Abstract
Rationale: Obliterative bronchiolitis (OB) is a major cause of mortality after lung transplantation. Depletion of airway stem cells (SCs) may lead to fibrosis in OB.
Objectives: Two major SC compartments in airways are submucosal glands (SMGs) and surface airway p63 (also known as TP63 [tumor protein 63])-positive/K5 (also known as KRT5 [keratin 5])-positive basal cells (BCs). We hypothesized that depletion of these SC compartments occurs in OB.
Methods: Ferret orthotopic left lung transplants were used as an experimental model of OB, and findings were corroborated in human lung allografts. Morphometric analysis was performed in ferret and human lungs to evaluate the abundance of SMGs and changes in the expression of phenotypic BC markers in control, lymphocytic bronchiolitis, and OB airways. The abundance and proliferative capacity of proximal and distal airway SCs was assessed using a clonogenic colony-forming efficiency assay.
Measurements and Main Results: Ferret allografts revealed significant loss of SMGs with development of OB. A progressive decline in p63+/K5+ and increase in K5+/K14+ and K14+ BC phenotypes correlated with the severity of allograft rejection in large and small ferret airways. The abundance and proliferative capacity of basal SCs in large allograft airways declined with severity of OB, and there was complete ablation of basal SCs in distal OB airways. Human allografts mirrored phenotypic BC changes observed in the ferret model.
Conclusions: SMGs and basal SC compartments are depleted in large and/or small airways of lung allografts, and basal SC proliferative capacity declines with progression of disease and phenotypic changes. Global airway SC depletion may be a mechanism for pulmonary allograft failure.
Keywords: lung transplantation, obliterative bronchiolitis, chronic rejection, bronchiolitis obliterans syndrome
At a Glance Commentary
Scientific Knowledge on the Subject
Lung transplantation is an excellent treatment option for patients with end-stage lung disease, yet 50% of recipients die within 5 years as a result of obliterative bronchiolitis (OB). OB is characterized by progressive fibrotic occlusion of the bronchiolar airways, and bronchiolar stem cell (SC) depletion has been proposed as one mechanism of pathogenesis. Here we use a recently developed ferret lung transplant model of OB to evaluate phenotypic changes to SC compartments in the conducting airways and compare these changes to human lung allografts with terminal OB.
What This Study Adds to the Field
Our findings demonstrate that the development of OB in ferrets and humans coincides with the progressive depletion of p63 (also known as TP63 [tumor protein 63])-positive/K5 (also known as KRT5 [keratin 5])-positive basal cells throughout all levels of the conducting airways and the destruction of submucosal glands, a site of SC niche in the larger, cartilaginous airways. Our findings of global destruction of SC compartments in the conducting airways may have diagnostic utility in monitoring OB progression.
Lung transplantation is a viable treatment option for end-stage lung diseases for which no other effective medical therapies exist (1). However, long-term survival is limited because of the development of obliterative bronchiolitis (OB), which is considered a form of chronic rejection (2, 3). OB is a histological diagnosis characterized by fibrosis of the respiratory and terminal bronchioles of the small airways (4). A clinical surrogate of OB and allograft dysfunction is bronchiolitis obliterans syndrome, which will develop in approximately half of lung transplant recipients within 5 years of transplantation (3). Currently, OB is fatal, and there are no effective treatments for slowing its progression. Questions abound regarding the pathogenesis of OB; perhaps chief among them is whether airway epithelial stem cells (SCs) are targeted for destruction in the allograft.
Progress in investigating mechanisms underlying epithelial injury in OB has been hindered by limitations in accurately phenocopying human OB lesions in an animal model with SC biology similar to human airways (5). An animal model that displays both immune and nonimmune mechanisms that contribute to the pathogenesis of OB (2, 5) is needed, especially because several studies suggest that a loss of epithelial SCs may play a role (6, 7). For example, previous studies have suggested that club cells are lost in lungs of patients with OB (7) and that there exists an inverse relationship between circulating CCSP+ cells with bronchiolitis obliterans syndrome severity (6). The ferret model of lung transplantation fits these criteria and reproduces the natural progression of OB in human allografts (8).
Discrete anatomic regions of the lung possess distinct stem/progenitor-cell populations (9–11). For example, along the surface of the conducting airways, basal cells serve as progenitor cells and have been recognized as a source of multipotent SCs (12, 13). Multipotent basal cells along the surface airway epithelium (SAE) are chiefly identified based on coexpression of K5 (also known as KRT5 [keratin 5]) and the tumor suppressor protein p63 (also known as TP63 [tumor protein 63]) (12–15). These basal cells play an important role in maintaining homeostasis of the SAE because of their ability to self-renew and differentiate into epithelial cells of various lineages (13). In the mouse lung, the distribution of basal cells is largely limited to the trachea and bronchi, whereas in human lungs these cells are present down to the terminal bronchioles (16, 17). At steady state, the majority of basal cells are K5+p63+, and only a small population express K14; however, K14+ basal cells expand after epithelial injury (18–20). Interestingly, ablation of basal cells by inhalation of chlorine gas resulted in the development of OB-like lesions in mice (21).
In addition to surface airway basal cells, submucosal glands (SMGs) are another important source of stem/progenitor cells that have the capacity to differentiate into all SAE cell types (9, 18, 22–26). These glands contain slowly cycling progenitors (25–26,), which are capable of repeatedly responding to injury (27). In mice, however, SMGs are largely restricted to above the proximal three tracheal rings, whereas in humans, they are abundant throughout the cartilaginous airways, and in this regard ferrets are highly similar (28). How surface airway basal SCs and glandular SC compartments are impacted by OB progression is not understood.
Here we use the ferret model of orthotopic lung transplantation to define changes in SMG and SAE basal SCs in allograft versus native lung tissues. We show that SMGs are progressively destroyed as OB develops, and with their destruction it is likely that the glandular SC niche is also lost. In addition, we demonstrate that an expansion of K14+ basal cells in large and small ferret allograft airways accompanies progressive depletion of clonogenic airway basal SCs during the development of OB.
Methods
Ferret Lung Transplantation and Denervation Surgeries
Animal procedures were approved by the University of Iowa Institutional Animal Care and Use Committee. Orthotopic left lower lobe transplantation was performed as previously described on adult outbred sable ferrets (8). Additional details regarding ferret transplantation and follow-up procedural care are provided in the online supplement. The timeline for each animal is shown in Table E1 in the online supplement. Denervation was performed in adult male ferrets by dividing the bronchus, followed by reanastomosis, thereby severing innervation to the left lower lobe. Animals were killed 5 months after denervation.
Tissue Processing and Histology
Ferret lungs were fixed in 10% neutral buffered formalin and processed into 10-μm paraffin sections. Allograft rejection was graded using the international grading scheme for pulmonary allograft rejection recommended by the International Society for Heart and Lung Transplantation (Table E2) (4). Allografts were categorized as having no airway rejection (NRt, n = 3), acute rejection with lymphocytic bronchiolitis in the absence of OB (LBt, n = 6), or chronic rejection in the presence of OB (OBt, n = 5). Additional detail on the scoring method for making these measurements is provided in the online supplement. Human studies were performed after approval from the Institutional Review Board. SMG quantification was done in five human allografts with end-stage OB (three repeat lung transplants, two autopsy), and two allografts without OB from autopsy specimens (died from nonpulmonary causes). Human autopsy lung samples from patients who died of nonpulmonary pathology were used as controls (n = 7).
Gene Expression Analysis
Gene expression analysis was performed using the QuantiGene Plex Assay Kit (Affymetrix) using ferret lung sections prepared per manufacturer’s “FFPE Tissues” protocol. Gene expression was internally normalized to two housekeeping genes (HPRT and PPIB) (Table E3). This expression dataset and additional detail on the method for making these measurements are provided in the online supplement.
Immunofluorescence and Morphometric Analysis
Deparaffinized sections underwent antigen retrieval in 10 mM sodium citrate, 0.1% Triton X-100 at 95°C for 5 minutes under pressure. Sections were blocked and stained with primary antibodies against K5 (Covance), K14 (Thermo Scientific), p63 (Thermo Scientific), Ki67 (eBiosciences), Keratin 8 (K8) (abcam), and/or cleaved-caspase 3 (Cell Signaling), followed by secondary fluorochrome-linked antibodies. Sections were mounted using Aqua-Mount medium (Lerner Laboratories) with nuclear Hoechst 33342 counterstaining (Thermo Fisher). Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) was performed using a DeadEnd Fluorometric TUNEL assay kit (Promega) per manufacturer’s protocol. Morphometric analysis of images was performed using the cell scoring application in MetaMorph Image Analysis software. Additional detail on the method for making these measurements is provided in the online supplement.
Ferret Airway Epithelial Colony-Forming Efficiency Assay
Resected large and small airways were digested with pronase (1.5 mg/ml) in Dulbecco’s modified Eagle medium for 24 hours at 4°C, as previously described, to isolate basal cells (29). Dissociated cells were suspended in F-media, counted, and seeded at serial dilutions (100–2,000 cells) onto irradiated NIH-3T3 J2 fibroblast feeders in triplicate (30). After 7 days, cultures were fixed with formalin, stained with DAPI, and imaged. Proliferative capacity is presented as the number of nuclei formed per cells seeded. Additional detail on the methods for performing these experiments is provided in the online supplement.
Statistical Analyses
Statistical significance testing and normality testing were performed in Graphpad Prism. Specific tests and their results are indicated in the relevant figure legends. When appropriate, ferret data were paired with size-matched control airways from each animal’s native lobe, and human data were scored using size-matched (±0.3 mm), age-matched (±5 yr), and sex-matched airways.
Results
Ferret Allografts Feature Significant SMG Loss
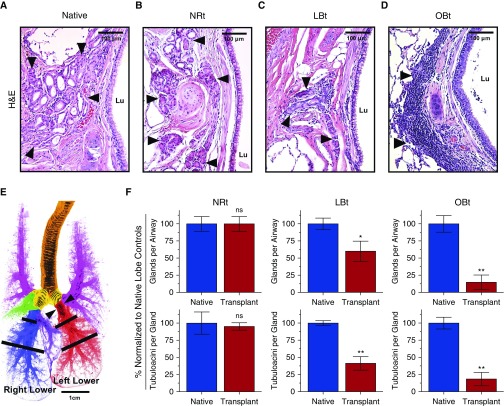
Histologic assessment of cartilaginous large airways in ferret allografts revealed that SMGs become atrophic compared with native lobes. Moreover, large airway SMGs of the allograft featured extensive cellular inflammatory infiltrates, with the extent of damage being more severe in samples with histologic OB (Figures 1A–1D), the majority of which stained CD3+, a marker of T lymphocytes (data not shown). Notably, the region of the native airway shown in Figure 1A is just proximal to the anastomosis, whereas the allograft airways shown in Figures 1B–1D are just distal to the anastomosis and juxtaposed at the lobar bronchus anastomosis. At the bronchial anastomosis, we observed a destruction of allograft SMGs, whereas SMGs in the native bronchus were spared (Figure E1). Histological sections were used to compare SMGs in size-matched airways between allograft and contralateral native lobes, as diagrammed in Figure 1E. Compared with contralateral native lobes, there was a significant decrease in the number of glands per airway in LBt (P = 0.0411) and OBt (P = 0.0079), whereas in NRt, there was no difference (P > 0.9999) from that of native lobes (Figure 1F). Similarly, glands of both LBt (P = 0.0022) and OBt (P = 0.0079) allografts were composed of significantly fewer tubuloacinar structures than those of native lobes, but again no difference was observed in NRt lobes (Figure 1F). These findings demonstrate that SMG destruction in the proximal airways increases with severity of allograft rejection.
Figure 1.
Loss of submucosal glands (SMGs) in ferret pulmonary allografts demonstrating progressive obliterative bronchiolitis (OB) pathology. (A–D) Representative hematoxylin and eosin (H&E)-stained sections of large airways of native lungs (A), transplant lobes with no allograft airway rejection (NRt) (B), transplant lobes with lymphocytic bronchiolitis (LBt) (C), and transplant lobes with OB (OBt) (D). Arrowheads indicate SMGs, and Lu denotes airway lumen. (E) Illustration of normal ferret lung block with color-coded lobar anatomy indicating the trachea (orange), mainstem bronchi (yellow), bilateral upper lobes (pink), right middle lobe (green), right mediastinal lobe (purple), right lower lobe (blue, native control lobe), and left lower lobe (red, experimental transplant lobe). Black bars indicate where sections were obtained for analysis of SMG abundance. Arrowheads indicated where the transplant anastomosis is performed. (F) Quantification of SMGs in ferret allografts (red) versus native control lobes (blue), with respect to number of well-defined glands per airway (top) and number of tubuloacinar structures per gland (bottom) across the same spectrum of disease found in A–D (NRt, n = 3; LBt, n = 6; OBt, n = 5). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01 by Mann-Whitney U test. ns = not significant.
Human Allografts with End-Stage OB Feature Significant SMG Loss
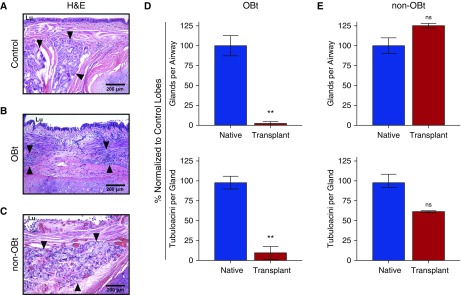
To evaluate whether SMG loss in human allografts was similar to that observed in ferrets, we performed similar quantification on five end-stage OB lung allografts as well as two allografts without evidence of OB. In contrast to age-matched, sex-matched, and size-matched cartilaginous airway controls (Figure 2A), SMGs of the cartilaginous airways in human OB allografts were atrophic and consumed by an intense inflammatory infiltrate (Figure 2B). In human samples from two individuals who had received lung transplants but died as a result of nonpulmonary complications, the SMGs remained intact (Figure 2C). Compared with control lungs, OB allografts showed a significant decline in the number of glands per airway (P = 0.0012) and acini per gland (P = 0.0012) (Figure 2D). However, there was little difference in the SMG architecture between control lobes and allografts from patients who had no OB (Figure 2E). Taken together, these data demonstrate that the extent of SMG destruction correlates with the severity of transplant rejection in both ferrets and humans.
Figure 2.
Loss of submucosal glands (SMGs) in human pulmonary allografts demonstrating progressive obliterative bronchiolitis (OB) pathology. (A–C) Representative hematoxylin and eosin (H&E)-stained sections of large airways from control pulmonary tissue (A), pulmonary allografts with OB (OBt) (B), and pulmonary allografts without OB (non-OBt) from patients who received a lung transplant but died from nonpulmonary complications (C). Arrowheads indicate SMGs, and Lu denotes airway lumen. (D and E) Quantification of SMGs in human pulmonary allografts (red) versus control lobes (blue), with respect to the number of gland clusters per airway (top), and the number of tubuloacinar structures per gland (bottom) in seven OBt allografts (D) and in two non-OBt allografts (E). Blue columns indicate size-matched, age-matched, and sex-matched nontransplanted lung tissue from patients who died of nonpulmonary pathology (n = 7). Data are presented as mean ± SEM. **P = 0.0012 by Mann-Whitney U test. ns = not significant.
Expression of SMG-Specific Genes Declines with Worsening OB
Analysis of mRNA expression of 11 different cell-type marker genes and 5 cell fate–specific genes was generally consistent with our histological and immunofluorescence findings. Principal component analysis of collective gene expression revealed a grouping of native lobes with allografts having limited (LBt) or no (NRt) histological signs of rejection apart from allografts with worsening OB (OBt) (Figure E2A). Specifically, expression of markers for ciliated cells (FOXJ1) and club cells (SCGB1A1) were significantly reduced in OBt allografts (Figures E2B and E2C). In addition, consistent with the destruction of SMGs, markers for serous cells (LYZ), mucous cells (MUC5B), and myoepithelial cells (ACTA2) were also significantly reduced in OBt allografts as compared with native lobes (Figures E2D–E2F). Furthermore, increased MKI67 and CASP3 expression indicates an increase in proliferation and apoptosis in OBt allografts, respectively (Figures E2G and E2H). These data corroborate our histological and immunofluorescent findings.
Denervation Alters the SMG Structure
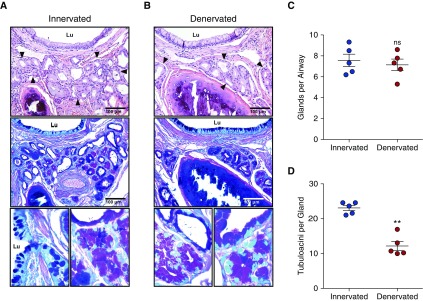
To interrogate if surgical denervation affects SMG structure and abundance, we denervated the left lower lobe of five ferrets and evaluated airway SMGs 5 months later. Compared with the nonsurgical right lower lobe (Figure 3A), gland abundance in denervated lobes (Figure 3B) was not altered (Figure 3C). However, there was a significant (P = 0.0011) decrease in tubuloacinar structures per gland unit (Figure 3D). Notably, no pathology was present in distal airways, indicating that denervation alone was insufficient to produce OB-like lesions.
Figure 3.
Effects of airway vagal nerve interruption (denervation) on submucosal glands (SMGs) in ferret pulmonary lung lobes. (A and B) Representative hematoxylin and eosin–stained sections (top), serial sections stained with periodic acid–Schiff/Alcian blue (PAS/ab) (middle), and high-magnification PAS/ab images (bottom) of large airways of innervated (nonsurgical) ferret lobes (A), and denervated ferret lobes (B). Arrowheads indicate SMGs, and Lu denotes airway lumen. (C and D) Quantification of the innervated control lobes (blue) and denervated lobes (red) indicating the number of glands (C) and the number of tubuloacinar structures per gland (D). Data are shown as the raw number of glands and tubuloacinar structures in each animal and presented as the mean ± SEM of five animals. No denervated lobes demonstrated histopathological evidence of overt obliterative bronchiolitis. **P = 0.0011 by paired t test. ns = not significant.
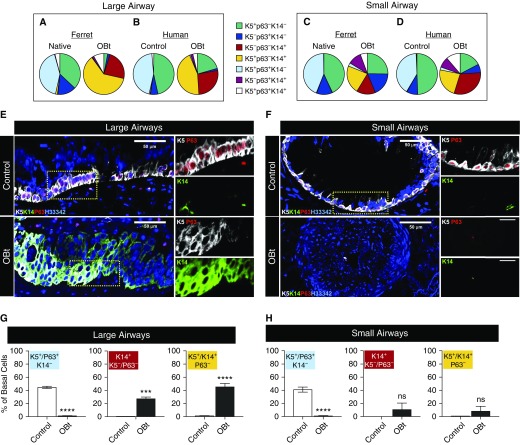
Ferret and Human Allografts with OB Lose K5+p63+ Basal Cells in Large and Small Airways
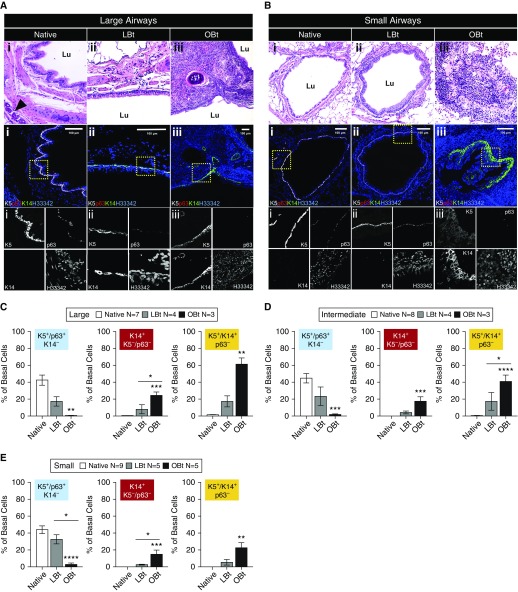
Immunofluorescence staining of basal cell markers in native ferret airways revealed an abundance of K5+p63+ cells, with few K14+ cells in the SAE (Figures 4A and 4B). In large airways of allografts from animals with LBt, there was a more pronounced diversity in basal cell phenotypes including K5+K14+p63+ and K5+K14+p63− cells, with a decline in K5+p63+K14− basal SCs (Figure 4A). In large airways of allografts with OBt, a majority of basal SCs were K14+ and K5+K14+, and p63 staining was nearly absent (Figure 4A). Morphometric quantification confirmed a significant increase in the abundance of K14+K5−p63− (P < 0.001) and K14+K5+p63− (P < 0.01) basal cells and significant decline in K5+p63+K14− (P < 0.01) basal cells within large OBt airways as compared with native lobes (Figure 4C). These phenotypic changes to basal cells were intermediate within LBt large airways, demonstrating that the phenotypic change was progressive with the severity of allograft rejection (Figure 4C).
Figure 4.
Changes in the expression of basal cell markers in airways of native and allograft in ferrets with varying allograft disease severity. (A and B) Hematoxylin and eosin–stained sections and serial section immunofluorescence images of (A) large and (B) small airways from (Ai and Bi) native, (Aii and Bii) lymphocytic bronchiolitis allografts, and (Aiii and Biii) obliterative bronchiolitis (OB) allografts. Columns indicate representative airways used for basal cell quantification in the left lower lobe pulmonary allograft in lymphocytic bronchiolitis without OB (LBt) and in OB (OBt) versus mirror-image sections from the native right lower lobe used for quantification of native airway controls. Arrowhead indicates submucosal gland, and Lu denotes airway lumen. (C–E) Quantification of basal cell phenotypic changes in large (C), intermediate (D), and distal (E) airways in ferret allografts as compared with native lobes of the same animals. Sections were stained for K5, p63, and K14 as marked. Statistical analysis was performed by two-way analysis of variance and Tukey multiple comparison post hoc test: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Data shown are mean ± SEM for number of animals/lobes for each dataset as indicated in the figure.
The composition of basal cell phenotypes in the native intermediate and small airways was similar to that of large airways (Figures 4B, 4D, and 4E). The abundance of K5+p63+K14− basal cells significantly declined within OBt intermediate and small airways (P < 0.001 and P < 0.0001, respectively), as compared with native lobes (Figures 4D and 4E). The opposite trend was observed for K14+K5−p63− and K14+K5+p63− basal cells, with significant increases in both OBt intermediate and small airways (Figures 4D and 4E) and more subtle changes in less severe LBt airways. Given that K5+p63+ basal cells have been shown to be multipotent SCs for small and large airways (14, 15, 20, 31), their progressive loss in this model suggests that the development of OB is accompanied by airway SC depletion. The phenomenon was observed globally throughout all levels of the conducting airways and was accompanied by a concomitant increase in the proportion of K14+ basal cell phenotypes.
We next evaluated how closely basal cell phenotypic changes in ferret allograft airways mirrored those of human allografts with OB. Importantly, the percentage of basal cells with a K5+p63+K14− was strikingly similar (∼40%) in both large and small airways of both human control and ferret native lobes (Figures 5A–5D). Human OB large and small airways also demonstrated a nearly complete absence of K5+p63+K14− basal cells (Figures 5B, 5D, and 5E–5H), as observed in ferret OB allografts (Figures 4, 5A, and 5C). As also seen in ferret, there was a significant (P < 0.001) increase in K14+K5−p63− and K14+K5+p63− basal cells of the large human OB airways (Figures 5G and 5H). In contrast to ferret OB allografts, K14+K5−p63− and K14+K5+p63− basal cells did not significantly increase in the distal airways (Figure 5H), because the stage of human OB evaluated had nearly complete loss of all basal cell markers and fibrosis of the distal airway lumens (Figure 5F).
Figure 5.
Changes in the expression of basal cell markers in large and small airways of control lungs and allograft human lungs with obliterative bronchiolitis (OB). (A–D) Proportion of total basal cells expressing one or more phenotypic markers in healthy lungs or pulmonary allografts with OB (OBt) in large airways of ferret (A) and human (B) or small airways of ferret (C) and human (D). Immunofluorescence images for K5, p63, and K14 expression in human large (E) and small (F) airways of control and OBt lungs. (G and H) Quantification of basal cell phenotype changes in large (G) and small (H) airways. ***P < 0.0001; ****P < 0.00001 by two-way analysis of variance and Tukey multiple comparison post hoc test. Data shown are mean ± SEM for seven patients for control lobes and three patients for OBt lobes. ns = not significant.
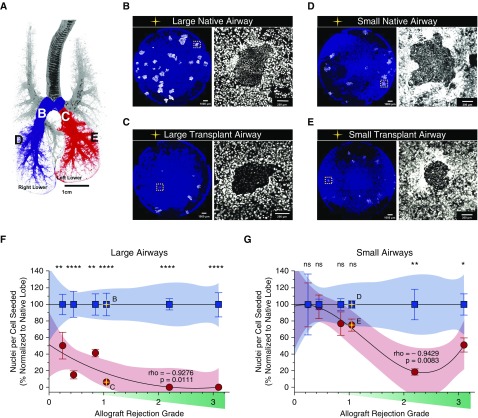
Clonogenic Potential of Basal SCs Is Compromised Early in Allograft Airways
The ability of single airway basal cells to form clones on fibroblast feeders and the number of cells per clone is an indication of the abundance and proliferative capacity of basal SCs in the airway, respectively. Given that SMGs and K5+p63+K14− basal SCs are depleted in OB allografts, we sought to formally demonstrate that clonogenic SCs are depleted from OB airways. As an index of the regenerative capacity of airway SCs, we used a colony-forming efficiency (CFE) assay to compare the total number of basal cells generated between native and allograft airway epithelia from large airways that contain SMGs and small airways devoid of SMGs (Figure 6A). As expected, native large airways contained more clonogenic SCs than distal native airways (Figures 6B and 6D). As hypothesized, the number of clonogenic SCs (i.e., clone number) generated from large and small airway allografts was reduced as compared with native airways (Figures 6B–6E). Importantly, large and small allograft airway SCs had a significantly reduced proliferative capacity as compared with the native lobe (Figures 6F and 6G). Declining proliferative capacity of both large and small airway SCs correlated significantly with allograft rejection grade by International Society for Heart and Lung Transplantation criteria (Spearman rho = −0.9276, P = 0.0111 and rho = −0.9428, P = 0.0083, respectively). However, the decline was more prominent in large airway SCs than in small airway SCs (nonlinear cubic regression models). Furthermore, even in animals that developed no histological signs of pulmonary allograft airway rejection, there was a significant decline in proliferative capacity of large airway SCs compared with native lobes, whereas a difference in small airway SC expansion was only evident in OBt animals and not in NRt or LBt animals (Student t test, P < 0.05) (Figures 6F and 6G).
Figure 6.
Proliferative capacity of ferret allograft progenitor cells versus native lobe controls in the large and small airways. (A) Diagrammatic representation of the location of progenitor cell harvesting for use in the colony forming efficiency assay in the large (white letters) and small (black letters) airways. (B–E) Immunofluorescence images of representative wells in the large native (B), large transplant (C), small native (D), and small transplant (E) airways of the animal with an allograft rejection grade of 1.05 (yellow star). The irradiated NIH-3T3 J2 feeder fibroblasts are blue, with colonies in white. Higher-magnification images of a representative colony are displayed to the right of each low-power image. (F and G) Correlation of nuclei per cell seeded with increasing allograft rejection grade (green wedge) of native (blue) and transplant (red) airways in large (F) and small (G) airways. Nuclei per cell seeded is graphed as the mean of three wells ± SEM of individual animals. Allograft rejection grade corresponds to the sum of the allograft histological scores for acute rejection, airway inflammation, chronic airway rejection, and chronic vascular rejection (Table E2). Proliferative capacity is represented as the percent nuclei per cell seeded, normalized to the average across all native control lobes (n = 6). Significance testing between native and allograft airways was determined by two-tailed Student t test (ns = not significant; *P < 0.01; **P < 0.001; ****P < 0.00001). Spearman correlation was used to describe the decline in the proliferative capacity of progenitor cells in large (F; rho = −0.9276; P = 0.0111) and small (G; rho = −0.9429; P = 0.0083) airways. Nonlinear (cubic) regression was used to model the declining trend in allograft (red) progenitor cell proliferative capacity versus native (blue) airways. Shaded regions represent the 95% confidence interval of the regression models.
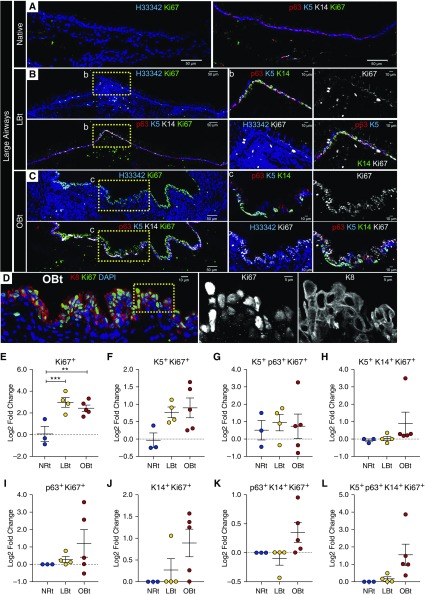
In addition, we sought to evaluate if there was a concordant decrease in basal SC proliferation in vivo. Interestingly, in large airways, localization of a proliferation marker (Ki67) increased with greater OB severity (Figures 7A–7C), and this was most evident in suprabasal cells that expressed Krt8 (K8) (Figure 7D). Compared with NRt samples, LBt (P < 0.0002) and OBt (P = 0.0014) airways had a significantly greater increase in Ki67+ cells that were negative for K5, p63, and K14 relative to native lobes (Figure 7E). However, the fold change in other phenotypic subsets of proliferating cells did not reach significance (Figures 7F–7L). By contrast, apoptosis did not appear to increase in the SAE of large or small airways (Figure E3), although apoptotic cells were observed in the lumen and interstitium of the airways consistent with an increase in CASP3 mRNA expression with OB severity (Figure E2). In addition, we observed a decline in CCSP+ club cells in distal allograft airways (Figure E4). These findings demonstrate that basal cell phenotypes that increase in OB airways (e.g., K14+K5−p63− and K14+K5+p63− cells) retain the proliferative capacity in vivo but lose clonogenic potential for expansion in vitro. Such findings are consistent with allograft airways progressively losing self-renewing basal SCs while expanding transient amplifying basal cells with limited potential for self-renewal. In SMGs, the phenotypic markers p63, K14, and K5 generally mirror the phenotypic changes we see of surface airway basal cells, especially increasing K14 staining in LBt allografts as compared with native lobes and NRt allografts (Figure E5).
Figure 7.
Proliferation in large airways of ferret pulmonary allografts. (A–C) Shown are representative immunofluorescent images of ferret airways stained for Ki67 in addition to basal cell markers, K5, p63, K14, and nuclear Hoechst 33342 (H33342) contrasting large airways from a native lobe (A), an allograft with lymphocytic bronchiolitis (LBt) (B), and an allograft with obliterative bronchiolitis (OBt) (C). Lower-magnification panels indicate Ki67 (green) staining with H33342 (blue) alone or Ki67 (green) costaining with p63 (red), K5 (blue), and K14 (white). Higher-magnification images of the boxed regions indicated in B and C are displayed to the right and include triple-channel p63 (red), K5 (blue), and K14 (green) images, single-channel Ki67 (white) images, double-channel H33342 (blue) and Ki67 (white) images, and four-channel p63 (red), K5 (blue), K14 (green), and Ki67 (white) images. Scale bars indicate 50 μm in lower-magnification images and 10 μm in higher-magnification images. (D) Representative OBt airway with costaining of Ki67, K8, and nuclear Hoechst 33342 (H33342). To the right of D are higher-magnification, single-channel images of Ki67 and K8 staining from the indicated boxed region, and the scale bars indicate 10 μm in the lower-magnification image and 5 μm in the higher-magnification images. (E–L) Quantification of Ki67+ cell phenotypes lacking basal cell markers (E), or coexpressing K5 (F), K5 and p63 (G), K5 and K14 (H), p63 alone (I), K14 alone (J), p63 and K14 (K), or all three markers (L). Statistical analysis was performed by two-way analysis of variance and Tukey multiple comparison post hoc test: **P < 0.01; ***P < 0.001. Data points indicate the comparative log2 fold change of individual animal allografts to native lobes, with black lines indicating mean = SEM. NRt = transplant lobes with no allograft airway rejection.
Discussion
Processes specific to small airways are central to the current paradigm of OB pathogenesis. Although our findings support SC depletion in distal allograft airways, they also challenge the existing paradigm by suggesting that pathogenesis is more global than previously believed—affecting SC compartments throughout the transplanted lung. Indeed, the destruction of SMGs and the loss of K5+p63+ basal SCs in large and small airway epithelia associate with OB severity in both ferret and human allografts. Notably, progressive variation in the phenotypic profile of basal SCs and a loss of clonogenic potential in vitro correlated with OB severity in ferret allografts, supporting the notion that SC compartments throughout the conducting airways are exposed to a common injury.
Presumably, phenotypic changes to basal cells in the proximal SAE are related to the destruction of SMGs. Multiple lines of evidence suggest that within large airways, SMGs are a niche for SCs with the capacity to regenerate surface epithelia (18, 22–26, 32). Thus, SMG destruction could deplete a reserve of basal cell precursors for the SAE in the setting of increased inflammatory stress in the transplanted allograft. Previous findings in mouse, ferret, and pig models of cystic fibrosis (CF), as well as in human CF tissue, discovered that adaptation of surface airway SC niches occurs in the setting of SMG pathology because of altered expression of the neuropeptide calcitonin gene–related peptide (CGRP), which stimulates glandular secretions and serves as a mitogen for slowly cycling SMG SCs (25). In the setting of allograft SMG destruction, similar processes may lead to basal cell phenotypic changes in the SAE.
Although the etiology of SMG destruction remains unclear, it is plausible that denervation of the transplanted lung alters the SMG SC niche by preventing neuroendocrine feedback needed for niche maintenance (33). Our findings show that SMGs are altered after denervation; however, although denervation may contribute to the pathogenesis of OB, it alone was not sufficient to lead to the development of OB. Although there was not a significant decline in SMG abundance, denervation did lead to a significant decrease in tubuloacinar structures, suggesting that disruption of neuroendocrine signaling can lead to remodeling of the SMG composition, potentially through altered SMG secretory properties much like that observed in CF (25). Alternatively, given that SMGs are a hub for away innate immunity (34), they may be especially susceptible to immune-mediated attack in allografts. Gene expression analysis showed a decline in SMG-specific genes, further strengthening our conclusion that SMG destruction progresses with OB severity. The precise nature of their destruction merits further study. For example, it is currently unknown whether SMGs are targeted by the recipient’s acquired alloimmune response or are damaged by some innate immune reaction(s).
Previous reports have suggested that the number of Scgb1a1+ (CCSP) variant club progenitors declines in human patients who develop OB (6, 7, 35), and we also observe this in ferret OB lung samples (Figures E2C and E4). However, this is the first report of a decline in K5+p63+ basal SC populations in the context of OB. Although K5+p63+ basal cells are less abundant in the distal airways of mice (12), our data are consistent with other studies in humans (16) and demonstrate that ferrets and humans share a highly similar distribution of basal cells in large and small airways. In addition, like humans, ferrets possess abundant SMGs throughout the cartilaginous airways (16, 28, 36, 37), whereas mice possess SMGs only in the proximal trachea (36, 38). These similarities in SC biology may account for the similarity between ferret and human OB development after lung transplantation.
Subsets of basal SCs with distinctive phenotypic expression of K5, p63, and/or K14 may have diverging capacities to proliferate and differentiate. Interestingly, several recent studies have shown that a subset of undifferentiated parenchymal cells within the mouse and human lung acquire p63 and K5 expression after severe injury and can regenerate alveolar epithelia without fibrosis (14, 39, 40). A shift from K5−p63− at steady state to K5+p63+ after injury suggests that these phenotypic markers are functionally important to epithelial regeneration pathways. It is important to note, however, that these observations were made in the context of severe parenchymal damage after influenza infection and in idiopathic pulmonary fibrosis, whereas in OB the parenchyma is largely spared. The observation of a decrease in p63+ basal cells and an increase in K14+ basal cells suggests that there is a shift in the predominant BC phenotype(s) in vivo. This in vivo shift corresponded to a decline in the ability to culture highly proliferative clonogenic basal cells. One potential pitfall of our data is that we did not test the phenotype of the cells that were plated in the CFE assays (Figure 6); however, in vivo immunofluorescence and histological staining suggest that most of the proliferating SAE cells in the animals with OB were Krt8 positive (Figure 7D).
The phenotypic and functional changes in basal cells during OB pathogenesis are apparent at the population level, and it is unclear if the decline in clonogenicity is a result of a decrease in a specific BC subtype that is capable of self-renewing or if there is a more generalized decline in the capacity to self-renew shared among the remaining BC phenotypes. However, given the phenotypic changes that correlate with the severity of OB and epithelial cell loss in the small airways, we posit that K14+K5−p63− and K5+K14+p63− basal cells, which are present in advanced stages of the disease, are limited in their capacity to self-renew as compared with K5+p63+K14− basal cells. Support for this hypothesis includes evidence that p63 plays a critical role in maintaining progenitor cell proliferation and multipotency in the airway (20, 31, 41). This hypothesis is further supported by a significant reduction in the number and proliferative capacity of clonogenic SCs of the proximal and distal allograft airways (Figure 6). The existence of both multipotent and unipotent basal cell lineages was previously demonstrated in human (42) and mouse (20) airways using lineage tracing. Whether these previously discovered unipotent lineages represent K14+K5−p63− and/or K5+K14+p63− basal cell populations in the OB airway remains to be determined and would require the ability to lineage trace in the ferret OB model.
In general, changes in the phenotypic profile of basal cells were similar in large and small airways as allograft rejection severity increased. Why then are the histological changes that define OB limited to the small airways? One hypothesis that is consistent with our current data is that although the relative abundance of the different basal cell phenotypes is similar in large and small airways, small airways have fewer total basal SCs. Indeed, clonogenic basal cells in distal airways were less abundant than in proximal airways (Figure 6). Thus, smaller airways could be more rapidly depleted of self-renewing basal SCs in the setting of heightened regenerative stress during continuous immune-mediated injury. Alternatively, fibroinflammatory airway constriction may progress more severely in the small airways because they lack SMGs, which may act as a reserve SC niche in large airways. The notion that self-renewing airway SCs are depleted during the development of OB through exhaustive proliferation is supported by a decrease in the CFE of allograft basal cells (Figure 6) coupled with an increase in basal cell and suprabasal cell proliferation in vivo (Figure 7). These findings suggest that transient amplifying basal cells with limited capacity to self-renew and/or clonogenically expand in vitro repopulate the airways of the OB lung.
This study demonstrates for the first time that, as pulmonary allograft rejection progresses in lung allografts, both SMGs and basal SCs are progressively lost. Importantly, our findings regarding phenotypic changes in basal cells and SMGs of the large airway during early OB may have diagnostic value, given that large airways are easily accessible for biopsy.
Footnotes
Supported by NHLBI grants K08 HL114725 (K.R.P.), R24 HL123482 (J.F.E.), and R01 HL121105 (J.A.K.-T.); National Institute of Diabetes and Digestive and Kidney Diseases grants P30 DK054759 (J.F.E.) and R01 DK047967 (J.F.E.); the Cystic Fibrosis Foundation (K.R.P.); the Thoracic Surgery Foundation for Research and Education (K.R.P.); the American Society of Transplant Surgeons (A.M.S.); National Cancer Institute grant P30 CA086862; and Roy J. Carver Charitable Trust through the University of Iowa Central Microscopy Research Facilities.
Author Contributions: A.M.S.: acquisition, analysis, interpretation, writing, and revision; T.J.L.: acquisition, analysis, interpretation, writing, and revision; A.K.C.: acquisition and analysis; P.J.A.: acquisition and analysis; S.R.T.: analysis and interpretation; L.B.: acquisition, analysis, interpretation, and revision; M.I.: acquisition, analysis, and editing; J.A.K.-T.: acquisition, revision, and editing; M.E.: acquisition and editing; T.P.: acquisition, interpretation, and editing; D.K.M.: acquisition, analysis, and editing; J.F.E.: conception, analysis, interpretation, revision, and editing; and K.R.P.: conception, analysis, acquisition, interpretation, writing, revision, editing, and overall supervision.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1368OC on December 13, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., III Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 3.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–508. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 5.De Vleeschauwer S, Vanaudenaerde B, Vos R, Meers C, Wauters S, Dupont L, et al. The need for a new animal model for chronic rejection after lung transplantation. Transplant Proc. 2011;43:3476–3485. doi: 10.1016/j.transproceed.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Gilpin SE, Lung KC, Sato M, Singer LG, Keshavjee S, Waddell TK. Altered progenitor cell and cytokine profiles in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2012;31:222–228. doi: 10.1016/j.healun.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kelly FL, Kennedy VE, Jain R, Sindhwani NS, Finlen Copeland CA, Snyder LD, et al. Epithelial clara cell injury occurs in bronchiolitis obliterans syndrome after human lung transplantation. Am J Transplant. 2012;12:3076–3084. doi: 10.1111/j.1600-6143.2012.04201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui H, Olivier AK, Klesney-Tait JA, Brooks L, Tyler SR, Sun X, et al. Ferret lung transplant: an orthotopic model of obliterative bronchiolitis. Am J Transplant. 2013;13:467–473. doi: 10.1111/j.1600-6143.2012.04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Driskell RR, Engelhardt JF. Stem cells in the lung. Methods Enzymol. 2006;419:285–321. doi: 10.1016/S0076-6879(06)19012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:718–725. doi: 10.1513/pats.200605-117SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Matsumoto K, Stripp BR. Bronchiolar progenitor cells. Proc Am Thorac Soc. 2009;6:602–606. doi: 10.1513/pats.200907-078RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuo W, Zhang T, Wu DZ, Guan SP, Liew AA, Yamamoto Y, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wansleeben C, Barkauskas CE, Rock JR, Hogan BL. Stem cells of the adult lung: their development and role in homeostasis, regeneration, and disease. Wiley Interdiscip Rev Dev Biol. 2013;2:131–148. doi: 10.1002/wdev.58. [DOI] [PubMed] [Google Scholar]
- 17.Widdicombe JH, Wine JJ. Airway gland structure and function. Physiol Rev. 2015;95:1241–1319. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 18.Hegab AE, Ha VL, Gilbert JL, Zhang KX, Malkoski SP, Chon AT, et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;29:1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, et al. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45:403–410. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- 21.O’Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol. 2013;49:788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch TJ, Anderson PJ, Xie W, Crooke AK, Liu X, Tyler SR, et al. Wnt signaling regulates airway epithelial stem cells in adult murine submucosal glands. Stem Cells. 2016;34:2758–2771. doi: 10.1002/stem.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, et al. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Transl Med. 2012;1:719–724. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 25.Xie W, Fisher JT, Lynch TJ, Luo M, Evans TI, Neff TL, et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121:3144–3158. doi: 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelhardt JF. Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol. 2001;24:649–652. doi: 10.1165/ajrcmb.24.6.f206. [DOI] [PubMed] [Google Scholar]
- 27.Lynch TJ, Engelhardt JF. Progenitor cells in proximal airway epithelial development and regeneration. J Cell Biochem. 2014;115:1637–1645. doi: 10.1002/jcb.24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajighasemi-Ossareh M, Borthwell RM, Lachowicz-Scroggins M, Stevens JE, Finkbeiner WE, Widdicombe JH. Distribution and size of mucous glands in the ferret tracheobronchial tree. Anat Rec (Hoboken) 2013;296:1768–1774. doi: 10.1002/ar.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, et al. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest. 2012;122:2724–2730. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegab AE, Ha VL, Bisht B, Darmawan DO, Ooi AT, Zhang KX, et al. Aldehyde dehydrogenase activity enriches for proximal airway basal stem cells and promotes their proliferation. Stem Cells Dev. 2014;23:664–675. doi: 10.1089/scd.2013.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wine JJ. Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system. Auton Neurosci. 2007;133:35–54. doi: 10.1016/j.autneu.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 35.Bourdin A, Mifsud NA, Chanez B, McLean C, Chanez P, Snell G, et al. Donor clara cell secretory protein polymorphism is a risk factor for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2012;94:652–658. doi: 10.1097/TP.0b013e31825ffca6. [DOI] [PubMed] [Google Scholar]
- 36.Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 2001;198:207–221. doi: 10.1046/j.1469-7580.2001.19820207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widdicombe JH. Transgenic animals may help resolve a sticky situation in cystic fibrosis. J Clin Invest. 2010;120:3093–3096. doi: 10.1172/JCI44235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Innes BA, Dorin JR. Submucosal gland distribution in the mouse has a genetic determination localized on chromosome 9. Mamm Genome. 2001;12:124–128. doi: 10.1007/s003350010244. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi Y, Kim T, Brumwell AN, Driver IH, Wei Y, Tan V, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol. 2017;19:904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arason AJ, Jonsdottir HR, Halldorsson S, Benediktsdottir BE, Bergthorsson JT, Ingthorsson S, et al. deltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS One. 2014;9:e88683. doi: 10.1371/journal.pone.0088683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]