Abstract
This is a case report of an 8-year-old boy who developed an atypical, rare subphenotype of autoimmune inflammatory acute juvenile dermatomyositis (JDM), initially masquerading as viral polymyositis (PM)-like presentation, that was complicated by a hitherto unreported fulminant, life-threatening pediatric systemic capillary leak syndrome (SCLS). We highlight the close differential between viral PM and JDM, the baffling clinical syndromic constellation of hypotension with hemoconcentration – a “shock”-like syndrome, hypoalbuminemia without albuminuria, and generalized edema with the atypical JDM presentation, and stress crucial need to implement early aggressive, multipronged immunomodulatory treatment along with intensive fluid resuscitation which saved the life, this patient from a stormy, and turbulent 4-week clinical illness. This is the first published case description in the current literature of the association of an aggressive subphenotype of JDM and life-threatening pediatric SCLS. This report opens the Pandora's Box to explore the genetic and pathomechanisms of both disorders.
Keywords: Aggressive subphenotype, capillary leak syndrome, Clarkson syndrome, generalized pitting edema, juvenile dermatomyositis, muscle weakness, myositis, subcutaneous edema, systemic capillary leak
INTRODUCTION
Juvenile dermatomyositis (JDM) is the most common idiopathic, autoimmune, inflammatory, myopathic disease of childhood; albeit rare, occurring in 3/1 million children which is diagnosed when the disease has begun before the age of 15 years.
Having an underlying pathologic denominator of a immune-mediated vasculopathic process triggering microvascular and endothelial injury, JDM is a potentially life-threatening autoimmune multisystem disease, primarily affecting muscles and skin, that is associated with considerable morbidity and mortality if not recognized early and aggressively treated, in some cases as high as 50% with one-third of survivors left with mild-to-severe disability.[1,2,3,4]
Although the distinctive dermatologic features of bilateral periorbital/upper eyelid edema and focal mild facial swelling has been well described in acute cases of JDM,[5] widespread and generalized subcutaneous edema (anasarca) as a presenting feature of in polymyositis (PM)/dermatomyositis (DM) has not been listed in the standard textbooks of rheumatology and is indeed a rare presentation in the published literature. To the best of our knowledge, there is only <30 cases in the literature till date, but none were associated with the clinical phenotype of SCLS such a subphenotype clinical variant of JDM is said to have a florid disease course, poorer prognosis with significant morbidity and mortality, and is postulated to represent a more aggressive disease course with underlying immune complex-mediated vascular endothelial damage as the contributor of significant generalized edema (anasarca).[6,7,8,9] To the best of our knowledge, this is the first published report of an 8-year-old boy with acute JDM, generalized edema, and complicated by fulminant, life-threatening systemic capillary leak syndrome (SCLS; Clarkson Syndrome). There is a report of a similar co-occurrence, being described in a 69-year-old Caucasian woman with acute exacerbation of chronic DM causally linked to C1 inhibitor deficiency.[10]
CASE REPORT
This boy, premorbidly healthy presented to his nearby regional hospital with a viral prodrome. This included high fever (101°F–102°F), associated with constitutional symptoms of malaise, fatigue, anorexia, and myalgias. A week later, he presented to our hospital with reddish maculopapular skin rashes forearms and ankles; grotesque pitting edema (anasarca) involving the face trunk and extremities along with pure motor symmetrical progressive weakness, initially affecting pelvifemoral region where his muscles became excruciating painful. Within the next few days, he progressed to flaccid weakness to be bedbound. Other systems examination proved essentially unremarkable. He was presumed to have viral PM till there were additional catastrophic manifestations when he developed generalized pitting anasarca with normal renal functions. There was no family history of relevant autoimmune disorders. There was negative history of Raynaud's phenomenon, polyarthralgias, and/or polyarthritis; bulbar symptomatology such as dysphonia, pharyngeal dysphagia, respiratory muscle weakness, soft-tissue calcifications, or oral ulceration. There was no positive history of preceding vaccination, endocrine, nutritional or metabolic disorders, or exposure to drugs or toxic substances.
Physical examination revealed a fully conscious, oriented, sick looking, distraught boy with flaccid quadriparesis with Grade 1 deep tendon reflexes, diffuse Grade 1/5 Medical Research Council muscular weakness with exquisite pain, his muscles were diffusely tender to palpation and he had symmetric proximal and axial weakness and had quite grotesque pitting swelling of the face, neck, abdomen, extremities, and scrotum. Multiple inconspicuous erythematous maculopapular rashes were observed extensor surfaces of the elbows and the medial malleoli of the ankles with no evidence of calcinosis. No other focal neurological signs were elicited. Systemic examination was unremarkable. At the time of admission with flaccid quadriparesis and anasarca, he had hypoalbuminemia (1.8 mg/dl), hypotension (90/60 mmHg), tachycardia (140/min) with normal echocardiogram, and renal functions without albuminuria. Serum procalcitonin and sepsis work up, and viral serologies proved negative. The laboratory and biochemical investigations, complete blood counts, serologic tests for antinuclear antibodies (ANA), rheumatoid factor, ANA profile, perinuclear antineutrophil cytoplasmic antibody (ANCA), cytoplasmic-ANCA, antistreptolysin-O titers, thyroid functions, and hepatitis serologies were normal. Electrocardiography and cardiac injury enzymes were normal that conclusively ruled out subclinical myocardial involvement. His chest radiography and abdominal ultrasonography were unremarkable. His creatine phosphokinase and lactate dehydrogenase were considerably elevated at 12000U/L and 6980U/L, respectively, indicating rhabdomyolysis.
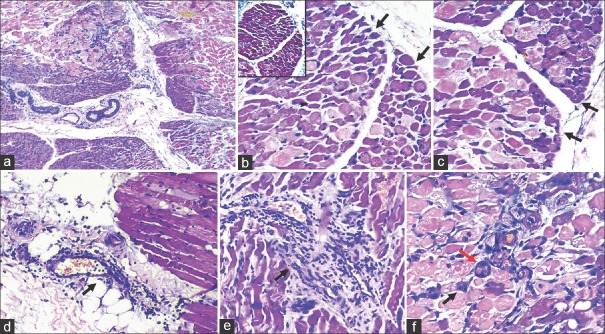
Nerve conduction studies were normal, and electromyography (EMG) revealed evidence of increased muscle membrane irritability as depicted by increased insertional activity and spontaneous fibrillations, low amplitude short duration polyphasic myopathic MUPs. An emergent left vastus lateralis muscle biopsy was done that conclusively confirmed the diagnosis of JDM [Figure 1]. By this time, Jo-1 IgG antibody and anti-Mi2 antibody were positive with reduced C3 and C4 complement levels which unambiguously established the clinical diagnosis of acute JDM with the baffling clinical syndromic constellation of hypotension with hemoconcentration (high hematocrit of 56%), a “shock”-like syndrome, hypoalbuminemia without albuminuria and massive generalized pitting edema attributed to a fulminant SCLS.
Figure 1.
(a) Preserved fascicular architecture (H and E, ×40); (b and c) perifascicular atrophy (black arrow) (H and E, ×200); (b) inset– normal endomyseal collagen (Masson Trichrome, ×200); (d) perimyseal perivascular lymphocytic infiltrate (black arrow) (H and E, ×200); (e) endomyseal perivascular lymphocytic infiltrate (black arrow) (H and E, ×200); (f) basophilic regenerating myofibre (black arrow) and myonecrosis (red arrow) (H and E, ×200)
In view of the intense pain and myalgias and the incessant crying, he received parenteral Tramadol (50 mg IV Q8H) and paracetamol around the clock. The patient's hemodynamic instability was intensively managed in neurointensive care unit with cardiovascular, central venous pressure monitoring, aggressive fluid resuscitation, and high-dose catecholamine therapy. There were occasions when the patient's blood pressure (BP) went below systolic BP (SBP) of 90 mmHg despite volume infusion and moderate dose dopamine; norepinephrine infusion was started and titrated to support a SBP of 90 mm Hg. In view of the aggressive and stormy nature of JDM and complicated by SCLS, a combination of pulse methylprednisolone (30 mg/kg/day, augmented with high-dose intravenous immunoglobulin (IVIg, 2 g/kg/day) was instituted in acute critical phase. The SCLS was managed with theophylline 200 mg BD, salbutamol 2 mg BD, leukotriene inhibitors (montelukast), fresh frozen plasma, and 20% albumin. His hemodynamic indices showed signs of improvement after 72 h of vigorous treatment. Thereafter, he was initiated on immunomodulatory therapy with 1.5 mg/kg Prednisolone and 2.5 mg/kg/day of Azathioprine. After 2 weeks of our intensive treatment, he started showing motoric recovery with amelioration of edema to 50% from baseline. He started ambulating and was discharged after a month of hospital admission. Two months following his discharge, he had normal motoric power and was switched to alternate-day glucocorticoid schedule. Subsequently, a cautious reduction of maintenance dose followed by a gradually taper of both prednisolone and azathioprine was done over 6 months. He has been on regular neurology follow-up now for about 2 years, remains in clinical remission, doing well in his school and daily life with immunomodulatory therapy tapered off by 1 year.
DISCUSSION
This report highlights an acute florid presentation of “viral PM” masquerade[11] either presenting with or triggered by a viral flu-like illness associated with severe and overwhelming constitutional symptoms. This unique case report does teach several clinical takeaways and clinical pearls, that we will discuss in this section. First, the “PM” phenotype without the denominative feature of typical cutaneous/dermatologic signs with exquisite muscle tenderness in this boy did mimic a viral PM. It is prudent to differentiate viral polymyositis from immune-meditated PM/DM, the masqueraders[11] that were conclusively proved negative in this patient. Second, the PM was hallmarked by a life-threatening clinical course associated with severe anasarca (JDM with anasarca) and complicated by another immune-mediated syndrome of SCLS (Clarkson's syndrome). The absence of other disorders causing generalized edema such as nephrotic syndrome, heart failure, liver and renal diseases, and hypothyroidism did rule out other possibilities. Although immune and inflammatory mediators are hypothesized to play a key role in SCLS although the exact mechanism of the capillary leak syndrome is still not elucidated, cytokines, and inflammatory mediators and oxidation injury-induced apoptosis are plausible postulated or likely mechanism for endothelial injury.[12,13,14]
The unremarkable emergent infectious and viral serology work up, normal precalcitonin levels with elevated C-reactive protein, hypocomplementemia (C3, C4), normal white blood cell counts alerted us to consider an atypical presentation of an abacterial, nonviral inflammatory autoimmune “acute” PM which is readily treatable. This was readily unequivocally established by EMG, muscle biopsy [Figure 1] (confirming JDM) and positive Jo-1 IgG antibody and anti Mi2 antibody levels. Despite an aggressive clinical course of JDM and SCLS, it was not complicated by any internal organ involvement/organ dysfunction. To the best of our knowledge, this is the first published case description in the current literature of the association of an aggressive subphenotype of JDM and life-threatening pediatric SCLS. Third, this report reiterates the need to recognize such clinical associations, not only from a challenging therapeutic standpoint, but also opens the Pandora's Box to explore the genetic, molecular, and immunologic underpinnings of this rare co-occurrence to help elucidate the underlying pathogenesis of both disorders.
Finally, this clinical overlap of JDM with SCLS does underscore the overall clinical course to be florid, life-threatening, and a clinical phenotype of severe disease activity that is associated with significant morbidity and mortality. Such an overlap syndrome undoubtedly warrants timely, aggressive, and multi-pronged immunomodulatory therapy with not only pulse methylprednisolone therapy but concomitant use of adjuvant immunosuppressive drugs and intravenous immunoglobulin to arrest the underlying autoimmune process. Early diagnosis, good supportive care, monitoring of fluid electrolyte status, vasopressor therapy, antibiotics, theophyllines, terbutaline/salbutamol, and leukotriene inhibitors are absolutely essential in the management of SCLS.[15] The early, timely diagnosis, clinical reasoning, and “pattern recognition” did certainly ensure a gratifying and complete neurologic functional recovery in a life-threatening clinical course of approximately 1 month without residual deficits or functional impairment in this young boy.
We did a search of the published literature using Medline and PubMed over the past 40 years; and to the best of our informed knowledge, there are approximately fifty published adult cases of DM and PM with edema, and is reported that such patients have a florid disease course, with significant morbidity and mortality. Out of the 50 cases, about 24 cases have been associated with generalized subcutaneous edema.[6] The underlying pathogenesis for the subcutaneous edema has been hypothesized to be related to increased vascular permeability in the tissues and muscle from the inflammatory autoimmune process, deposition of immune complexes, complement activation, and vascular endothelial damage leading to an extensive leakage of fluid into surrounding structures. However, none of these reported cases have been reported to be associated with the typical features of SCLS. Timely diagnosis and emergent multi-pronged treatment of these cases are quintessential to decrease morbidity. With the diagnosis of acute JDM established with muscle biopsy, Jo-1 IgG antibody and anti-Mi2 antibody in this patient, the atypical, yet unreported, acute fulminant presentation with “shock” syndrome, hypoalbuminemia, and increased hematocrit should alert clinicians to the diagnosis of SCLS. It is interesting to note that JDM can also be associated with other autoimmune diseases such as autoimmune hemolytic anemia, Evans syndrome, and myasthenia gravis.
In summary, we have presented a unique and rare presentation of acute and rapid onset JDM with certain peculiarities and atypical features whose clinical course to be complicated by another immune-mediated catastrophic disorder of SCLS, both of these autoimmune disorders that we postulate to have the common denominator of severe endothelialitis. This life-threatening overlap is often treatment refractory and quintessentially mandates a multipronged prompt aggressive immunosuppressive therapy.
CONCLUSION
On a research imperative, this association of an aggressive clinical variant of acute JDM with SCLS, we feel, is not a coincidence or a chance association, but certainly needs more research insights. There is a knowledge gap to gain insight into the pathophysiology and shared immunomechanisms between JDM subphenotype with SCLS. We reiterate this extremely rare combination of “Acute JDM with anasarca” and SCLS that presented to us as a clinical nightmare is being reported for greater inter-specialty awareness and timely recognition and aggressive treatment of this enigmatic life-threatening fatal clinical disorder.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Informed patient consent was obtained for the publication of the case details.
REFERENCES
- 1.Maugars YM, Berthelot JM, Abbas AA, Mussini JM, Nguyen JM, Prost AM, et al. Long-term prognosis of 69 patients with dermatomyositis or polymyositis. Clin Exp Rheumatol. 1996;14:263–74. [PubMed] [Google Scholar]
- 2.Sultan SM, Ioannou Y, Moss K, Isenberg DA. Outcome in patients with idiopathic inflammatory myositis: Morbidity and mortality. Rheumatology (Oxford) 2002;41:22–6. doi: 10.1093/rheumatology/41.1.22. [DOI] [PubMed] [Google Scholar]
- 3.Marie I, Hachulla E, Hatron PY, Hellot MF, Levesque H, Devulder B, et al. Polymyositis and dermatomyositis: Short term and longterm outcome, and predictive factors of prognosis. J Rheumatol. 2001;28:2230–7. [PubMed] [Google Scholar]
- 4.Murthy JM. Drug treatment of polymyositis and dermatomyositis. Neurol India. 2010;58:3–5. doi: 10.4103/0028-3886.60386. [DOI] [PubMed] [Google Scholar]
- 5.Hall VC, Keeling JH, Davis MD. Periorbital edema as the presenting sign of dermatomyositis. Int J Dermatol. 2003;42:466–7. doi: 10.1046/j.1365-4362.2003.01696.x. [DOI] [PubMed] [Google Scholar]
- 6.Tu J, McLean-Tooke A, Junckerstorff R. Increasing recognition of dermatomyositis with subcutaneous edema-is this a poorer prognostic marker? Dermatol Online J. 2014;20:21244. [PubMed] [Google Scholar]
- 7.Mourão AF, Pinto TL, Falcão S, Ribeiro C, Vieira H, Caetano-Lopes J, et al. Juvenile dermatomyositis associated with anasarca – A clinical case. Acta Reumatol Port. 2009;34:276–80. [PubMed] [Google Scholar]
- 8.Saygi S, Alehan F, Baskin E, Bayrakci US, Ulu EM, Ozbek N, et al. Juvenile dermatomyositis presenting with anasarca. J Child Neurol. 2008;23:1353–6. doi: 10.1177/0883073808318544. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell JP, Dennis GJ, Rider LG. Juvenile dermatomyositis presenting with anasarca: A possible indicator of severe disease activity. J Pediatr. 2001;138:942–5. doi: 10.1067/mpd.2001.113363. [DOI] [PubMed] [Google Scholar]
- 10.Gradwohl-Matis I, Illig R, Salmhofer H, Neureiter D, Brunauer A, Dünser MW, et al. Fulminant systemic capillary leak syndrome due to C1 inhibitor deficiency complicating acute dermatomyositis: A case report. J Med Case Rep. 2014;8:28. doi: 10.1186/1752-1947-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh H, Talapatra P, Arya S, Gupta V. Viral myositis as a close mimicker of polymyositis. Ann Trop Med Public Health. 2013;6:324–6. [Google Scholar]
- 12.Clarkson B, Thompson D, Horwith M, Luckey EH. Cyclical edema and shock due to increased capillary permeability. Am J Med. 1960;29:193–216. doi: 10.1016/0002-9343(60)90018-8. [DOI] [PubMed] [Google Scholar]
- 13.Iwasa T, Ohashi H, Kihira K, Koike Y, Otake K, Inoue M, et al. 10-year-old girl with life-threatening idiopathic systemic capillary leak syndrome: A case report. BMC Pediatr. 2014;14:137. doi: 10.1186/1471-2431-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Druey KM, Greipp PR. Narrative review: The systemic capillary leak syndrome. Ann Intern Med. 2010;153:90–8. doi: 10.1059/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zancanaro A, Serafini F, Fantin G, Murer B, Cicardi M, Bonanni L, et al. Clinical and pathological findings of a fatal systemic capillary leak syndrome (Clarkson disease): A case report. Medicine (Baltimore) 2015;94:e591. doi: 10.1097/MD.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]