Abstract
Rationale: A major barrier to a more complete understanding of acute respiratory distress syndrome (ARDS) pathophysiology is the inability to sample the distal airspace of patients with ARDS. The heat moisture exchanger (HME) filter is an inline bacteriostatic sponge that collects exhaled moisture from the lungs of mechanically ventilated patients.
Objectives: To test the hypothesis that HME filter fluid (HMEF) represents the distal airspace fluid in patients with ARDS.
Methods: Samples of HMEF were collected from 37 patients with acute pulmonary edema (either from ARDS or hydrostatic causes [HYDRO; control subjects]). Concurrent undiluted pulmonary edema fluid (EF) and HMEF were collected from six patients. HMEF from 11 patients (8 ARDS and 3 HYDRO) were analyzed by liquid chromatography–coupled tandem mass spectometry. Total protein (bicinchoninic acid assay), MMP-9 (matrix metalloproteinase-9), and MPO (myeloperoxidase) (ELISA) were measured in 29 subjects with ARDS and 5 subjects with HYDRO. SP-D (surfactant protein-D), RAGE (receptor for advanced glycation end-products) (ELISA), and cytokines (IL-1β, IL-6, IL-8, and tumor necrosis factor-α) (electrochemiluminescent assays) were measured in six concurrent HMEF and EF samples.
Measurements and Main Results: Liquid chromatography–coupled tandem mass spectrometry on concurrent EF and HMEF samples from four patients revealed similar base peak intensities and m/z values indicating similar protein composition. There were 21 significantly elevated proteins in HMEF from patients with ARDS versus HYDRO. Eight proteins measured in concurrent EF and HMEF from six patients were highly correlated. In HMEF, total protein and MMP-9 were significantly higher in ARDS than in HYDRO.
Conclusions: These data suggest that HMEF is a novel, noninvasive method to accurately sample the distal airspace in patients with ARDS.
Keywords: acute respiratory distress syndrome; airspace fluid; biomarkers; heat moisture exchanger filter; pulmonary edema fluid
At a Glance Commentary
Scientific Knowledge on the Subject
A detailed understanding of the natural history, pathophysiology, and prognosis of acute respiratory distress syndrome (ARDS) in humans is limited by the inability to easily sample the distal airspace.
What This Study Adds to the Field
Fluid that is collected from heat moisture exchanger filters that are used by mechanically ventilated patients with ARDS accurately represents fluid in the distal airspace. Heat moisture exchanger fluid represents a novel, simple, noninvasive method to repeatedly sample the distal airspace in patients with ARDS and is a powerful tool to facilitate mechanistic understanding of this devastating disease.
Acute respiratory distress syndrome (ARDS) continues to be a major challenge in critical care medicine with high short- and long-term mortality rates (1, 2). There are currently no targeted pharmacologic therapies to treat this syndrome, and treatment is focused on supportive care, protective mechanical ventilation (3), and conservative fluid management (4). A major limitation impeding development of new therapies for ARDS is the inability to easily, safely, and reliably sample the injured distal airspace. Each of the currently available methods for studying the airspace of patients with ARDS has significant drawbacks. BAL fluid (5) can be difficult to obtain, is not practical for serial collections, and introduces an unknown dilution factor to measured analytes. The direct aspiration of undiluted pulmonary edema fluid (EF) is possible (6, 7), but is only feasible early in the disease course and only in patients with the most severe pulmonary edema. Exhaled breath condensate may be useful for characterizing the airspace (8), but current collection equipment is costly, sample volume is limited, and collection is labor-intensive. Identification of a simple, noninvasive way to accurately sample the distal airspace in patients with ARDS would be a major advance for studying the biology of the injured alveolus.
The heat moisture exchanger (HME) filter is an inline, disposable, hygroscopic, bacteriostatic sponge routinely placed between the patient and the ventilator to minimize loss of heat and moisture from exhaled breath (Figure 1). During mechanical ventilation, moisture from the patient’s exhaled breath condenses on this filter until the filter is exchanged after several hours. Various HME filter devices are commercially available, with different filter designs and dead space volumes. HME filters are produced for neonatal and pediatric use as well. There is no universal standard of care for the routine changing of HME filters. Previous research has demonstrated the safety of leaving HME filters in place in the ventilator circuit for 48 hours (9). However, the American Association for Respiratory Care does recommend changing visibly soiled HME filters (10). The standard of care for the routine changing of HME filters and practices may vary by institution.
Figure 1.
Depiction of a heat moisture exchanger (HME) filter placement inline in the ventilator circuit between the patient and mechanical ventilator.
Once the filter is removed, condensed fluid can be collected from the filter and analyzed. Previous studies have demonstrated a potential use for HME filter fluid (HMEF) in the detection of ventilator-associated pneumonia (11, 12), but studies of the protein composition of HMEF in ARDS are lacking. Because HMEF captures exhaled airspace contents, we hypothesized that HMEF represents distal airspace fluid in patients with acute pulmonary edema from either ARDS or hydrostatic causes (HYDRO). To test this hypothesis, we compared the protein composition of simultaneously collected undiluted pulmonary EF and HMEF from patients with acute pulmonary edema due to ARDS or hydrostatic causes by using liquid chromatography–coupled tandem mass spectrometry (LC-MS/MS) with confirmatory immunoassays of selected proteins. To test the biological and pathological relevance of HMEF, we measured three analytes that have been reported to be elevated in the airspace when ARDS is present: total protein, MPO (myeloperoxidase), and MMP-9 (matrix metalloproteinase-9). To test the similarity between HMEF and EF, we performed high-resolution LC-MS/MS on concurrent matched HMEF and EF samples and individual protein and cytokine quantification on time-matched HMEF and EF samples. Finally, to explore the utility of HMEF as a platform for discovery, we compared HMEF mass spectroscopy (MS) between ARDS and HYDRO and analyzed differentially expressed proteins in the context of both biological pathways and protein–protein interaction networks.
Methods
Study Design
The Vanderbilt University Medical Center Institutional Review Board approved this minimal-risk study with a waiver of informed consent because HME filter exchanges are part of routine clinical care and used filters are typically discarded. Subjects were eligible if they were invasively mechanically ventilated and had bilateral radiographic infiltrates consistent with pulmonary edema. Subjects were then designated as having ARDS (13) or hydrostatic (cardiogenic, HYDRO) pulmonary edema or a mixed cause of pulmonary edema (mixed) as defined through a two-physician review (14, 15). HME filters (AirLife Adult HME filters, CareFusion) were collected after being in position for up to 12 hours. For patients in the adult ICUs at the Vanderbilt University Medical Center, HME filters are replaced every 12 hours or more frequently if they become soiled or saturated. When clinically available, a sample of directly aspirated, undiluted pulmonary EF was collected (16) concurrently with HME filter collection from two patients with ARDS, one patient with HYDRO, and three with mixed pulmonary edema. EF differs from BAL fluid (BALF) in that it is not diluted with saline for collection and is more representative of the contents of the distal airspace. Patient data were prospectively collected from the medical record and included demographics, ARDS risk factor, ventilator settings at the time of HME filter collection, hospital and ICU length of stay, and hospital mortality. Murray’s lung injury score (17) and the APACHE II (Acute Physiology and Chronic Health Evaluation II) score (18) were calculated.
Sample Preparation and Storage
Filters were transported to the laboratory on ice and centrifuged at 2,000 × g for 10 minutes to collect condensed fluid. HMEF was then aliquoted and stored at −80°C for further analysis. Undiluted pulmonary EF was centrifuged at 2,000 × g for 10 minutes. Supernatants were aliquoted and stored at −80°C.
Mass Spectrometry
LC tandem MS/MS
To compare the protein composition of HMEF from eight patients with ARDS with the protein composition of HMEF from three patients with HYDRO, we used LC-MS/MS. We first measured total protein concentration in each sample by bicinchoninic acid (BCA) assay. Then an equal volume of fluid from each sample underwent trichloroacetic acid (TCA) precipitation by the addition of 25% final volume TCA. Pellets were then washed twice with acetone and, after reduction of disulfide bonds using BondBreaker (Pierce) and alkylation with iodoacetamide, subjected to a 2M urea-denaturing trypsin digestion. After digestion, the amount of protein in the pellet was approximated on the basis of the previously measured total protein of the HMEF sample. MS data were collected on the resulting peptide mixture using a 90-minute LC separation coupled directly to a ThermoFisher LTQ tandem mass spectrometer operating in a data-dependent mode for selection and acquisition of tandem (MS/MS) spectra. This was performed on equal amounts of protein from the first filter fluid collected from 11 patients (8 ARDS and 3 HYDRO). Resulting MS/MS spectra were searched against the UniprotKb human reference database using SEQUEST, and the resulting identifications filtered and collated into proteins using Scaffold (Proteome Software).
High-resolution MS
To compare matched EF and HMEF samples from four patients, we used a 3-hour LC gradient coupled to data acquisition on a high-resolution QExactive (ThermoFisher) mass spectrometer. These were performed on 1 μg of protein precipitated from both EF and HMEF collected simultaneously from four patients. This both allowed for more experimental depth and opened up the potential to perform intensity-based comparisons at the full-scan MS amount. Spectra were visualized and compared using the BioConductor MSnBase package for R (19). Proteome-wide label-free quantification was performed using MaxQuant with follow-up data analysis and visualization using Perseus (20).
Total Protein and Individual Protein Quantification
Total protein, myeloperoxidase, and matrix metalloproteinase-9
All protein quantification was done directly on HMEF without any precipitation or protein extraction. Total protein concentration was measured by BCA assay (Bio-Rad). MPO (Abcam) and MMP-9 (R&D) were measured in duplicate by ELISA in HMEF from 29 patients with ARDS and 5 patients with HYDRO.
Individual protein quantification in concurrent HMEF and EF
MPO, MMP-9, SP-D (surfactant protein-D; Biovendor), and RAGE (receptor for advanced glycation end-products; R&D) were measured in duplicate by ELISA. Cytokines (IL-1β, IL-6, IL-8, and tumor necrosis factor(TNF)-α) were measured using electrochemiluminescent assays (Meso Scale Diagnostics). These measurements were made in concurrent samples of pulmonary EF and HMEF from six patients (two ARDS, one HYDRO, and three mixed).
Statistical Analysis
Mass spectroscopy
LC-MS/MS spectral counts were compared by Fisher’s exact test with a Benjamini-Hochberg correction for multiple comparisons using Scaffold (Proteome Software). A quasi-Poisson linear model was constructed for each detected protein to independently confirm significance.
Individual protein comparisons
Protein and cytokine concentrations were compared between ARDS and HYDRO by a Mann-Whitney U test using SPSS version 24 (IBM) for Mac.
Pathway and protein interaction analysis
Kegg pathway analysis was performed on significantly elevated proteins in ARDS HMEF (P < 0.05) using the DAVID online bioinformatics resource (21, 22). The string-db online tool was used to map individual proteins to their interaction networks (23). The minimum required interaction score was set to 0.9, with interactions determined by text mining, databases, experiments, co-expression, and co-occurrence. The interaction network was visualized using Cytoscape (24).
Results
Patient and Filter Characteristics
A total of 37 patients were enrolled. Table 1 shows patient characteristics of the 29 patients with ARDS and 5 patients with HYDRO (3 mixed patients were excluded). HMEF had a median volume of 3.0 ml for patients with ARDS and 2.0 ml for patients with HYDRO.
Table 1.
Characteristics of 34 Patients with ARDS or HYDRO
| Variable | ARDS (n = 29) | HYDRO (n = 5) |
|---|---|---|
| Patient characteristics | ||
| Age, yr | 48 (48–56) | 68 (50–68) |
| Male, % | 59 | 40 |
| Primary risk factor for ARDS, n | ||
| Pneumonia/aspiration | 19 | |
| Nonpulmonary sepsis | 8 | |
| Primary graft dysfunction | 1 | |
| Acute chest syndrome | 1 | |
| APACHE II | 22 (22–40) | 24 (22–40) |
| LIS | 2.8 (2.8–3.3) | 2.1 (1.8–2.4) |
| PaO2/FiO2 | 112 (112–148) | 240 (184–288) |
| ICU LOS | 9 (9–12) | 4 (3–4) |
| Hospital LOS | 14 (14–20) | 13 (6–13) |
| Mortality, n (%) | 8 (28) | 0 (0) |
| Filter characteristics | ||
| Collection time from intubation, h | 25 (25–50) | 8 (7–12) |
| Average fluid volume, ml | 3.0 (3.0–4.0) | 2.0 (1.5–3.6) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ARDS = acute respiratory distress syndrome; HYDRO = hydrostatic pulmonary edema; LIS = Murray Lung Injury Score; LOS = length of stay.
Data are shown as median (interquartile range) except where otherwise indicated.
HMEF Protein Composition Is Similar to the Protein Composition of Undiluted Pulmonary EF
To determine whether the protein content of HMEF is similar to that of undiluted EF collected directly from the distal airspaces, we compared pulmonary EF and HMEF collected simultaneously from four patients with pulmonary edema (one ARDS, one HYDRO, and two mixed) in an unbiased analysis. To determine whether the proteome of HMEF was similar to that of EF, we compared the location and intensity of ion base peaks in the spectral compositions from LC-MS/MS. The comparison of peaks between samples demonstrated a close similarity between matched HMEF and EF samples from each of the four individuals who had paired HMEF and EF samples (Figure 2). Plots comparing spectral intensities for each detected protein in HMEF and EF for individual patients were linear (r2 0.849–0.951), consistent with a very similar protein composition between HMEF and EF for each individual patient (Figure 3, white boxes). By contrast, comparison of HMEF and EF from different patients (Figure 3, gray boxes) shows deviation of points from the line of unity (r2 range = 0.121–0.835) suggesting a greater difference in identified peptides between patients than between matched samples from the same patient.
Figure 2.
Direct spectral comparison of matched heat moisture exchanger filter fluid (HMEF) and edema fluid (EF) samples. Visual comparison of matched pulmonary EF (upgoing peaks, blue) and HMEF (downgoing peaks, red) reveals similar base peak intensities and locations in a patient with acute pulmonary edema from acute respiratory distress syndrome (ARDS) (A) and one with hydrostatic pulmonary edema (HYDRO) (B). Bold peaks bearing a dot are reflected in spectra from both HMEF and EF samples. There were 118 common peaks in matched ARDS samples and 157 common peaks in matched HYDRO samples.
Figure 3.
Correlation of spectral intensities between matched heat moisture exchanger (HME) filter fluid and edema fluid samples. Label-free quantitation of spectral intensities from four patients with matched HME filter fluid and edema fluid collection reveals correlation of spectral intensities within each patient (white squares, r2 range = 0.849–0.951) and less correlation between patients (gray squares, r2 range = 0.121–0.835). Patient 4, acute respiratory distress syndrome; Patient 2, hydrostatic pulmonary edema; Patients 1 and 3, mixed.
To confirm that concentrations of a panel of specific proteins were similar between HMEF and EF in ARDS, we measured total protein and eight biomarkers of lung injury (IL-1β, IL-6, IL-8, MMP-9, MPO, RAGE, SP-D, and TNF-α) in HMEF and EF from six patients (two ARDS, one HYDRO, and three mixed) who had concurrent HMEF and EF samples collected. Concentrations of all nine analytes were similar between the EF and HMEF with a trend toward slightly higher concentrations of each analyte detected in EF (Figures 4A and 5). A Bland-Altman comparison was performed between HMEF and EF concentrations for total protein (Figure 4B) and for each of the remaining eight individual analytes. Figure E1 (see the online supplement) shows close agreement between measurements made on HMEF compared with EF. Together, these data demonstrate that, regardless of etiology of pulmonary edema (ARDS, HYDRO, or mixed), HMEF and EF are similar in terms of both overall protein composition and concentration of specific proteins.
Figure 4.
Total protein concentrations in matched edema fluid (EF) and heat moisture exchanger filter fluid (HMEF). (A) Total protein on matched EF (solid circles) and HMEF (open triangles) from six patients. Patients 1 and 3, acute respiratory distress syndrome; Patient 4, hydrostatic pulmonary edema; Patients 2, 5, and 6, mixed. (B) A Bland-Altman plot of agreement between HMEF and EF total protein (dashed lines represent upper and lower 95% confidence intervals). The diamonds show the difference between the measurements on each fluid for an individual patient.
Figure 5.
Individual protein quantification of eight analytes in matched heat moisture exchanger filter fluid (HMEF) and edema fluid (EF). Individual proteins were measured by ELISA and electrochemiluminescence in six patients with matched samples of HMEF and EF. Patients 1 and 3, acute respiratory distress syndrome; Patient 4, hydrostatic pulmonary edema; Patients 2, 5, and 6, mixed. MMP-9 = matrix metalloproteinase-9; MPO = myeloperoxidase; RAGE = receptor for advanced glycation end-products; SP-D = surfactant protein-D; TNF-α = tumor necrosis factor-α.
ARDS and HYDRO HMEF Have Distinct Protein Profiles
We compared the proteomic profile of HMEF in patients with ARDS and patients with HYDRO using LC-MS/MS. A total of 222 proteins (see Table E1 of the online supplement) were detected in the 11 HMEF samples examined by LC-MS/MS. Twenty-one proteins, including MPO and MMP-9, were significantly elevated in ARDS versus HYDRO (P < 0.008) when adjusted for multiple comparisons with a Benjamini-Hochberg (false discovery rate) procedure (Table 2). Because some proteins are expressed at very low concentrations in HMEF and thus could lead to a false positive result by Fisher’s exact test, we used a quasi-Poisson generalized linear model for each analyte as a more stringent test (Table 2). This analysis confirmed that 12 proteins were differentially expressed in HMEF between ARDS and HYDRO. In addition to the 21 proteins that were higher in ARDS, MS analysis identified 9 proteins that had a lower abundance in ARDS than in HYDRO (see Table E2 of the online supplement).
Table 2.
Proteins Elevated in ARDS HMEF Compared to HYDRO HMEF
| Elevated Proteins in ARDS | HMEF |
|
|---|---|---|
| Fisher's Exact | Quasi-Poisson | |
| S100A9 | <0.001 | <0.001 |
| S100A8 | <0.001 | <0.001 |
| Myeloperoxidase | <0.001 | <0.001 |
| Leukocyte elastase inhibitor | <0.001 | <0.001 |
| Matrix metalloproteinase-9 | <0.001 | <0.001 |
| Immunoglobulin M | <0.001 | <0.001 |
| Plastin-2 | <0.001 | <0.001 |
| Immunoglobulin A2C | <0.001 | <0.001 |
| α-Amylase 1 | <0.001 | <0.001 |
| Cathepsin | <0.001 | 0.023 |
| Myosin-9 | <0.001 | 0.026 |
| Immunoglobulin A1C | <0.001 | <0.001 |
| Gelsolin | 0.002 | 0.002 |
| α-Actinin 1 | 0.004 | 0.076 |
| Actin, cardiac | 0.004 | 0.002 |
| Glucose 6 phosphate isomerase | 0.005 | 0.16 |
| Leucine-rich α2 | 0.005 | 0.303 |
| Fibronectin | 0.005 | 0.042 |
| Filamin A | 0.005 | 0.16 |
| Actin, cytoplasmic 1 | 0.006 | |
| Brain acid-soluble protein 1 | 0.007 | 0.17 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; HMEF = heat moisture exchanger filter fluid; HYDRO = hydrostatic pulmonary edema.
Bold indicates significant P values by Benjamini-Hochberg; proteins are shown in order by Fisher’s exact P value.
HMEF Demonstrates Elevated Total Protein and Biomarker Concentrations in ARDS as Compared with Hydrostatic Pulmonary Edema
It has been well described that patients with ARDS have a higher total protein in undiluted pulmonary EF than those with HYDRO (25). The total protein concentration in HMEF was significantly elevated in ARDS compared with HYDRO (Figure 6). MMP-9 was upregulated in HMEF in patients with ARDS compared with HYDRO (Figure 7A). This replicates previous observations of upregulated MMP-9 in BALF and EF in patients with ARDS (26). In addition, elevated MPO amounts in the lung have been reported in lung tissue from patients with ARDS (27) and in a murine model of influenza-induced ARDS (28). We found a trend toward an increased MPO in ARDS versus HYDRO (Figure 7B). Together, these data suggest that HMEF analysis can replicate findings from BALF analysis of biomarkers underlying ARDS pathogenesis.
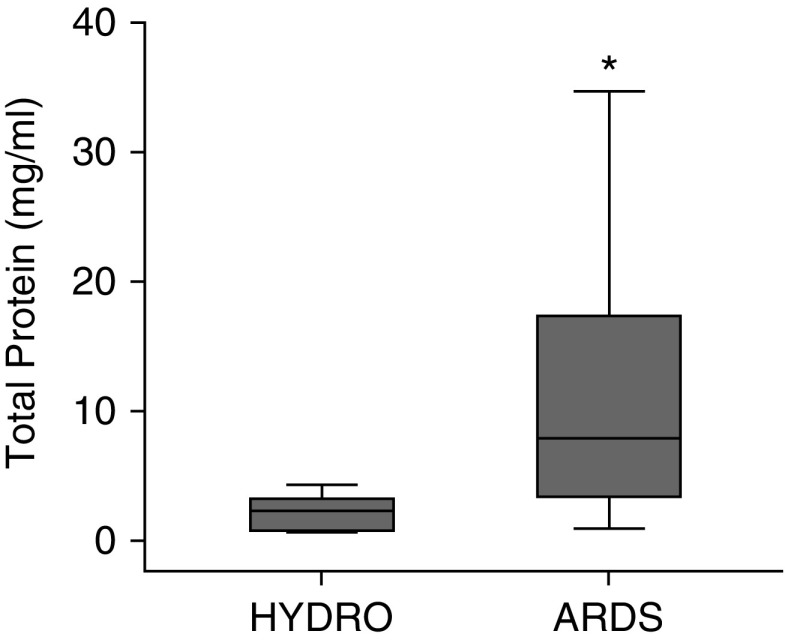
Figure 6.
Total protein comparison in heat moisture exchanger filter fluid between patients with acute respiratory distress syndrome (ARDS) and those with hydrostatic pulmonary edema (HYDRO). Total protein was higher in heat moisture exchanger filter fluid from patients with ARDS (n = 29) than in that from patients with HYDRO (n = 5). *P = 0.008. Box-and-whisker plots show median (line), interquartile range (IQR) (box ends), and 1.5 × IQR (whiskers).
Figure 7.
MMP-9 (matrix metalloproteinase-9) and MPO (myeloperoxidase) quantification. Protein quantification of (A) MMP-9 and (B) MPO in heat moisture exchanger filter fluid from 29 patients with acute respiratory distress syndrome (ARDS) and 5 patients with hydrostatic pulmonary edema (HYDRO). *P = 0.022; MPO, P = 0.179. Box-and-whisker plots show median (line), interquartile range (IQR) (box ends), and 1.5 × IQR (whiskers).
Analysis of HMEF May Reveal New Biomarkers in ARDS
Finally, having established that HMEF protein composition is similar to EF, we tested whether further analysis of LC-MS/MS data could reveal new insights into the pathogenesis of ARDS. Kegg pathway analysis of enriched proteins in ARDS compared with HYDRO revealed 10 significantly enriched pathways (see Figure E2 of the online supplement). These enriched pathways provide an assessment of protein pathways that are altered in the airspace during ARDS and may provide insight into the etiology and progression of the disease. Mapping individual proteins elevated in ARDS HMEF (P < 0.05 by Fisher’s exact test) to their protein–protein interaction networks revealed multiple notable interactions between detected proteins even when the minimum required interaction score was set to >0.9 (Figure 8). This analysis identified a clustering of cytoskeletal components as well as proteins involved in carbohydrate metabolism. These processes shared a mutual interaction with MMP-9, a protein proposed to play a pathogenic role in ARDS (27).
Figure 8.
Protein interactions in heat moisture exchanger filter fluid for patients with acute respiratory distress syndrome. High-confidence interactions between proteins elevated in heat moisture exchanger filter fluid demonstrate notable interactions between detected proteins. The green area highlights MMP-9, a previously described mediator of lung injury.
Discussion
The major finding from this study is that fluid collected from HME filters from mechanically ventilated patients with acute pulmonary edema due to ARDS or hydrostatic pulmonary edema accurately reflects distal airspace fluid. There was a strong correlation between both the proteomic profile as well as individual biomarker quantification of HMEF and undiluted pulmonary EF collected simultaneously from individual patients. This strong correlation was present regardless of the etiology of pulmonary edema. The proteome from HMEF from mechanically ventilated patients with ARDS was distinct from the proteome of patients with HYDRO. These findings help to establish HMEF as a noninvasive alternative to direct aspiration of pulmonary EF or BAL for sampling the distal airspace in ARDS.
Use of HMEF as a research tool overcomes many of the previous challenges to studying the distal airspace. In particular, HMEF collection is inexpensive, is noninvasive, is readily available, and can be collected serially over time. There are multiple potential applications for HMEF analysis. First, HMEF may be used to discover important, novel biologic mechanisms of lung injury. The proteomic analysis supports HMEF as a matrix for identification of potentially important biologic proteins and pathways. In addition, it is possible that nonprotein biomarkers such as DNA, RNA, lipids, carbohydrates, or other mediators are present in HMEF, although these were not specifically measured in our study. Second, HMEF may be useful in further defining and characterizing distinct ARDS endotypes that may have different underlying pathogenesis and different responses to therapies (29). For example, comparison of proteomic profiles between the eight patients with ARDS found that 15 proteins were upregulated in at least three patients with ARDS (see Table E3 of the online supplement). Detailed study of the protein composition of HMEF in a larger cohort may help to identify distinct phenotypes of ARDS based on biomarkers present in the airspace rather than in the plasma. As such, HMEF analysis could classify molecular phenotypes of ARDS (29) on a patient-to-patient basis, allowing for personalized care and targeted treatments as they become available. Third, serial HMEF analysis may help to define the natural history of ARDS by providing a window into the airspace during acute injury, progression, resolution, and repair. Finally, HMEF analysis could be an important adjunct to clinical trials by providing a way to test biologic effects of therapies in subjects enrolled in clinical trials.
Another potential application of HMEF is in the identification of lung pathogens in mechanically ventilated patients. Previous work has shown utility for HMEF in the detection of pulmonary microbial pathogens, with a significant correlation between pathogens identified by standard microbiological sampling using BALF compared with those identified in HMEF in a cohort of 14 mechanically ventilated adults from a surgical ICU (11). May and colleagues reported that bacterial DNA was detectable in HMEF in 48 mechanically ventilated adult patients undergoing diagnostic bronchoscopy for suspected ventilator-acquired pneumonia. They demonstrated that bacterial DNA in HMEF was highly concordant with bacterial DNA in matched BALF (concordance >95% by K-statistic) as well as with results of traditional microbiologic culture techniques confirming concordance of identified pathogens between HMEF and more traditional methods (12). These studies demonstrate that HMEF has high sensitivity and specificity for noninvasive identification of pulmonary pathogens implicated in ventilator-associated pneumonia and that HMEF may potentially replace BALF for the purpose of pathogen detection. The current study extends these published studies of HMEF in pneumonia diagnosis to protein analysis, which can likewise be done in a high-throughput, noninvasive manner.
Kegg Pathway analysis of proteins upregulated in HMEF from patients with ARDS versus HYDRO reveals a proteome with elevated proteins of coagulation, complement activation, actin rearrangement, and cell migration (Figure E2), demonstrating the usefulness of HMEF for mechanistic discovery in mechanically ventilated patients with ARDS. There were nine significantly downregulated proteins in ARDS, which showed no notable Kegg Pathway enrichments. Mapping upregulated proteins to known protein–protein interaction networks (Figure 8) showed clustering of proteins involved in carbohydrate metabolism as well as cytoskeletal/actin rearrangement. Bhargava and colleagues reported an association of proteins involved in pathways of coagulation, carbohydrate metabolism, complement activation, cell migration, and actin organization in BALF with outcomes of patients with ARDS (30); each of these findings was confirmed in our analysis of HMEF from patients with ARDS. Two of the most significantly elevated proteins in HMEF in ARDS were S100A8 and S100A9, two proteins known to be elevated in the BALF of patients with ARDS (31, 32). In addition to showing elevation of several proteins already associated with ARDS, our studies identified several novel proteins upregulated in the airspace during ARDS, including myosin-9, glucose 6 phosphate isomerase, and filamin A. Taken together, these findings suggest that HMEF shows great promise as a tool to study the pathophysiology of ARDS and potentially other pulmonary diseases.
This study has several strengths, including the unbiased nature of the MS analysis, the direct comparison of HMEF to simultaneously collected pulmonary EF as the gold standard of distal airway sampling, and the replication of previously published findings in ARDS. There are also some limitations to this study. First, the HME filters analyzed were in place for variable lengths of time because they were collected as part of routine clinical care. Variability in the duration of HME filter placement could impact analyte concentration. In HME filters with well-defined ventilator dwell times, there was statistically significant correlation between filter dwell time and the amount of fluid collected from the filter (Figure E3). Despite this, there remained a tight correlation between protein concentrations in EF and HMEF from the same patient with filter dwell times ranging from 1 hour to 12 hours, suggesting that the duration of HME filter placement has only a minor impact on the proteins detected. It is important to note, however, that the effect of dynamic changes in analyte concentrations was not specifically studied. Future studies on samples obtained after set intervals of dwell time along the trajectory of the disease course will be necessary to understand temporal changes in analytes.
Second, this pilot study has a relatively small sample size. Aspiration of undiluted EF is only possible in the minority of mechanically ventilated patients, thereby limiting the ability to directly compare concurrent EF and HMEF in a larger number of patients. In addition, increased use of noninvasive ventilation for hydrostatic pulmonary edema in the clinical setting limits the opportunity to collect HME filters from these patients. Although we were able to detect protein concentrations down to the pg/ml range, it is possible that HMEF is not ideal for analysis of low-abundance proteins. Future studies will test the lower limits of protein or other biomarker analysis in HMEF. Finally, our study only enrolled subjects with pulmonary edema; whether HMEF analysis is useful in other mechanically ventilated patients remains unknown.
Conclusions
In summary, HMEF is a readily available and novel method for serial, noninvasive sampling of fluid from the distal airspace of mechanically ventilated patients with pulmonary edema. The proteins in HMEF are strikingly similar to those in pulmonary EF collected directly from the distal airspace. Closer examination of elevated proteins within HMEF not only supports previous findings from studies in BALF and EF, but also may identify previously undiscovered mediators of ARDS. Because HMEF collection is a noninvasive, inexpensive, and simple method for sampling the distal airspace in mechanically ventilated patients with pulmonary edema, it could revolutionize our understanding of the pathogenesis of clinical ARDS.
Acknowledgments
Acknowledgment
The authors thank the Department of Respiratory Care at the Vanderbilt University Medical Center for assistance with HME filter collection.
Footnotes
Supported by NIH grants HL126671 (J.A.B.), HL103836 (L.B.W.), T32HL105346-07 (D.W.R.), and HL136888 (C.M.S), and UL1TR000445 from the National Center for Advancing Translational Sciences (J.B.M.).
Author Contributions: L.B.W. and J.A.B. conceived and designed the study; J.B.M., V.E.K., C.M.S., D.W.R., B.S.G., M.A.W., and N.E.W. collected, processed, and analyzed samples; W.H.M. performed proteomics measurements and analysis; J.B.M., C.M.S., L.B.W., and J.A.B. analyzed data and wrote the manuscript; all authors reviewed and approved the final submission.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201707-1474OC on December 18, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pierrakos C, Vincent JL. The changing pattern of acute respiratory distress syndrome over time: a comparison of two periods. Eur Respir J. 2012;40:589–595. doi: 10.1183/09031936.00130511. [DOI] [PubMed] [Google Scholar]
- 2.Wang CY, Calfee CS, Paul DW, Janz DR, May AK, Zhuo H, et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med. 2014;40:388–396. doi: 10.1007/s00134-013-3186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Wattiez R, Falmagne P. Proteomics of bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;815:169–178. doi: 10.1016/j.jchromb.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Bowler RP, Duda B, Chan ED, Enghild JJ, Ware LB, Matthay MA, et al. Proteomic analysis of pulmonary edema fluid and plasma in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1095–L1104. doi: 10.1152/ajplung.00304.2003. [DOI] [PubMed] [Google Scholar]
- 7.Fein A, Grossman RF, Jones JG, Overland E, Pitts L, Murray JF, et al. The value of edema fluid protein measurement in patients with pulmonary edema. Am J Med. 1979;67:32–38. doi: 10.1016/0002-9343(79)90066-4. [DOI] [PubMed] [Google Scholar]
- 8.Kurova VS, Anaev EC, Kononikhin AS, Fedorchenko KY, Popov IA, Kalupov TL, et al. Proteomics of exhaled breath: methodological nuances and pitfalls. Clin Chem Lab Med. 2009;47:706–712. doi: 10.1515/CCLM.2009.166. [DOI] [PubMed] [Google Scholar]
- 9.Djedaini K, Billiard M, Mier L, Le Bourdelles G, Brun P, Markowicz P, et al. Changing heat and moisture exchangers every 48 hours rather than 24 hours does not affect their efficacy and the incidence of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;152:1562–1569. doi: 10.1164/ajrccm.152.5.7582295. [DOI] [PubMed] [Google Scholar]
- 10.Restrepo RD, Walsh BK American Association for Respiratory Care. Humidification during invasive and noninvasive mechanical ventilation: 2012. Respir Care. 2012;57:782–788. doi: 10.4187/respcare.01766. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs RJ, Debelak K, Norris PR, Jenkins JM, Rooks JC, Young TR, et al. Non-invasive detection of pulmonary pathogens in ventilator-circuit filters by PCR. Am J Transl Res. 2012;4:72–82. [PMC free article] [PubMed] [Google Scholar]
- 12.May AK, Brady JS, Romano-Keeler J, Drake WP, Norris PR, Jenkins JM, et al. A pilot study of the noninvasive assessment of the lung microbiota as a potential tool for the early diagnosis of ventilator-associated pneumonia. Chest. 2015;147:1494–1502. doi: 10.1378/chest.14-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 14.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaver CM, Woods J, Clune JK, Grove BS, Wickersham NE, McNeil JB, et al. Circulating microparticle levels are reduced in patients with ARDS. Crit Care. 2017;21:120. doi: 10.1186/s13054-017-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 17.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 19.R Development Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2010. R: a language and environment for statistical computing. [Google Scholar]
- 20.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, et al. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2:2366–2382. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the aetiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J. 2010;35:331–337. doi: 10.1183/09031936.00098709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 27.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome: clinical and pathophysiologic significance. Am Rev Respir Dis. 1986;133:218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- 28.Sugamata R, Dobashi H, Nagao T, Yamamoto K, Nakajima N, Sato Y, et al. Contribution of neutrophil-derived myeloperoxidase in the early phase of fulminant acute respiratory distress syndrome induced by influenza virus infection. Microbiol Immunol. 2012;56:171–182. doi: 10.1111/j.1348-0421.2011.00424.x. [DOI] [PubMed] [Google Scholar]
- 29.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. Distinct molecular phenotypes of direct vs. indirect ARDS in single-center and multicenter studies. Chest. 2015;147:1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhargava M, Becker TL, Viken KJ, Jagtap PD, Dey S, Steinbach MS, et al. Proteomic profiles in acute respiratory distress syndrome differentiates survivors from non-survivors. PLoS One. 2014;9:e109713. doi: 10.1371/journal.pone.0109713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang DW, Hayashi S, Gharib SA, Vaisar T, King ST, Tsuchiya M, et al. Proteomic and computational analysis of bronchoalveolar proteins during the course of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008;178:701–709. doi: 10.1164/rccm.200712-1895OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Torre C, Ying SX, Munson PJ, Meduri GU, Suffredini AF. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics. 2006;6:3949–3957. doi: 10.1002/pmic.200500693. [DOI] [PubMed] [Google Scholar]