There has been recent interest in protocols for enhanced-recovery after surgery (ERAS), designed to achieve a faster recovery and limit the acute stress response to surgery. These are multimodal, including changes in anesthesia and surgical practice, standardized additional sterile techniques, nursing protocols, medication changes, and mobility protocols. Regional anesthesia is often part of enhanced-recovery after surgery and theoretically blocks sympathetic response and postoperative inflammation. Data also suggest the use of regional anesthesia in the treatment of breast cancer and colon cancer can decrease tumor recurrence rates and improve overall survival (OS).1, 2 The goal of this study is to determine the effects of thoracic epidural anesthesia (TEA) versus intravenous patient-controlled anesthesia (IVPCA), on oncologic outcomes after resection of colorectal hepatic metastases.
With approval from the Wake Forest University Health Sciences Institutional Review Board, this is a retrospective review of a prospective hepatic surgery database of patients with colorectal liver metastases who underwent resection between 1996 and 2015 at our institution and who had either IVPCA or TEA for postoperative pain control. Those patients who underwent radiofrequency ablation only or those without specific documentation of type of perioperative pain control in the chart were excluded. Demographic data, potential risk factors, and outcomes were obtained from the database. The primary surgical team generally manages IVPCA at our institution, whereas the anesthesia acute pain team manages TEA. The narcotic agent most commonly used for IVPCA is dilaudid but could also be morphine or fentanyl per physician preference. TEA infusions were typically local anesthetics such as bupivacaine and/or hydromorphone.
One hundred seventy nine patients underwent hepatic resection for colorectal metastases, of which 44 received TEA and 128 received IVPCA. Seven (3.9%) received both TEA and IVPCA. Mean use of TEA was 3.1 ± 1.2 days and IVPCA was 3.1 ± 2.2.
OS and disease-free survival (DFS) at five years was 45.2 ± 4.9 per cent and 23.9 ± 4.0 per cent with a median OS and DFS of 4.4 and 1.5 years, respectively. OS and DFS at five years for TEA and IVPCA patients was 36.2 ± 9.4 per cent and 17.0 ± 7.2 per cent, and 47.8 ± 5.7 per cent (P = 0.47) and 26.2 ± 4.8 per cent (P = 0.48), respectively (Fig. 1). Univariate analyses can be seen in Table 1. There was an increased length of IVPCA for major resection with a median of three days for a major resection and two for minor resections (P = 0.026). There was not, however, a difference in days of TEA (3.5 days for major and three days for minor, P = 0.68). Extra hepatic procedures were associated with longer IVPCA and TEA (P = 0.031). However, extrahepatic procedures did not predict type of anesthesia used (P = 0.34) and was not associated with increased operating room time (P = 0.78) with means of 6.2 hours in both groups. In addition, extrahepatic procedures were not in themselves significant for DFS (P = 0.71) and OS (P = 0.83) on univariate analysis.
Fig. 1.
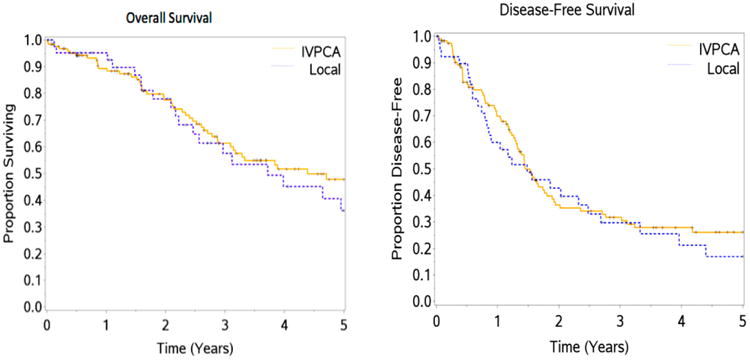
Survival curves for OS and DFS in the IVPCA and TEA patients.
Table 1. Univariate Analysis for OS and DFS.
| Variable | ||||
|---|---|---|---|---|
|
|
||||
| OS | n | HR | 95% confidence interval | P value |
| Age | 179 | 1.07 | 0.96, 1.18 | 0.23 |
| Gender | 179 | 1.05 | 0.66, 1.66 | 0.85 |
| Body mass index | 138 | 1.06 | 1.006, 1.12 | 0.029 |
| COPD | 179 | 0.98 | 0.45, 2.14 | 0.96 |
| Smoker | 161 | 1.21 | 0.74, 1.97 | 0.61 |
| Diabetes | 179 | 1.74 | 1.01, 3.01 | 0.047 |
| Renal failure | 179 | 5.88 | 1.35, 25.6 | 0.018 |
| Number of tumors | 157 | 1.07 | 0.98, 1.18 | 0.15 |
| Maximum tumor size | 152 | 0.99 | 0.90, 1.09 | 0.86 |
| operating room time | 148 | 1.07 | 0.91, 1.27 | 0.42 |
| Blood loss | 165 | 0.997 | 0.97, 1.03 | 0.88 |
| Transfusions | 143 | 0.87 | 0.66, 1.15 | 0.33 |
| Laparoscopic procedure | 151 | 059 | 0.28,1.25 | 0.16 |
| Major resection | 178 | 0.75 | 0.48, 1.18 | 0.21 |
| R0 resection | 179 | 0.45 | 0.20, 0.99 | 0.046 |
| Overall complications | 179 | 1.26 | 1.08, 1.48 | 0.0039 |
| Length of stay | 13 | 1.12 | 0.99, 1.26 | 0.076 |
| Eastern Cooperative Oncology Group status | 173 | 1.64 | 0.88, 3.07 | 0.013 |
| 6.54 | 1.50, 28.6 | |||
| >2 comorbidities | 179 | 1.3 | 0.76, 2.25 | 0.34 |
| Epidural | 179 | 1.12 | 0.71, 1.77 | 0.62 |
| Carcinoembryonic antigen | 162 | 1.11 | 0.96, 1.28 | 0.15 |
| Chronic pain | 125 | 1.47 | 0.75, 2.89 | 0.26 |
| Preoperative heart rate | 119 | 1.57 | 1.22, 2.02 | 0.0005 |
| American Society of Anesthesiologists | 129 | 1.82 | 1.05, 3.16 | 0.033 |
| TEA days | 24 | 1.31 | 0.66, 2.58 | 0.44 |
| Patient-controlled anesthesia days | 89 | 1.2 | 1.06, 1.37 | 0.0059 |
| DFS | ||||
| Age | 163 | 1 | 0.91, 1.09 | 0.93 |
| Gender | 163 | 1.07 | 0.72, 1.57 | 0.75 |
| Body mass index | 126 | 1.03 | 0.99, 1.07 | 0.12 |
| COPD | 163 | 0.91 | 0.55, 2.17 | 0.8 |
| Smoker | 145 | 1.08 | 0.72, 1.63 | 0.7 |
| Diabetes | 163 | 1.58 | 1.004, 2.50 | 0.051 |
| Renal failure | 163 | 2.13 | 0.67, 6.76 | 0.2 |
| Number of tumors | 142 | 1.1 | 1.01, 1.21 | 0.037 |
| Maximum tumor size | 139 | 1.004 | 0.91, 1.11 | 0.94 |
| Operating room time | 138 | 1.14 | 0.999, 1.29 | 0.052 |
| Blood loss | 150 | 0.99 | 0.97, 1.02 | 0.69 |
| Transfusions | 143 | 0.80 | 0.63, 1.04 | 0.10 |
| Laparoscopic procedure | 151 | 1.30 | 0.80, 2.11 | 0.29 |
| Major resection | 162 | 0.72 | 0.49, 1.06 | 0.099 |
| Overall complications | 163 | 1.17 | 1.01, 1.35 | 0.036 |
| Length of stay | 12 | 1.1 | 0.98, 1.24 | 0.11 |
| Eastern Cooperative Oncology Group status | 157 | 0.72 | 0.44, 1.20 | 0.093 |
| 3.48 | 0.83, 14.7 | |||
| >2 comorbidities | 163 | 1.1 | 0.69, 1.76 | 0.68 |
| Epidural | 163 | 1.18 | 0.77, 1.79 | 0.45 |
| Carcinoembryonic antigen | 148 | 1.08 | 0.95, 1.21 | 0.24 |
| Chronic pain | 116 | 1.47 | 0.90, 2.42 | 0.13 |
| Systolic blood pressure | 107 | 1.012 | 0.96, 1.07 | 0.66 |
| Preoperative heart rate | 111 | 1.23 | 1.03, 1.48 | 0.02 |
| Beta blocker use | 117 | 1 | 0.56, 1.76 | 0.99 |
| American Society of Anesthesiologists | 121 | 1.07 | 0.72, 1.58 | 0.75 |
| TEA days | 23 | 1.59 | 1.04, 2.44 | 0.033 |
| Patient-controlled anesthesia days | 86 | 1.13 | 1.01, 1.27 | 0.035 |
Research surrounding narcotics shows that opioids depress immune function by altering natural killer cells and increasing tumor cell proliferation.3 Many surgeons have been using regional anesthesia for postoperative pain control as opioids also affect bowel function and mental status. Previous researchers have found that regional anesthesia improves both overall and DFS in breast and colon cancer.1, 2 In a retrospective review looking specifically at liver resections for colorectal metastases, Zimmitti et al.4 showed improved OS and DFS with regional anesthesia. This study, however, demonstrated no difference in OS and DFS between patients with IVPCA versus TEA.
Interestingly, on univariate analysis, longer use of pain medication correlates with decreased OS and DFS, which has not been seen in previous literature. Both our anesthesia and surgery teams generally continue the epidural and IVPCA until patients are consistently tolerating a diet. Our study is looking at patients undergoing hepatectomy, where slow return of bowel function is less common than in colorectal surgery. One consideration may be that patients who had longer TEA and IVPCA use may have had more difficult to control pain whereas transitioning to oral medications. Patient with a laparoscopic surgery had a shorter length of TEA/ IVPCA time (P = 0.007); mean 2.5 ± 1.6 for laparoscopic surgery versus 3.5 ± 2.3 for open procedures. This suggests that patients with open procedures may have worse pain control or delayed bowel function, which highlights the known advantages of minimally invasive surgery. Extrahepatic procedures were associated with longer IVPCA and TEA time (P = 0.03). These extrahepatic cases may involve increased chance of ileus, which would lengthen the use of TEA and IVPCA. Extrahepatic procedures were not associated with a difference in DFS or OS. Ileus, or lack of nutrition in the early postoperative period, rather than extrahepatic procedures may be a confounder for worse outcomes.
Previous work from our institution on the role of TEA in hepatectomy demonstrated that open procedures are associated with more use of TEA. It would be expected that larger tumors or more tumors may require open procedures and are more likely to get TEA. Increased number of tumors was an independent predictor of DFS on univariate analysis. Major resection and maximum tumor size, however, had no effect on DFS or OS. In addition, there was no difference between TEA and IVPCA groups on laparoscopic versus open procedures (P = 0.23) or major resections (P = 0.999). Larger tumor size in the TEA group may, however, have skewed OS and DFS results as surgeons may choose TEA if expecting more pain from an open procedure, even if a larger resection is not performed. More pain would lead to more immunosuppression and may close the gap between the two groups on survival. Because pain scores are not available, the data obtained in this study are only hypothesis generating.
This study has all several limitations. Anesthesia type was recommended by the surgeon, but patients may have self-selected based on patient preference and clinical eligibility for epidural. In addition, the hospital records before the conversion to the electronic medical record are less comprehensive and there are some missing data.
The advantages seen in TEA may be related to avoidance of the immunosuppression that comes in pain states and avoidance of opioids. There may be multiple other confounders to immunosuppression, such as diabetes, cardiovascular disease, and poor overall health. TEA does have real side effects, including hypotension but the potential benefits of decreasing narcotic exposure at this point may outweigh the risk. It is important to have a discussion with patients about their pain management options including advantages and disadvantages of each. Future prospective research trials are needed to compare regional versus narcotic anesthesia for patients undergoing hepatectomy for malignant conditions to better understand the role of perioperative analgesia and its effect on oncologic outcomes.
Brief Reports.
Brief Reports should be submitted online to www.editorialmanager.com/ amsurg. (See details online under “Instructions for Authors”.) They should be no more than 4 double-spaced pages with no Abstract or sub-headings, with a maximum of four (4) references. If figures are included, they should be limited to two (2). The cost of printing color figures is the responsibility of the author.
In general, authors of case reports should use the Brief Report format.
Contributor Information
Mary Garland, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Dylan Addis, Department of Anesthesiology, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Greg Russell, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Clancy Clark, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Edward Levine, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Russell Howerton, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Konstantinos Votanopoulos, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Sean Dobson, Department of Anesthesiology, Wake Forest University School of Medicine, Winston-Salem, North Carolina
Perry Shen, Department of Surgery, Wake Forest University School of Medicine, Winston-Salem, North Carolina
References
- 1.Christopherson R, James KE, Tableman M, et al. Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg. 2008;107:325–32. doi: 10.1213/ane.0b013e3181770f55. [DOI] [PubMed] [Google Scholar]
- 2.Exadaktylos AK, Buggy DJ, Moriarty DC, et al. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? [Accessed December 14, 2016];Anesthesiology. 2006 105:660–4. doi: 10.1097/00000542-200610000-00008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17006061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page AJ, Ejaz A, Spolverato G, et al. Enhanced recovery after surgery protocols for open hepatectomy–physiology, immunomodulation, and implementation. J Gastrointest Surg. 2015;19:387–99. doi: 10.1007/s11605-014-2712-0. [DOI] [PubMed] [Google Scholar]
- 4.Zimmitti G, Soliz J, Aloia TA, et al. Positive impact of epidural analgesia on oncologic outcomes in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23:1003–11. doi: 10.1245/s10434-015-4933-1. [DOI] [PubMed] [Google Scholar]


