram (ECG), X-ray chest, and ultrasonography abdomen were done. All the patients underwent magnetic resonance imaging (MRI) spine study to rule out compressive lumbosacral radiculopathy. MRI brain was done in selected patients who presented with encephalopathy; nerve conduction study (motor nerve conduction, sensory nerve conduction, and F-wave analysis) and needle electromyography (EMG) (analysis of spontaneous discharges, motor unit potentials, and interference pattern) were done in all patients.
In all these patients, no specific etiology was found to explain the clinical features other than Siddha medicine exposure. As recent literature evidences supported the role of heavy metal toxicity in patients with native medicine intake, we sent the blood samples of all these patients to estimate the levels of mercury, lead, and arsenic which are the common heavy metal toxins found in native medicines. These heavy metals were measured in the blood by inductively coupled plasma mass spectrometry method. As the patients had features of neuromyotonia, blood anti-VGKC antibody (CASPR2) analysis was done in selected patients by indirect immunofluorescence antibody assay.
Patients with the same clinical features following Siddha medicine exposure who cannot afford to test heavy metal level in blood were excluded from the study; patients with incomplete blood investigations were excluded from the study analysis as other causes of neuropathy could not be ruled out in these patients. Patients with compressive radiculopathy due to lumbosacral disc prolapse were excluded from the study. Patients with uncontrolled hyperglycemia and those who were known to have symptoms of the diabetic neuropathy were excluded from the study. Totally fourteen patients (who had the same clinical features following Siddha medicine intake) were excluded from the study by following the exclusion criteria.
RESULTS
Patient's characteristics
Thirty-two patients fulfilled the inclusion criteria; 15 were females and 17 were males; Most of the patients (19 patients) were middle-aged adults [Table 1] in the 31–50 years' age group (59.4%).
Table 1.
Age distribution of the patients in the study group

Siddha medicine exposure
All 32 patients in the study took Siddha medicine during or before the onset of symptoms. Most of the patients consumed Siddha medicines for simple, chronic, and benign illnesses. They have consumed Siddha medicine for the management of psoriasis (3 patients), pimples (1), hyperpigmented skin lesions (1), spondylotic low back pain (9) and osteoarthritis of knee (3), anorexia (3), hydrocele (1), menstrual irregularities (1), migraine (3), and tiredness (4). Three patients consumed Siddha medicines for the management of type 2 diabetes mellitus (DM), Duchenne muscular dystrophy, and HIV infection, respectively.
Most of the patients took Siddha medicines for a duration, varying from 1 month to 3 months, and they stopped the intake of medicines after developing back pain, lower limb pain, or after admission in the hospital. For most of the patients, symptoms started during Siddha medicine intake, but 8 patients (25%) developed symptoms with a short interval (latent period) after stopping the Siddha medicine. The latent period varied minimally from 15 days to maximally 4 months. Only 2 patients had prolonged exposure of 8 months and 2 years.
All the patients in the study bought Siddha medicines from unauthorized dealers and unauthorized Siddha practitioners. None of the patients in the study group was treated by registered Siddha medicine practitioners.
Clinical features
All patients [Table 2] had subacute presentation with 15 days to 2 months' duration of symptoms.
Table 2.
Clinical features of the patients in the study group
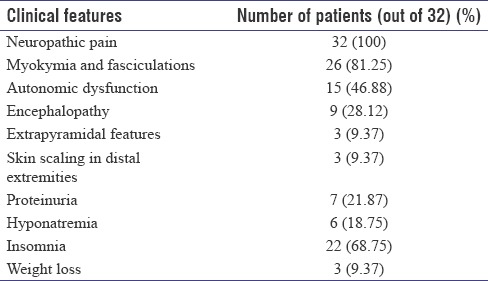
Neuropathic pain
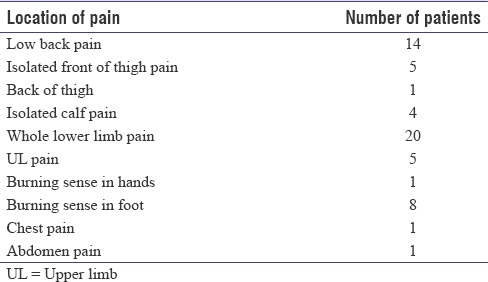
Neuropathic pain was the most common important symptom experienced by all the patients in the study group. Patients developed pain in the low back region and buttock and pain involved the bilateral lower limbs in most of the patients. Pain felt diffusely in whole lower limbs, only over the thigh, only over the calf, or only over the foot. Pain was severe, continuous, intractable, unbearable, and persistent throughout the day and sleep was severely disturbed. It was pulling dysesthesia, burning, or squeezing in nature. Pain was typically aggravated in the night or in the early morning time. Pain was typically aggravated by standing or walking for a short distance (lower limb pulses were normal). Hence, most of the patients preferred to lie down quietly in the cot although pain was not relieved by lying down posture. Pain was commonly felt in the low back, front of thigh, and calves [Table 3]. Some patients also had similar type of neuropathic pain in the bilateral shoulders, neck, and arm, and one patient had bilateral chest pain (cardiac evaluation was normal).
Table 3.
Location of neuropathic pain in the study group
Most of the patients cried developed depression and some patients had suicidal ideas due to intractable and drug refractory pain. Only five patients in the study complained numbness, two patients had patchy forearm numbness, two had leg numbness, one had perioral numbness, and none of these five patients had objective sensory loss. Most of these patients initially consulted in orthopedic hospitals, and MRI spine would have been done before admission in neurology unit. All patients in our study underwent MRI spine which was normal in all of these patients (no evidence of disc prolapse with root compression). Severe neuropathic pain did not respond to nonsteroidal anti-inflammatory drugs and usual dose of pregabalin. All the patients required larger doses of pregabalin or gabapentin and oral opioids; fentanyl patch (50 μg/day) was used in some patients with intractable pain. Combination of intravenous (IV) methylprednisolone with these drugs produced better pain relief.
Myokymia and fasciculations
Twenty-six out of 32 patients developed myokymia and fasciculations. In 12 patients, myokymia was confined only to the bilateral calves. In seven patients, myokymia involved the bilateral whole lower limbs. Seven patients had generalized myokymia and fasciculations involving all four limbs and trunk. Out of the 26 patients, 3 patients had perioral myokymia and fasciculations.
Characteristically, all the patients in our study had no evidence of motor or sensory deficits although they had peripheral nerve injury as evidenced by neuropathic pain, myokymia, and fasciculations; only one patient had bilateral calf muscle wasting without weakness. Nerve conduction studies and needle EMG were done in all patients; nerve conduction study was normal in all of the patients (no evidence of axonal loss, demyelination, or F-wave abnormalities). Needle EMG showed spontaneous discharges including fasciculations and myokymia in the clinically involved muscles in all the 26 patients who had clinical myokymia. No clinical or EMG evidence of myokymia was found in the remaining six patients. All patients had normal motor unit potentials and interference pattern.
Autonomic dysfunction
Fifteen patients in the study had resting tachycardia with heart rate varying from 120 to 140. ECG confirmed it to be sinus tachycardia. Cardiac evaluation including echo was normal. Two patients had severe postural hypotension and had postural dizziness and could not walk; five patients had paroxysmal episodes of excessive sweating involving whole body lasting for 5–10 min, several episodes/day. Episodes of sweating lasted for 15 days to 1 month. Two patients had severe hypertension (HT) only during the period of Siddha medicine toxicity symptoms. They had normal blood pressure before and afterwards. One of the patient developed seizure during severe HT and had features of posterior reversible encephalopathy syndrome in MRI brain.
Three patients had paroxysmal episodes of chills and rigor along with piloerection [Figure 1]. Rigor lasted for about 30 min and improved spontaneously. They had several episodes of rigor/day. They had no fever. One patient had frequent episodes of rigor that lasted for 1 month without fever. He was evaluated in a hospital as pyrexia of unknown origin although he had no fever. Rigor recovered spontaneously in 1 month.
Figure 1.
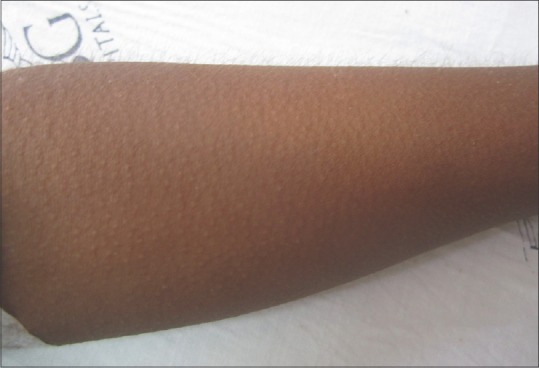
Generalized piloerection in a 36-year-old male with mercury toxicity due to autonomic dysfunction. He also had rigor and sweating during piloerection
Encephalopathy
Nine patients in the study group had encephalopathic features in the form of dullness, apathy, confusion, irrelevant talk, and outbursts of anger. Two patients had dysarthria. Four patients had features of severe depression although premorbid mood was normal. Four patients had drowsiness, and of these, two patients had persistent drowsiness and disorientation for almost a month period. MRI brain and cerebrospinal fluid analysis were normal in all the nine patients.
Extrapyramidal features and neuroleptic sensitivity
One patient with encephalopathy had mild extrapyramidal features (not drug induced) with limb rigidity and bradykinesia which gradually improved over 1 month. Patients with Siddha medicine toxicity showed extreme sensitivity to small dose of neuroleptic medicines. Two patients with encephalopathy and delirium received small dose of IV haloperidol (1 mg), and they developed severe extrapyramidal syndrome with orofacial dyskinesia, coarse tremors of the upper limbs, and severe rigidity of limbs; by witnessing the excessive sensitivity to neuroleptics, we avoided using haloperidol for patients with Siddha medicine toxicity and delirium, and instead, we started using IV benzodiazepine or oral quetiapine to control agitation and delirium.
Skin features
Three patients developed severe skin scaling and loss of epidermis in the distal extremities (palms and soles) [Figure 2]. One patient developed ichthyosis in the leg. No nail changes were found.
Figure 2.
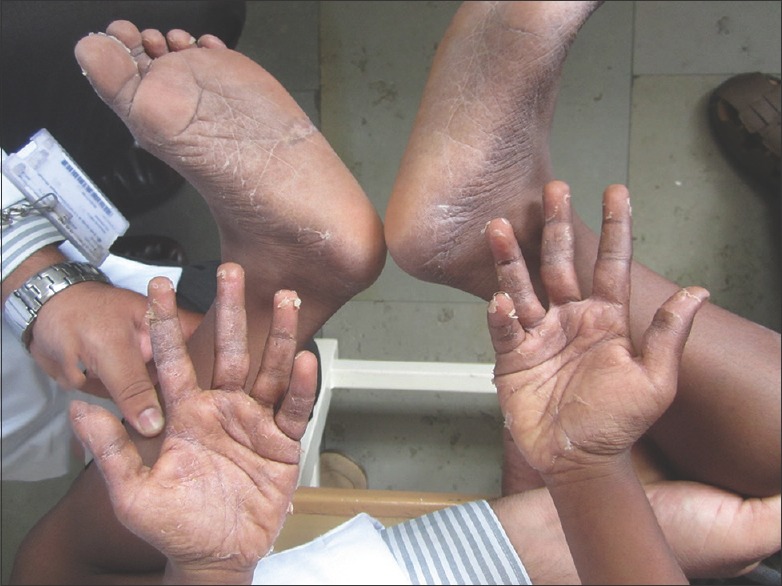
Severe scaling of palms and soles in an 11-year-old boy with mercury toxicity following Siddha medicine intake
Renal failure
Seven patients found to have proteinuria and no other causes (HT or DM) were found in these patients to explain proteinuria. One patient developed nephrotic syndrome with anasarca and he also had encephalopathy, tremors, myoclonic jerks, dysarthria, and myokymia. He developed severe hyponatremia, sepsis, and died. Renal biopsy could not be done for him.
Hyponatremia
Six patients had hyponatremia and three of them had severe symptomatic hyponatremia with high urine sodium and euvolemia suggestive of SIADH. With 3% saline, sodium level improved.
Other features
Insomnia was a common symptom. Twenty-two patients experienced severe insomnia. Three patients had tiredness, fatigue, and extreme lethargy. Three patients had severe weight loss, more than 10 kg in 3-month period. These three patients were investigated extensively with upper gastrointestinal scopy and whole-body positron emission tomography scan, and no cause was found.
Blood mercury level analysis
All the patient underwent detailed blood tests which were normal; no other causes were found to explain the clinical presentations other than the close temporal association with Siddha medicine intake.
All patients underwent blood mercury, arsenic, and lead level. Blood mercury level was high in all the patients in the study group. None of patients worked in industry involving mercurial products. In all the patients, only source of mercury exposure was Siddha medicine ingestion, obtained from unauthorized dealers. Twelve patients had blood mercury level in the range of 10–20 ng/ml (normal level is <10 ng/ml); 11 patients had blood mercury level in the range of 20–40 ng/ml; and 9 patients had very high blood mercury level (>40 ng/ml).
All patients with very high blood mercury level had prolonged and frequent intake of Siddha medicines. The level of mercury in the blood was directly correlated with the severity of clinical presentation. Patients with high blood mercury levels had very severe clinical features (prolonged and severe neuropathic pain, severe autonomic dysfunction with sweating and rigor, generalized myokymia, and prolonged encephalopathy).
Blood lead level was normal in all the patients. Blood arsenic level was high in one patient (15 ng/ml). Anti-VGKC Ab (CASPR2) was done in 20 patients (out of 26 total patients who had myokymia and fasciculations), out of which 12 patients had positive anti-VGKC Ab (CASPR2).
Treatment and outcome
Patients with prolonged exposure of Siddha medicine showed severe clinical features, but exposure of at least 15 days was sufficient to produce severe clinical features. All the patients were treated symptomatically with pregabalin, gabapentin, opioids, and short course of steroids. We have used steroids in all of our patients in the study group, irrespective of the VGKC Ab status. We used injection methylprednisolone 500 mg IV OD for 5 days followed by oral prednisolone 10 mg/day for next 4–6 weeks. When steroids were started, patients had better pain relief and reduction in myokymia (gradually) than treatment with pregabalin alone. Only one patient (with positive VGKC Ab) showed dramatic recovery of pain and myokymia in a single day after starting injection methylprednisolone 500 mg IV OD. Dose of pregabalin used was 150–300 mg/day according to the treatment response. Gabapentin was used with dose ranging from 200 to 600 mg/day.
All the patients improved gradually over several months (3–6 months). Severe neuropathic pain and myokymia reduced at least after a period of 3 months. Encephalopathy, tremor, sweating, and rigor improved over 1 month. Only four patients received penicillamine, which did not improve the symptoms. Except for one patient, no other patients showed dramatic recovery with steroids.
All patients became completely symptoms free 4–6 months after stopping the Siddha medicine intake. Patients with prolonged encephalopathy, severe generalized myokymia, and autonomic dysfunction had delayed recovery. Follow-up monitoring of blood mercury level was done only in six patients (due to economic reasons) who showed gradual decline in blood mercury level over 6 months.
Two patients died during the course of illness. One patient with encephalopathy and nephrotic syndrome died of sepsis. Other patient with severe extrapyramidal syndrome had severe autonomic instability and hypotension. He was taken home against medical advice and died in the house.
DISCUSSION
Mercury can produce toxicity to human beings through ingestion, inhalation, or rarely IV route. Minamata disaster due to mercury poisoning occurred in Japan in 1950 due to consumption of mercury-polluted fish from the bay of the Japanese coastal city, Minamata. Methylmercury released from a chemical factory contaminated this bay. Mercury is also found in industrial products such as battery, thermometer, agricultural fungicides, and some cosmetic products.[1] One more important and neglected source of mercury in ingested form is unauthorized Siddha medicines.
Indian traditional medicine systems use heavy metals and minerals in preparation of medicines after purification and detoxification.[2] Metals and minerals are used more predominantly in Siddha medicines than other systems (ayurvedic). Among the several heavy metals (lead, arsenic, gold, mercury), mercury is used extensively in Siddha medicine after detoxification.[3] The branch of Siddha science that deals with mercury-based medicine is called Rasa shastra or Rasavatham.[4] Rasa means elixir of life. Rasa denotes state of liquidness. Mercury is denoted as rasa and is considered as kingdom of minerals. Mercury-based Siddha medicines are widely used by Siddha physician. In Siddha medicine preparations, mercury is used in five forms such as rasam (mercury), lingam (red sulfide of mercury), veram (mercury perchloride), pooram (mercury subchloride), and rasa-chinduram (red oxide of mercury). They are known as Pancha sutha.[5]
Mercury in naturally occurred forms (elemental mercury) and organic forms is toxic to the human body. Hence, much importance is given to the purification of mercury to obtain inorganic mercury (nontoxic form – Vaalai rasam) to be used in medical preparation,[6] so ancient siddhars took utmost care to evolve the specific methods for the detoxification of mercury.[4] Various processes are involved in purification of mercury to remove 15 layers of toxicity.[5] This detoxification method is followed till now for preparation of Siddha medicines. Scientific analysis should be made to identify what chemical change occurs during detoxification process.[7,8] Poor quality drugs, inadequately detoxified drugs, can produce serious side effects to human beings. In our study, all the patients received Siddha medicines from unauthorized dealers (i.e., from persons who did not have qualified Siddha medicine degree, recognized by Indian Government).
Almost all patients in this study had clinical features mimicking acquired neuromyotonia (Isaacs' syndrome) and Morvan's syndrome. Neuromyotonia was first described by Isaacs in 1961.[9] It is characterized by severe muscle stiffness, muscle cramps, fasciculations, myokymia (continuous muscle fiber activity), and weakness of limbs. Morvan's syndrome (described by French Physician Augustine Marie Morvan in 1890) is also characterized by similar continuous muscle fiber activity and in addition central features such as insomnia, irritability, and delirium;[10] peripheral nerve motor axonal hyperexcitability is the reason for the clinical features in these conditions.[11] Needle EMG is characterized by spontaneous discharges in the form fasciculations, doublet, triplet, myokymia, and neuromyotonia.[12]
Acquired neuromyotonia is autoimmune in nature,[13,14,15] and antibodies against VGKC Ab are found in about 30%–40% cases of acquired neuromyotonia. Often, the condition is idiopathic or secondary to conditions such as neoplasm, lymphoma, and thymoma.[16] Toxin exposure (lead, silver, mercury, and gold) is also associated with neuromyotonia.[17] A large number of case reports and case series analysis are available in the world literature about the association of native medicines intake or heavy metal poisoning with neuromyotonia like presentation.[18,19,20]
In this study, 81.2% of patients had continuous muscle fiber activity in the form of myokymia and fasciculations. All patients in the study had severe neuropathic pain. None of the patients had muscle stiffness, neuromyotonia, or muscle weakness. All the patients in the study had strong temporal relationship with the Siddha medicine intake (clinical features developed along with Siddha medicine intake or followed it), and no other causative factor was identified; all the patients had high blood mercury levels. None of the patients in this study had exposure of other sources of mercury such as agricultural fungicides or other chemicals. Hence, mercury toxicity in all the patients in the study is unequivocally due to unauthorized Siddha medicine intake which always contains mercurial products. Mercury attached to the tissues will take long time to clear from the body. Delayed clearance of blood mercury level in some of the patients in the study is probably due to the slow daily release of tissue mercury into the bloodstream.
Literature evidence also supports the association of heavy metals in native medicines and neuromyotonia like presentation. Zhou et al. reported[18] three cases of mercury poisoning associated with motor nerve hyperexcitability; two of these three cases also had encephalopathic features. Panagariya et al. reported[19] the clinical profile of series of twenty cases of neuromyotonia from Northwest India. Eleven out of twenty cases had striking temporal association with ayurvedic medicine intake. However, routine heavy metal screening (mercury, lead, arsenic, and gold) was negative. Chaurasia et al. reported[20] a case of continuous muscle fiber activity in a patient after ayurvedic drug intake. Blood silver and lead level was high. Case reports are also available in the literature about the association of mercury toxicity and systemic HT,[21,22] like our cases.
In this study, strikingly all patients had only unauthorized Siddha medicine intake and none had exposure to other medicines such as ayurvedic or homeopathic medicines; mercury is used more extensively in Siddha medicines than preparation of other native medicines (ayurvedic or homeopathic). Interestingly 12 patients in the study had positive VGKC-CASPR2 antibodies. This finding indicated that mercury not only produced direct toxic damage of peripheral nerve terminals but also it triggered autoimmunity against the ion channels (VGKC), located in the peripheral nerve terminals, by exposing these ion channels into body's immune mechanism. Probably, two different pathogenic mechanisms operated in these patients (1. heavy metal mediated and 2. autoimmune mediated) to produce peripheral nerve damage. The presence of “latent period” before clinical presentation in some of the patients could be explained by autoimmunity (time required to generate autoimmunity). Action potential is generated in the peripheral nerve terminal by opening of the Voltage -gated calcium channels (VGCC). Repolarization of peripheral nerve terminal occurs due to opening of the VGKC [Figure 3]. In neuromyotonia and Siddha medicine-induced mercury toxicity with secondary autoimmunity, antibodies to VGKC result in inadequate opening of potassium channels,[23] poor repolarization, and prolonged opening of VGCC. This results in excessive entry of calcium in the nerve terminal, excessive release of acetylcholine quanta and ultimately leads to continuous muscle fiber activity.
Figure 3.
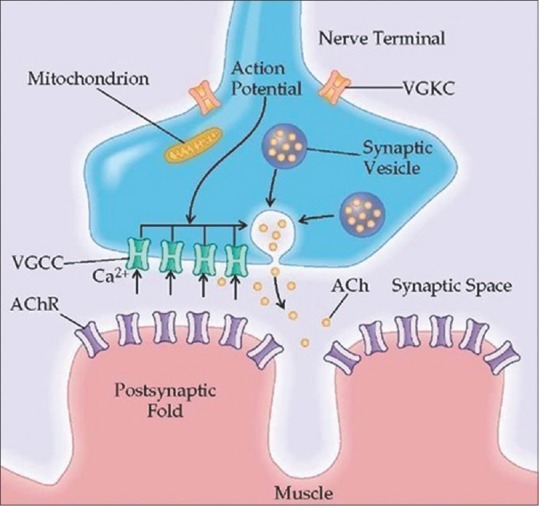
Motor nerve terminal showing voltage-gated potassium channels and voltage-gated calcium channels (Source: What-when-how, in-depth tutorials and information)
Although VGKC antibodies (CASPR2) are also found in idiopathic acquired neuromyotonia, several clinical features in this study distinguished Siddha medicine-induced mercury toxicity from idiopathic neuromyotonia. In idiopathic acquired neuromyotonia (Isaacs syndrome), motor features dominate and sensory and autonomic symptoms are less severe; muscle stiffness, muscle cramps, and neuromyotonia[23] are the cardinal clinical features; however, in this study, sensory symptoms dominated the clinical presentation, and severe, intractable neuropathic pain syndrome predominantly involving lower limbs was the most common presentation. Although myokymia was present in most of the patients, often, it was asymptomatic and not disabling; some patients had life-threatening autonomic dysfunction. None of the patients in the study had muscle stiffness and cramps which are the salient features of idiopathic neuromyotonia; Although VGKC antibodies (CASPR2) are found in these patients, it must be a triggered autoimmunity secondary to mercury toxicity.
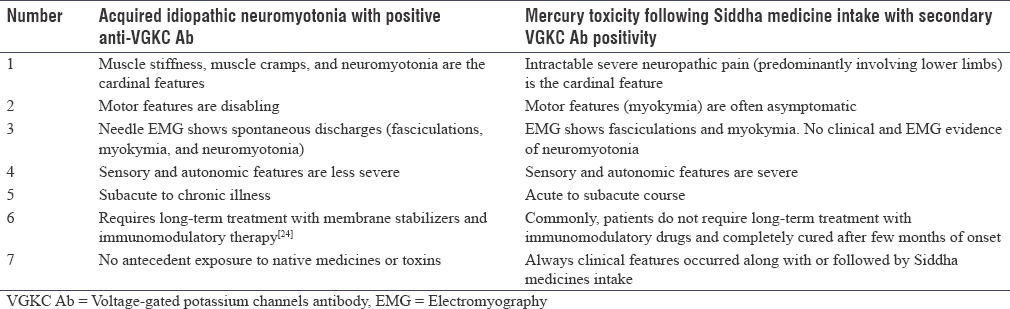
Table 4 clearly shows the distinguishing features between acquired neuromyotonia with VGKC Ab positivity and mercury toxicity following Siddha medicine intake with secondary VGKC Ab positivity. Patients in this study also had encephalopathy, delirium and extrapyramidal features, proteinuria, and renal failure which indicated that cerebral cortex, basal ganglia, and renal tubules[25] were also involved in the pathology. VGKC antibody level was done only in 20 out of 32 patients due to nonaffordability of some of the patients to do the test. Mercury level could not be measured in the Siddha medicine preparation due to nonavailability of such analysis in our region.
Table 4.
Differences between acquired neuromyotonia with voltage-gated potassium channels antibody positivity and mercury toxicity following Siddha medicine intake with secondary voltage-gated potassium channels antibody positivity
All the patients in this study took Siddha medicine only for trivial illness such as skin conditions or osteoarthritis, and medicines were obtained from unauthorized dealers; two patients in the study died due to the consequences of mercury toxicity (mortality 6.25%). All other patients had about three to 6 months of work loss due to intractable pain; high morbidity and mortality of this condition requires serious consideration.
Ancient Indian medicine system should be preserved. Ancient siddhars prepared Siddha medicines with utmost care to detoxify the mercury and were successful in treating various diseases without side effects. However, recent large emergence of mercury toxicity following Siddha medicine intake highly indicates that detoxification process in current Siddha medicine preparation is not followed meticulously. Licensing system should be established by the government to recognize large number of government and private Siddha medicine agencies. Unauthorized preparation and distribution of Siddha medicines should be strictly prohibited.
CONCLUSIONS
Mercury toxicity following Siddha medicine intake closely mimics acquired neuromyotonia; severe intolerable neuropathic pain, predominantly involving lower limbs, is the hallmark feature; although VGKC-CASPR2 antibody is found in some of these patients, it must be a triggered autoimmunity secondary to mercury toxicity due to Siddha medicine intake. Blood mercury level should be analyzed in all patients with neuromyotonia and positive VGKC Ab. Licensing system should be established by the government to prevent the distribution of unauthorized and poorly detoxified Siddha medicine.
Future research
This study has raised several questions and is the potential area for future research.
Exact chemical changes during mercury detoxification process while preparing Siddha medicines should be analyzed and surveillance method to assess the adequacy of detoxification process should be analyzed
Analysis of mercury content in Siddha medicine should be done
Analysis of motor nerve terminals (by skin biopsy) in patients with Siddha medicine toxicity to identify the nature of pathology should be done. Cellular level changes, changes in ion channels in nerve endings, and measuring of mercury content in the nerve endings should be done.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are very grateful to our beloved Dean, Dr. S. Ramalingam. M.D, PSGIMSR, for giving permission and supporting us to perform this study.
REFERENCES
- 1.Sathe K, Ali U, Ohri A. Acute renal failure secondary to ingestion of ayurvedic medicine containing mercury. Indian J Nephrol. 2013;23:301–3. doi: 10.4103/0971-4065.114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma PV. Siddha medicine. In: Sharma PV, editor. History of Medicine in India. New Delhi: The Indian National Science Academy; 1992. pp. 445–50. [Google Scholar]
- 3.Subbarayappa BV. Chemical practices and alchemy. In: Bose DM, Sen SN, Subbarayappa BV, editors. A Concise History of Science in India. New Delhi: Indian National Science Academy; 1971. pp. 315–35. [Google Scholar]
- 4.“Rasa Shastra – Freedom Vidya”. [Last accessed on 2017 Jul 15]. Available from: http://www.Shrifreedom.org .
- 5.Medicinal Preparations. [Last accessed on 2017 Jul 15]. Available from: http://www.siddha-medicine.org/index.html .
- 6.Kadam A. Mercury in Ayurveda: A poison turned nectar. Rasamruta (ayurveda e journal) Ed. 2013 [Google Scholar]
- 7.Koch I, Moriarty M, Sui J, Rutter A, Saper RB, Reimer KJ, et al. Bioaccessibility of mercury in selected Ayurvedic medicines. Sci Total Environ. 2013;454-455:9–15. doi: 10.1016/j.scitotenv.2013.02.089. [DOI] [PubMed] [Google Scholar]
- 8.Jayawardene I, Saper R, Lupoli N, Sehgal A, Wright RO, Amarasiriwardena C, et al. Determination of in vitro bioaccessibility of Pb, As, Cd and Hg in selected traditional Indian medicines. J Anal At Spectrom. 2010;25:1275–82. doi: 10.1039/C003960H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaacs H. A syndrome of continuous muscle-fibre activity. J Neurol Neurosurg Psychiatry. 1961;24:319–25. doi: 10.1136/jnnp.24.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liguori R, Vincent A, Clover L, Avoni P, Plazzi G, Cortelli P, et al. Morvan's syndrome: Peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain. 2001;124:2417–26. doi: 10.1093/brain/124.12.2417. [DOI] [PubMed] [Google Scholar]
- 11.Irani PF, Purohit AV, Wadia NH. The syndrome of continuous muscle fiber activity. Evidence to suggest proximal neurogenic causation. Acta Neurol Scand. 1977;55:273–88. doi: 10.1111/j.1600-0404.1977.tb05647.x. [DOI] [PubMed] [Google Scholar]
- 12.Khwaja GA, Batla A, Patidar Y, Choudhary N, Gupta M, Chowdhury D, et al. Clinical and electrophysiological profile of Isaac's syndrome: A report of six cases. JIACM. 2015;16:261–4. [Google Scholar]
- 13.Hart IK. Acquired neuromyotonia: A new autoantibody-mediated neuronal potassium channelopathy. Am J Med Sci. 2000;319:209–16. doi: 10.1097/00000441-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Shillito P, Molenaar PC, Vincent A, Leys K, Zheng W, van den Berg RJ, et al. Acquired neuromyotonia: Evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol. 1995;38:714–22. doi: 10.1002/ana.410380505. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S, Newsom-Davis J, Mills K, Byrne N, Lang B, Vincent A, et al. Autoimmune aetiology for acquired neuromyotonia (Isaacs' syndrome) Lancet. 1991;338:75–7. doi: 10.1016/0140-6736(91)90073-x. [DOI] [PubMed] [Google Scholar]
- 16.Newsom-Davis J, Mills KR. Immunological associations of acquired neuromyotonia (Isaacs' syndrome).Report of five cases and literature review. Brain. 1993;116(Pt 2):453–69. doi: 10.1093/brain/116.2.453. [DOI] [PubMed] [Google Scholar]
- 17.Grisold W, Mamoli B. The syndrome of continuous muscle fibre activity following gold therapy. J Neurol. 1984;231:244–9. doi: 10.1007/BF00313659. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Zhang X, Cui F, Liu R, Dong Z, Wang X, et al. Subacute motor neuron hyperexcitability with mercury poisoning: A case series and literature review. Eur Neurol. 2014;72:218–22. doi: 10.1159/000363290. [DOI] [PubMed] [Google Scholar]
- 19.Panagariya A, Kumar H, Mathew V, Sharma B. Neuromyotonia: Clinical profile of twenty cases from Northwest India. Neurol India. 2006;54:382–6. doi: 10.4103/0028-3886.28110. [DOI] [PubMed] [Google Scholar]
- 20.Chaurasia RN, Abbas A, Shukla R. Toxin induced continuous muscle fiber activity syndrome. Ann Neurosci. 2008;15:4. [Google Scholar]
- 21.Sharifian M, Zoorisafa M, Kiahosseni M. Hypertensive encephalopathy induced by mercury poisoning; a report of 3 cases (in an Iranian family) Iran J Child Neurol. 2007;1:53–9. [Google Scholar]
- 22.Torres AD, Rai AN, Hardiek ML. Mercury intoxication and arterial hypertension: Report of two patients and review of the literature. Pediatrics. 2000;105:E34. doi: 10.1542/peds.105.3.e34. [DOI] [PubMed] [Google Scholar]
- 23.Vincent A. Understanding neuromyotonia. Muscle Nerve. 2000;23:655–7. doi: 10.1002/(sici)1097-4598(200005)23:5<655::aid-mus1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Hayat GR, Kulkantrakorn K, Campbell WW, Giuliani MJ. Neuromyotonia: Autoimmune pathogenesis and response to immune modulating therapy. J Neurol Sci. 2000;181:38–43. doi: 10.1016/s0022-510x(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 25.Kazantzis G. Mercury and the kidney. Trans Soc Occup Med. 1970;20:54–9. doi: 10.1093/occmed/20.2.54. [DOI] [PubMed] [Google Scholar]


