To the Editor:
Bronchopulmonary dysplasia (BPD), the most common form of chronic lung disease during childhood, leads to substantial morbidity in premature infants (1). Inflammation is a major antecedent risk factor for BPD, yet the molecular mechanisms that regulate the inflammatory cascade in the preterm lung are not well described (2). Further, biomarkers that accurately identify infants at high risk for BPD are also not well defined.
RAGE (receptor for advanced glycation end products) is a membrane-spanning receptor that mediates inflammatory signaling in multiple organs. In the lung, RAGE is predominantly expressed on alveolar epithelial cells, where it binds a variety of ligands, including AGEs (advanced glycation end products), resulting in activation of inflammatory signaling pathways (3). Along with its full-length form, RAGE also exists in soluble forms (sRAGE [soluble RAGE]) produced by alternate splicing (esRAGE [endogenous sRAGE]) or by proteolytic cleavage of the extracellular portion of RAGE (cleaved sRAGE). Soluble forms of RAGE possess a ligand-binding domain but lack transmembrane and cytoplasmic domains, which prevents them from activating intracellular signaling (4). Thus, sRAGE functions as a “decoy” to bind and sequester RAGE ligands, thereby attenuating inflammation.
Reduced levels of sRAGE are found in chronic pulmonary conditions such as chronic obstructive pulmonary disease (COPD) and neutrophilic asthma (5). Expression of sRAGE in the preterm lung and its relationship with BPD have not been well characterized. Therefore, we performed a study in which we quantified the levels of sRAGE in the lungs of intubated preterm infants and examined the association between these measurements and subsequent development of severe BPD. Some of the results of these studies have been previously published as an abstract (6).
Methods
Preterm infants born between the ages of 23 0/7 and 28 6/7 weeks were prospectively enrolled in the PROP (Prematurity and Respiratory Outcome Program) study at Vanderbilt University Medical Center from September 2011 to December 2014 (7). Infants who remained intubated at 1 week of age and had tracheal aspirate (TA) samples collected at that time were eligible for inclusion in this single-center study. Concentrations of esRAGE and total sRAGE in TA samples were measured using commercially available ELISA kits (B-Bridge International [esRAGE] and R&D Systems [sRAGE]) and normalized to the total protein content of each sample.
Results
Forty-nine eligible preterm infants had an archived week 1 TA sample of sufficient volume. One infant with a congenital airway anomaly and three infants with TA samples containing low protein content (<0.1 mg/ml) were excluded. Of the remaining 45 infants, 15 were diagnosed with severe BPD, defined as the need for mechanical ventilation or significant noninvasive positive pressure support (>2 L/min Vapotherm [Vapotherm] or continuous positive airway pressure with an FiO2 > 0.3) at 36 weeks postmenstrual age (PMA). Four infants who died before 36 weeks PMA were included in the severe-BPD group. Twenty-six premature infants without severe BPD or death comprised the control group. Table 1 displays the distribution of clinical variables for infants in the two groups.
Table 1.
Demographics and Clinical Characteristics
| Control (n = 26) | Severe Bronchopulmonary Dysplasia/Death (n = 19) | P Value | |
|---|---|---|---|
| Gestational age* | 26.1 (±1.2) | 25.2 (±1) | 0.01 |
| Birth weight* | 791 (±189) | 677 (±146) | 0.02 |
| Race, white | 20 (77%) | 11 (57%) | 0.21 |
| Sex, male | 13 (50%) | 8 (42%) | 0.76 |
| Antenatal steroids | 21 (81%) | 17 (89%) | 0.68 |
| Days on oxygen† | 50 (41–99) | 120 (86–155) | 0.01 |
| Days on ventilation† | 26 (11–35) | 43 (20–75) | 0.02 |
| Length of stay† | 98 (80–121) | 126 (96–176) | 0.05 |
Mean (±SD).
Median (25–75th percentile).
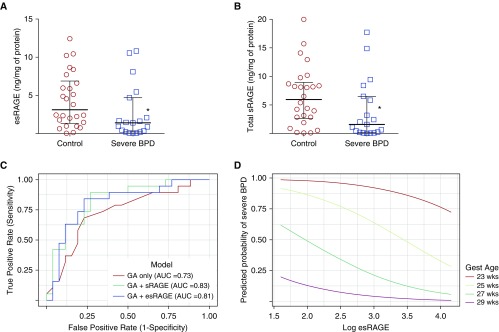
Levels of esRAGE and total sRAGE were lower in the TAs of infants with severe BPD compared with controls (Figures 1A and 1B), irrespective of whether the four infants who died before 36 weeks PMA were included in the analysis. In both groups, esRAGE accounted for the majority of measured sRAGE, and levels of esRAGE and total sRAGE were highly correlated (r = 0.7, P < 0.001). Levels of esRAGE and total sRAGE did not correlate with gestational age (GA) (r = −0.03, P = 0.83; and r = 0.09, P = 0.57, respectively).
Figure 1.
(A and B) Measured levels of esRAGE (endogenous sRAGE [soluble receptor for advanced glycation end products]) and total sRAGE (normalized to total protein) in tracheal aspirates (TAs) from premature infants at approximately 1 week of age. Lines indicate the median with upper and lower quartiles. Absolute values of esRAGE and total sRAGE were significantly lower in TAs from infants who developed bronchopulmonary dysplasia (BPD) or died compared with control infants (not shown). (C) Receiver-operating characteristic curves of logistic regression models using gestational age (GA), GA + esRAGE, and GA + total sRAGE to predict the outcome of severe BPD/death. (D) Predicted probability of severe BPD/death by log esRAGE and GA. Each colored curve represents the results of logistic regression modeling that depicts the probability of the outcome of severe BPD/death as a continuum at the specified GA. *P < 0.05 versus control. AUC = area under the receiver operating characteristic curve; Gest = gestational.
As GA is a known predictor of BPD/death, we estimated the association of esRAGE or total sRAGE with BPD/death when controlling for GA using separate logistic regression models. A twofold increase in esRAGE or total sRAGE was associated with decreased adjusted odds of severe BPD/death (odds ratio [OR], 0.61; 95% confidence interval [CI], 0.44–0.84; and OR, 0.60; 95% CI, 0.42–0.86, respectively). Likelihood ratio tests were used to determine whether a model using GA and esRAGE (or total sRAGE) was better than GA alone for predicting severe BPD/death. We found that the area under the curve (AUC) was significantly greater for the GA + esRAGE model than for the GA-alone model (0.81 vs. 0.73, P = 0.03). The AUC for GA + total sRAGE was also significantly higher than that for the model with GA alone (0.83 vs. 0.73, P = 0.01; Figure 1C). The predicted probability of severe BPD/death based on esRAGE levels and GA is shown in Figure 1D.
To determine whether the expression of esRAGE and total sRAGE in TA samples was dependent on the severity of lung disease, we calculated the respiratory severity score (RSS = mean airway pressure × FiO2) for each infant on the day of sample collection and compared it with the measured sRAGE levels. We found no correlation between the RSS and TA esRAGE or total sRAGE levels (esRAGE and RSS, r = −0.1, P = 0.49; total sRAGE and RSS, r = −0.2, P = 0.11).
Discussion
Our findings indicate that esRAGE and total sRAGE levels are reduced in the airways of preterm infants at risk for developing BPD. Further, lower esRAGE (and total sRAGE) levels were an independent predictor for severe BPD in our study. These findings are consistent with accumulating data showing that the RAGE/sRAGE axis is important in the pathogenesis of pulmonary diseases. Total sRAGE is reduced in the lungs of individuals with COPD and idiopathic pulmonary fibrosis, and membrane-bound RAGE and its ligands AGE and HMGB1 are increased in the lungs of patients with COPD (5, 8, 9). Similarly, prior studies have reported that HMGB1 is increased in the TAs of preterm infants at risk for BPD (10), and that plasma total sRAGE levels may negatively correlate with FiO2 need in preterm infants in the first week of life (11). Because soluble forms of RAGE act as decoy receptors, these studies suggest a common theme in which increased RAGE activation plays a role in both neonatal and adult lung diseases.
We found that esRAGE accounts for the majority of total sRAGE in preterm infant TA samples (both control and BPD). This is in contrast to adult conditions such as acute lung injury, where cleaved sRAGE appears to be the predominant soluble form (12). In this setting, cleaved sRAGE, likely produced by activity of proteases, may represent an acute inflammatory response to tissue injury. In comparison, in chronic lung diseases such as BPD, concentrations of total sRAGE are reduced because of downregulation of the esRAGE isoform. Thus, expression of individual sRAGE forms may vary substantially depending on the specific disease state.
Because TA samples could only be collected from intubated infants, only patients who were on ventilator support at the time of sample collection were included in this study, potentially limiting the applicability of our findings. In addition, although we accounted for GA in logistic regression models, our study had limited statistical power to examine other clinical variables that could affect the risk for BPD, including the cumulative oxygen dose and duration of mechanical ventilation. In the future, larger studies will be needed to specifically address whether the strength of association between TA esRAGE and BPD varies by time of TA collection or correlates with additional pertinent clinical variables.
In summary, we found that preterm infants with severe BPD had reduced airway levels of esRAGE (and total sRAGE) in the first week of life. TA esRAGE and total sRAGE may be useful biomarkers for early identification of infants at risk for severe BPD. Further studies are required to determine how the expression of different forms of sRAGE changes over time in the preterm lung. In addition, a promising area of inquiry is the possibility that sRAGE levels can be pharmacologically augmented to reduce lung inflammation and prevent BPD.
Acknowledgments
Acknowledgment
The authors thank Amanda Stinnett for technical assistance, Theresa Rogers and Amy Beller for assistance in collecting tracheal aspirate samples, and Alexander Agthe for critically reviewing the manuscript.
Footnotes
Supported by Prematurity and Respiratory Outcomes Program/NIH grant U01-HL101456 (J.L.A.), Prematurity and Respiratory Outcomes Program/NIH scholars grant U01-HL101794 (J.T.B.), and NIH grants K12 HD087023 (Research Scholar, J.T.B.), K08 HL133484 (J.T.B.), and HL119503 (L.R.Y.). Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University (Vanderbilt Institute for Clinical and Translational Research; supported by grant UL1TR000445 from the National Center for Advancing Translational Sciences/NIH).
Author Contributions: J.T.B., T.S.B., and L.R.Y. conceived the study; J.T.B., J.L.A., P.E.M., T.S.B., and L.R.Y. planned the work; R.v.d.M. and J.T.B. performed the laboratory work; J.T.B., J.C.S., E.J.P., and J.M.S. contributed to analysis and interpretation of the data; S.S. coordinated the collection of tracheal aspirate samples; J.T.B., P.E.M., J.L.A., T.S.B., and L.R.Y. drafted the manuscript. All authors approved the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201707-1445LE on October 16, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008;34:174–190. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 3.Yonchuk JG, Silverman EK, Bowler RP, Agustí A, Lomas DA, Miller BE, et al. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med. 2015;192:785–792. doi: 10.1164/rccm.201501-0137PP. [DOI] [PubMed] [Google Scholar]
- 4.Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukkar MB, Wood LG, Tooze M, Simpson JL, McDonald VM, Gibson PG, et al. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur Respir J. 2012;39:721–729. doi: 10.1183/09031936.00022011. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin JT, Meer RV, Steele S, Agthe A, Moore PE, Aschner JL, et al. Tracheal aspirate esRAGE is reduced in preterm infants at risk for severe BPD; Presented at the Pediatric Academic Society Annual Meeting; May 6–9, 2017, San Francisco, CA. [Google Scholar]
- 7.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and Respiratory Outcomes Program Investigators. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manichaikul A, Sun L, Borczuk AC, Onengut-Gumuscu S, Farber EA, Mathai SK, et al. Plasma soluble receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2017;14:628–635. doi: 10.1513/AnnalsATS.201606-485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med. 2011;105:329–336. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Aghai ZH, Saslow JG, Meniru C, Porter C, Eydelman R, Bhat V, et al. High-mobility group box-1 protein in tracheal aspirates from premature infants: relationship with bronchopulmonary dysplasia and steroid therapy. J Perinatol. 2010;30:610–615. doi: 10.1038/jp.2010.16. [DOI] [PubMed] [Google Scholar]
- 11.Rogers LK, Graf AE, Bhatia A, Leonhart KL, Oza-Frank R. Associations between maternal and infant morbidities and sRAGE within the first week of life in extremely preterm infants. PLoS One. 2013;8:e82537. doi: 10.1371/journal.pone.0082537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabaudon M, Blondonnet R, Roszyk L, Pereira B, Guérin R, Perbet S, et al. Soluble forms and ligands of the receptor for advanced glycation end-products in patients with acute respiratory distress syndrome: an observational prospective study. PLoS One. 2015;10:e0135857. doi: 10.1371/journal.pone.0135857. [DOI] [PMC free article] [PubMed] [Google Scholar]