To the Editor:
We performed a randomized trial of continuous positive airway pressure (CPAP) versus laparoscopic gastric banding (LGB) for patients with severe obstructive sleep apnea (OSA), hypothesizing that residual disease (effective apnea–hypopnea index [AHI]) (1–3) and Epworth Sleepiness Scale (ESS) scores would be significantly lower with LGB compared with CPAP at 9 and 18 months. Approval was obtained from the institutional review boards at Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center. The protocol was registered at clinicaltrials.gov (NCT01187771). All participants gave written informed consent.
The inclusion criteria were age 18–65 years, body mass index (BMI) 35–45 kg/m2, severe OSA (AHI ≥ 30 events/h [level 1 study] or AHI ≥ 20 events/h [level 3 study]), and at least one OSA symptom. The exclusion criteria were prior CPAP or bariatric surgery, hypoventilation syndrome, increased perioperative risk, drowsy driving, non-English fluency, or any unstable medical condition. Suitability for both treatments, as well as the equipoise of each patient, was established by a sleep specialist and bariatrician before consent was obtained.
Patients underwent attended polysomnography using the Compumedics E-Series at baseline, 9 months, and 18 months.
Randomization was stratified by recruitment clinic, baseline BMI (35–40 or 40–45 kg/m2), and sex. Sequences had block sizes of two and four, with a 50% chance of choosing either one.
Initiation and management of OSA care once treatment was assigned were performed by the managing clinician as per usual care, except that patients undergoing LGB were provided auto-CPAP (REMstar Auto M Series; Philips Respironics) during the perioperative period to minimize OSA complications. The effective AHI was calculated as (x × AHIon-CPAP) + [(1 − x) × AHIoff-CPAP], where x is (CPAP adherence)/(habitual sleep duration) (3). Adherence and AHIon-CPAP were downloaded from the device and averaged across the previous 30 days. AHIoff-CPAP was calculated from polysomnography data obtained at 9 or 18 months.
We performed an a priori power analysis to determine the difference in effective AHI between arms, indicating a sample size of 80. With the support of our Data Safety Monitoring Board and approval from the NHLBI, we performed a second power calculation while the study was underway. We assumed a mean baseline AHI [mean ± SD] of 50 ± 10 events/h. In the CPAP group, we assumed a mean CPAP adherence of 4 h/night and usual sleep duration of 8 h/night, resulting in an effective AHI of 25 events/h. In the LGB group, based on a published meta-analysis, we anticipated a 31% reduction in AHI, resulting in an effective AHI of 35 events/h (4). Under these assumptions, we would have >90% power to detect a benefit of LGB with a sample size of 50.
We made between-arm comparisons using linear models with prespecified adjustments for randomization stratification factors. Within-arm comparisons were based on the difference between baseline and end-trial data, using one-sample t tests or signed-rank tests. Tests were considered statistically significant when P < 0.05 (two-sided).
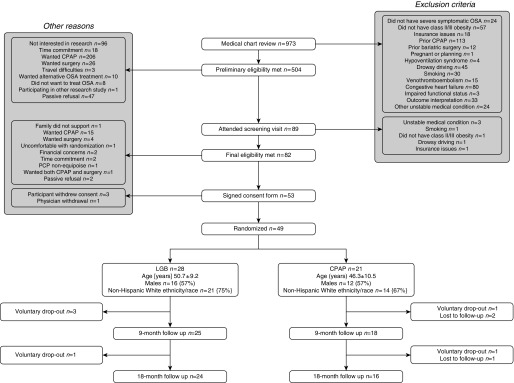
Figure 1 shows that 49 patients were randomized. In the LGB group, 14/28 underwent LGB only (50%), 10 underwent CPAP only (36%), and four underwent neither (14%). In the CPAP group, 20/21 received CPAP only (95%), and one began CPAP followed by gastric bypass surgery (5%).
Figure 1.
Consolidated Standards of Reporting Trials diagram. The numbers in the boxes represent sample size according to randomized arm. Age is presented as mean ± SD. CPAP = continuous positive airway pressure; LGB = laparoscopic gastric banding; OSA = obstructive sleep apnea; PCP = primary care provider.
LGB was associated with a greater drop in BMI (Table 1). In intention-to-treat analyses, the effective AHI at 9 months was 29.5 ± 23.4 and 20.0 ± 25.3 events/h in the LGB and CPAP groups, respectively (P = 0.02). At 18 months, the effective AHI was 20.9 ± 16.0 and 21.4 ± 17.6 events/h (P = 0.89). Although there were significant improvements in ESS scores with both treatments, there were no significant differences between arms. Self-reported sleep durations were 7.4 ± 1.4, 7.4 ± 1.3, and 7.3 ± 1.2 h/night at baseline, 9 months, and 18 months, respectively, in the surgery arm, and 8.5 ± 1.8, 8.0 ± 1.4, and 8.4 ± 1.5 h/night, respectively, in the CPAP arm.
Table 1.
Measures of Anthropometry and Sleep at Baseline, 9 Months, and 18 Months, according to Randomized Arm (Intention to Treat)
| |
|
Randomized LGB |
Randomized CPAP |
AdjustedP Values (between Arms) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 28) | 9 mo (n = 25) | 18 mo (n = 24) | Baseline (n = 21) | 9 mo (n = 18) | 18 mo (n = 16) | 9 mo | 18 mo | ||
| Anthropometry |
|||||||||
| BMI, kg/m2 |
39.1 ± 2.9 | 35.9 ± 3.5* | 35.7 ± 3.9* | 38.7 ± 3.1 | 37.4 ± 3.7 | 37.4 ± 4.5 | 0.01 | 0.09 | |
| Weight, kg |
115.4 ± 16.9 | 106.7 ± 18.0* | 106.1 ± 18.0* | 111.1 ± 16.1 | 106.0 ± 13.4 | 106.3 ± 16.0 | 0.45 | 0.60 | |
| Neck circumference, cm |
40.9 ± 4.3 | 40.1 ± 4.8 | 40.6 ± 3.8 | 42.6 ± 4.4 | 41.5 ± 4.1* | 42.3 ± 3.9 | 0.04 | 0.08 | |
| Waist circumference, cm |
123.5 ± 10.3 | 116.4 ± 13.2* | 115.0 ± 12.5* | 120.5 ± 8.1 | 115.7 ± 11.4* | 116.5 ± 10.2* | 0.70 | 0.52 | |
| Hip circumference, cm |
127.5 ± 8.6 | 120.8 ± 8.4* | 120.8 ± 8.9* | 124.1 ± 7.4 | 116.0 ± 16.9* | 120.7 ± 9.2 | 0.36 | 0.80 | |
| Sleep measures |
|||||||||
| AHI off CPAP treatment, events/h |
51.5 ± 23.5 | 39.3 ± 26.4* | 34.1 ± 24.6* | 47.5 ± 31.5 | 34.7 ± 31.6 | 36.4 ± 23.2 | 0.28 | 0.93 | |
| Effective AHI, events/h† |
N/A | 29.5 ± 23.4 | 20.9 ± 16.0 | N/A | 20.0 ± 25.3 | 21.4 ± 17.6 | 0.02 | 0.89 | |
| ESS, out of 24 |
10.4 ± 4.2 | 7.6 ± 4.7* | 8.0 ± 4.5* | 9.8 ± 5.0 | 7.8 ± 4.8 | 6.2 ± 3.7* | 0.53 | 0.19 | |
| Effective AHI <5 events/h, n (%) |
N/A | 1 (4%) | 3 (13%) | N/A | 4 (22%) | 3 (19%) | 0.14 | 0.67 | |
| Effective AHI <15 events/h, n (%) | N/A | 7 (28%) | 11 (46%) | N/A | 11 (61%) | 8 (50%) | 0.06 | 1.00 | |
| CPAP adherence, h/night | 4.5 ± 2.7 | 4.7 ± 2.8 | N/A | N/A | |||||
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CPAP = continuous positive airway pressure; ESS = Epworth Sleepiness Scale; LGB = laparoscopic gastric banding; N/A = not applicable.
Data are presented as mean ± SD unless otherwise indicated. Outcome data are not transformed.
Between-arm P values for continuous outcomes were obtained from models adjusted for stratification factors (recruitment clinic, baseline BMI category, and sex). AHI was log-transformed at 9 months only. BMI was log-transformed at 18 months only. Hip circumference, effective AHI, and ESS scores were log-transformed at 9 months and 18 months.
P < 0.05 testing if the within-arm difference from baseline was different from zero (within-arms t test or a signed-rank test depending on distribution).
Note that an effective AHI was calculated for any patient using CPAP regardless of randomized arm.
Analyses limited to patients who received their assigned treatment (n = 14 LGB; n = 20 CPAP) produced similar results, that is, a greater improvement in effective AHI with CPAP (18.6 ± 25.3 vs. 28.2 ± 15.7 at 9 mo, P = 0.02) and no difference in ESS scores (7.2 ± 4.3 vs. 5.9 ± 3.5 at 9 mo, P = 0.98). Changes in AHI varied linearly with changes in BMI, with a 3.3% (SEM, 1.5%) reduction in AHI for every 1% reduction in BMI and no difference in slope by treatment.
To our knowledge, this is the first randomized trial to compare bariatric surgery and CPAP as initial treatment for OSA. We found that the effective AHI at 9 months was significantly lower with CPAP, and there was no difference in daytime sleepiness between groups. Neither of these findings supported our hypotheses. The rate of OSA resolution with LGB was fairly low, in keeping with prior trials that evaluated the effects of LGB on AHI (5, 6). Compared with prior trials, the impact of LGB on average weight in our study was modest, at just under 10 kg. Despite this result, the mean reduction in AHI of 17.4 events/h in the surgery group is similar to the improvements observed in prior studies (5, 6). The linear relationship between changes in BMI and AHI supports the hypothesis that LGB improves OSA via weight loss.
Prior studies that compared medical treatment and surgical treatment of OSA were limited by failing to account for suboptimal adherence to CPAP. A strength of our study is the focus on effective AHI as a primary outcome. The comparative-effectiveness framework increases generalizability to standard local practice. High attrition rates are often a concern in comparative-effectiveness studies; however, our dropout rate of 12% is modest and unlikely to be a major source of bias.
Important limitations should be considered. First, an unexpectedly high number of patients did not undergo LGB, as the majority of these individuals opted to use CPAP. This, in large part, reflects the realities of bariatric surgery referral, with patient ambivalence, nonadherence to preoperative guidelines, detection of surgical contraindications, and insurance barriers all limiting the implementation of surgery. Given our results indicating increased effectiveness of CPAP, the crossovers from LGB to CPAP suggest that our intention-to-treat analyses may in fact underestimate the difference in effective AHI between CPAP and LGB. Our results indicating greater improvement with CPAP in per-protocol analyses support the robustness of our findings. It is possible that our results overestimate the benefit of CPAP, if those most likely to benefit from LGB did not undergo surgery. We believe this scenario to be unlikely. Finally, it is possible that our follow-up duration was not long enough to allow the full benefits of LGB to occur.
The superior impact of CPAP on the effective AHI means that, in our opinion, CPAP is a more effective treatment than LGB for controlling OSA. This finding does not eliminate weight-loss therapy, whether behavioral or surgical, as an adjunctive treatment. Of note, two recent randomized trials of behavioral weight-loss therapy in OSA indicated that the beneficial effects of weight loss on OSA severity may persist despite weight regain (7, 8). Furthermore, weight-loss interventions may have greater benefit on cardiovascular risk reduction than CPAP (9–11). Finally, our results do not provide insights into how more aggressive bariatric procedures, such as gastric bypass and sleeve gastrectomy, may compare with CPAP in the management of OSA.
Footnotes
Supported by NIH grants R01HL106410 and K24HL127307 (S.R.P.). Philips Respironics donated the CPAP machines and supplies used in the perioperative period for patients undergoing bariatric surgery.
Author Contributions: Study design: A.T., A.M., and S.R.P. Study conduct and data collection: J.P.B., A.T., M.R., R.A., A.M., R.L.O., A.A., K.A.D., and S.R.P. Analysis and interpretation: J.P.B., A.T., M.R., K.A.D., W.W., and S.R.P. Manuscript preparation: J.P.B., M.R., R.A., A.M., R.L.O., A.A., K.A.D., W.W., and S.R.P.
Originally Published in Press as DOI: 10.1164/rccm.201708-1637LE on October 16, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Boyd SB, Upender R, Walters AS, Goodpaster RL, Stanley JJ, Wang L, et al. Effective apnea-hypopnea index (“effective AHI”): a new measure of effectiveness for positive airway pressure therapy. Sleep. 2016;39:1961–1972. doi: 10.5665/sleep.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd SB, Walters AS. Effectiveness of treatment apnea-hypopnea index: a mathematical estimate of the true apnea-hypopnea index in the home setting. J Oral Maxillofac Surg. 2013;71:351–357. doi: 10.1016/j.joms.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi MT, Alameddine Y, Mojica J. Apnea burden: efficacy versus effectiveness in patients using positive airway pressure. Sleep Med. 2014;15:1579–1581. doi: 10.1016/j.sleep.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–542. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JB, Schachter LM, O’Brien PE, Jones K, Grima M, Lambert G, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142–1149. doi: 10.1001/2012.jama.11580. [DOI] [PubMed] [Google Scholar]
- 6.Feigel-Guiller B, Drui D, Dimet J, Zair Y, Le Bras M, Fuertes-Zamorano N, et al. Laparoscopic gastric banding in obese patients with sleep apnea: a 3-year controlled study and follow-up after 10 years. Obes Surg. 2015;25:1886–1892. doi: 10.1007/s11695-015-1627-5. [DOI] [PubMed] [Google Scholar]
- 7.Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, et al. Sleep AHEAD Research Group of the Look AHEAD Research Group. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–649A. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuomilehto H, Seppä J, Uusitupa M, Tuomilehto J, Gylling HKuopio Sleep Apnea Group Weight reduction and increased physical activity to prevent the progression of obstructive sleep apnea: a 4-year observational postintervention follow-up of a randomized clinical trial. [corrected] JAMA Intern Med 2013173929–930.[Published erratum appears in JAMA Intern Med 173:996.] [DOI] [PubMed] [Google Scholar]
- 9.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 11.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]