Abstract
Background
In twin pregnancies, the rates of adverse perinatal outcome and subsequent long-term morbidity are substantial, and mainly result from preterm birth (PTB).
Objectives
To assess the effectiveness of progestogen treatment in the prevention of neonatal morbidity or PTB in twin pregnancies using individual participant data meta-analysis (IPDMA).
Search strategy
We searched international scientific databases, trial registration websites, and references of identified articles.
Selection criteria
Randomised clinical trials (RCTs) of 17-hydroxyprogesterone caproate (17Pc) or vaginally administered natural progesterone, compared with placebo or no treatment.
Data collection and analysis
Investigators of identified RCTs were asked to share their IPD. The primary outcome was a composite of perinatal mortality and severe neonatal morbidity. Prespecified subgroup analyses were performed for chorionicity, cervical length, and prior spontaneous PTB.
Main results
Thirteen trials included 3768 women and their 7536 babies. Neither 17Pc nor vaginal progesterone reduced the incidence of adverse perinatal outcome (17Pc relative risk, RR 1.1; 95% confidence interval, 95% CI 0.97–1.4, vaginal progesterone RR 0.97; 95% CI 0.77–1.2). In a subgroup of women with a cervical length of ≤25 mm, vaginal progesterone reduced adverse perinatal outcome when cervical length was measured at randomisation (15/56 versus 22/60; RR 0.57; 95% CI 0.47–0.70) or before 24 weeks of gestation (14/52 versus 21/56; RR 0.56; 95% CI 0.42–0.75).
Author’s conclusions
In unselected women with an uncomplicated twin gestation, treatment with progestogens (intramuscular 17Pc or vaginal natural progesterone) does not improve perinatal outcome. Vaginal progesterone may be effective in the reduction of adverse perinatal outcome in women with a cervical length of ≤25 mm; however, further research is warranted to confirm this finding.
Keywords: 17-Hydroxyprogesterone caproate, individual participant data meta-analysis, preterm birth, twin pregnancy, vaginal progesterone
Introduction
In Europe and the USA about 3% of all pregnancies are twin pregnancies.1,2 In twin pregnancies, the rates of stillbirth, neonatal death, preterm birth, (very) low birthweight, and subsequent long-term morbidity are substantially higher than in singletons.3 Preterm birth is the principal factor contributing to these adverse outcomes, with 50% of twin pregnancies delivering before 37 weeks of gestation and 9% delivering before 32 weeks of gestation.4 Improving outcomes in twin pregnancies is a goal in modern obstetrics, but as yet no interventions have been proven to be of benefit in this group.
Randomised clinical trials (RCTs) have shown that antenatal progestogen therapy (vaginally administered natural progesterone and semi-synthetic progestogens such as intramuscular 17-hydroxyprogesterone caproate, 17Pc) reduces the rate of preterm delivery in women with singleton pregnancies who are at high risk because of preterm birth in a previous pregnancy,5–8 or because of a short cervix, measured sonographically, in the current pregnancy.9–11
There has been extensive international interest in determining whether the benefits of progestogens extend to twins: that is, whether these agents reduce the rate of preterm birth and thereby reduce perinatal morbidity. Two aggregated data meta-analyses (ADMA) have examined published trials of progestogens in twin pregnancies. One of these did not differentiate between 17Pc and vaginal progesterone.12 The other had too little information to investigate relevant subgroups.13 This is a common limitation of ADMA. Individual participant data meta-analysis (IPDMA) is a more robust design that more easily allows for subgroup analysis.14,15 A recent IPDMA focused on women with a short cervix and found that vaginal progesterone reduced the rate of early preterm birth and the rate of composite neonatal morbidity/mortality in singleton pregnancies.11 Based on the small number of twins in that analysis, there was a trend towards reduction of early preterm birth with progesterone and a significant reduction of neonatal morbidity/mortality. That meta-analysis did not include any studies with 17Pc. Moreover, none of the three previous meta-analyses of the effect of progestogens in twins included all published studies.11–13
The aim of the current study was to perform an IPDMA to investigate the effects of progestogens in women with a twin pregnancy and in prespecified subgroups. Analysis was performed separately for intramuscular 17Pc and vaginally administered natural progesterone.
Methods
The reporting of the IPDMA was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 The study was conducted based on a previously published protocol.17
Trial search and selection strategy
Trials were identified by searching the electronic databases Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE, Embase, ClinicalTrials.gov, and controlled-trials.com (all from inception until 1 March 2013). We used the following search strategy: preterm birth AND (progesterone OR 17-hydroxyprogesterone caproate OR progestogen OR 17P) AND (pregnancy multiple OR pregnancy multifetal OR pregnancy twin) AND (randomised controlled trial OR randomized controlled trial OR randomised trial OR randomized trial) AND human. The principal investigators, regarded as experts in their field, were contacted to identify further studies. No language restrictions were used.
We included RCTs that investigated the effectiveness of vaginally administered progesterone or intramuscular 17Pc, each versus placebo or non-intervention in the second or third trimester in women with a twin pregnancy, in the reduction of either preterm birth or adverse perinatal outcome. Trials that investigated the effectiveness in specific subgroups (e.g. women with a short cervix) were also eligible for inclusion. Two investigators (ES and SS) independently reviewed the identified papers for eligibility. Any disagreement was solved by a third reviewer (BWJM).
The risk of bias was assessed by two independent reviewers (ES and SS) using a risk-of-bias tool developed by the Cochrane collaboration, which contains specific items that assess random sequence allocation bias, allocation concealment, incomplete outcome data, selective reporting, other bias, blinding of participants and personnel, and blinding of outcome assessment.18 According to the Cochrane Handbook, each item of bias was scored as low, high, or unclear. All the items were scored twice, once based on the published paper or protocol and once by the principal investigator of the included study.
The principal investigators of all eligible RCTs were contacted to participate and were requested to provide individual participant data. The principal investigators had the choice to either complete an excel or spss data sheet or to send their data accompanied by a document clearly stating the definitions used. The data collected included relevant baseline characteristics and outcomes of interest, described in the following subsection. The principal investigator assessed data quality by comparison of the shared data with the numbers published. In case of discrepancies the principal investigator was contacted and corrections were made if necessary. If any questions were raised, the authors were contacted for clarification.
Outcomes and subgroups
The primary outcome of this IPDMA was a composite of adverse perinatal outcome and was defined differently for 17Pc and vaginal progesterone, based on the availability of different components of perinatal outcome in the individual studies. In the 17Pc analysis, the composite outcome included perinatal death, defined as intrauterine fetal death (IUFD) at any gestational age or neonatal death before discharge from the hospital, or significant neonatal morbidity, defined as one or more of the following: respiratory distress syndrome (RDS), requiring oxygen for ≥24 hours; bronchopulmonary dysplasia (BPD); intraventricular haemorrhage (IVH), grades III or IV; necrotising enterocolitis (NEC), grade II or more; or culture-proven sepsis. In the vaginal progestogen analysis the composite outcome included perinatal death, RDS, IVH, and NEC. Secondary outcomes included the individual neonatal morbidities listed above, IUFD, or preterm birth at <37, <35, <32, and <28 weeks of gestation, as well as time to delivery or death.
Secondary objectives were to assess the effect of progestogens in different prespecified subgroups, which were based on the results of included studies or previous studies.
Chorionicity, as assessed by ultrasonography and defined as mono- or dichorionic.19,20
Cervical length before 24 weeks of gestation ≤25 mm (yes/no).9,11
Prior spontaneous preterm birth <37 weeks of gestation (yes/no).5–8
The subgroup effects were investigated for the primary outcome, adverse perinatal outcome, and time to delivery or death. Dosage of vaginal progesterone was not prespecified as a separate subgroup analysis, as there is sufficient evidence that dosage does not affect results in twin pregnancies.21,22
Analysis
All analyses were performed on an intention-to-treat basis for 17Pc and vaginal progesterone, as compared with control, separately.
The effectiveness of progestogen treatment was estimated using mixed models for binomial outcomes with a log link, thus resulting in risk ratios (RRs) with 95% confidence intervals (95% CIs). A random intercept (to account for differences in prevalence between studies) and a random slope (to account for differences in treatment effect between studies) was included in these models. In the analysis on child level, we incorporated a compound symmetric residual error variance to account for the clustering of children with one shared mother.23
Heterogeneity across trials was assessed using the I2 measure and the values were interpreted as follows: 0% indicates no observed heterogeneity; 25, 50, and 75% indicate low, moderate, and high heterogeneity, respectively.24 The number needed to treat (NNT) calculation was planned when an association was found to be statistically significant.
As the interest of the effectiveness of progestogen is mainly focused on preterm birth and not necessarily on overall gestational age at delivery, we applied a two-part model to estimate the effect of progestogen on the combined end point of preterm birth (i.e. birth before 37, 35, 32, and 28 weeksof gestation), and gestational age at delivery for those who are born preterm.25 A two-part model combines the probability of preterm birth (estimated by a mixed log-binomial regression model) with the median gestational at delivery in those with a preterm birth, estimated using a mixed linear quantile regression model.26 Confidence intervals were constructed with bootstrapping (10 000 samples).
Subgroup effects were investigated using an interaction term between the subgroup of interest and treatment in the regression model. When the interaction was found to be statistically significant (P < 0.05), a stratified analysis was performed to investigate the effect of progestogen treatment in different strata of the subgroups.
Two post-hoc sensitivity analyses were performed: one in which we excluded all non-blinded studies, and one in which studies that included less than 100 participants were excluded. Statistical analyses were performed using r 2.15.2 (The R Foundation for Statistical Computing, 2012) and sas 9.2 (SAS Institute Inc., Cary, NC, USA, 2010).
Results
Included studies
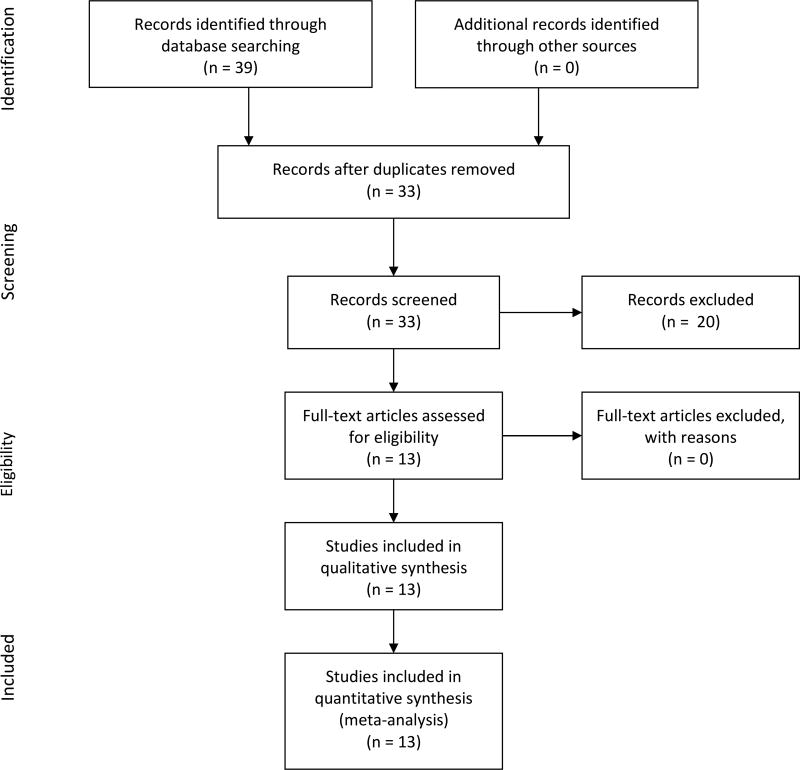
Thirty-nine studies were identified through database searching (Figure 1). Thirteen studies met all of the inclusion criteria (Figure 1; Table 1; Appendix S1).5,9,19,20,22,27–34 The other studies identified from the literature search (n = 20) did not investigate twins, described a study protocol, or were not expecting to complete the study within the next year.35 Data sets containing IPD were obtained for all 13 RCTs. All studies had institutional review board approval and informed consent from all participants.
Figure 1.
Flow of study identification.
Table 1.
Overview of the studies investigating progestogen treatment included in the IPDMA
| Type of progestogen |
Study | Type of study | n | Inclusion criteria | Dosage |
|---|---|---|---|---|---|
| 17Pc, weekly | Rouse20 | Placebo-controlled double-blind RCT, multicentre | 661 | Women carrying twins with a gestational age of 16+0–20+3 weeks | 250 mg |
| Lim30 | Placebo-controlled double-blind RCT, multicentre | 650 | Women with a twin pregnancy and a gestational age between 15 and 19 weeks, with chorionicity assessed by ultrasonography | 250 mg | |
| Nassar31 | Placebo-controlled double-blind RCT, single centre | 286 | Viable twin pregnancy between 16 and 20 weeks of gestation | 250 mg | |
| Combs29 | Placebo-controlled double-blind RCT, multicentre | 240 | Women with a dichorionic diamniotic twin pregnancy at 15–23 weeks of gestation with an ultrasound examination showing no major fetal anomalies | 250 mg | |
| Senat33 | Open-label RCT, single centre | 165 | Women carrying twins with cervical length of ≤25 mm between 20 and 32 weeks of gestation | 500 mg | |
| Briery28 | Placebo-controlled double-blind RCT, single centre | 30 | Twin pregnancy between 20 and 30 weeks of gestation with intact membranes | 250 mg | |
| Vaginal, daily | Rode32 | Placebo-controlled double-blind RCT, multicentre | 677 | Women with a live, diamniotic twin pregnancy and with chorionicity assessed by ultrasound before 16 weeks of gestation | 200-mg pessaries |
| Norman19 | Placebo-controlled double-blind RCT, multicentre | 500 | Women carrying twins with gestation and chorionicity established by scan before 20 weeks of gestation | 90 mg of gel | |
| Serra22 | Placebo-controlled double-blind RCT, multicentre | 290 | Dichorionic diamniotic twin pregnant women | 1× or 2× 200-mg pessaries | |
| Aboulghar27 | Placebo-controlled RCT, single centre | 92 | Nulliparous women who conceived after IVF or ICSI with a dichorionic twin between 18 and 24 weeks of gestation | 2× 200-mg suppositories | |
| Wood34 | Placebo-controlled double-blind RCT, single centre | 81 | Women with a multifetal pregnancy between 16+0 and 20+6 weeks of gestation and ultrasound-confirmed minimum of two live fetuses | 90 mg of gel | |
| Cetingoz5 | Placebo-controlled double-blind RCT, single centre | 67 | Women carrying twins | 100-mg suppositories | |
| Fonseca9 | Placebo-controlled double-blind RCT, multicentre | 24 | Women carrying twins who were undergoing routine ultrasonography at 20–25 weeks of gestation and had a cervical length of <15 mm | 200-mg capsules |
ICSI, intracytoplasmatic sperm injection; IVF, in vitro fertilisation.
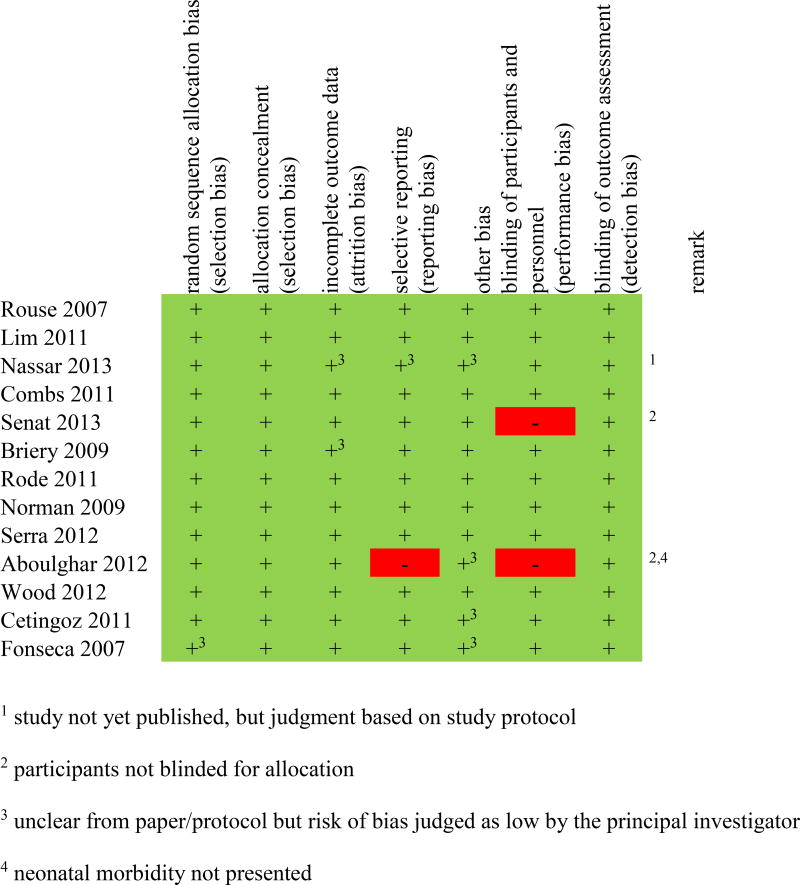
The studies randomised women to either 17Pc20,28–31,33 or vaginal progesterone,5,9,19,22,27,32,34 each versus placebo or non-intervention. The characteristics of the 17Pc and vaginal progesterone included in this IPDMA are shown in Table 1. Eleven studies were placebo-controlled double-blind RCTs,5,9,19,20,22,28–32,34 one was an open-label trial of 17Pc versus no treatment,33 and one was a placebo-controlled trial in which participants were not blinded to their treatment (Figure 2).27
Figure 2. Risk of bias for each included study.
Risk of bias was judged as low (green, +), high (red, −), or unclear (yellow, ?), and was assessed based on the published paper/protocol and queries to the principal investigator of the included study.
In general, all studies enrolled twin pregnancies with a gestational age between 16+0 and 23+6 weeks of gestation at randomisation. Several studies had additional inclusion criteria: three studies included only women with a dichorionic twin pregnancy,22,27,29 one vaginal progesterone study included women with a cervical length of <15 mm at 20–25 weeks of gestation,9 and one 17Pc study included women between 20 and 32 weeks of gestation with a cervical length of ≤25 mm.33 Two trials allowed randomisation of women who had a prophylactic (history-indicated) cerclage in situ.29,32 The exclusion criteria of the studies were similar with most of the studies eliminating twin pregnancies with suspected major fetal abnormalities, suspected twin-to-twin transfusion, serious maternal medical disease, cerclage in place or planned, contraindication to progestogens, or twin gestations that were the result of intentional fetal reduction (Appendix S1). Several studies used stratified randomisation (e.g. by centre,19,20,29,32 chorionicity,19,30,32 or parity30). One study was excluded from the analysis of the primary outcome, i.e. adverse perinatal outcome (but not the other outcomes), because only two components of the composite were registered.27 The availability of other data of interest in the individual studies is listed in Appendix S2.
One study found that progestogens (vaginal progesterone) significantly reduced the rate of preterm delivery before 37 weeks of gestation,5 and one study found that that progestogens (17Pc) reduced composite neonatal morbidity,31 results that were not repeated in the other 11 trials. Four out of six 17Pc studies and one out of seven vaginal progesterone studies showed that progestogens were associated with a non-significant increase in preterm births.19,20,28–30 Furthermore, one (17Pc) study found a significant decrease in median gestational age at delivery,29 and two (vaginal progesterone) studies showed a non-significant reduction in preterm birth in the progestogen group.9,32 In addition, two studies (one 17Pc, one vaginal progesterone) showed a non-significant increase in IUFD.19,20
The overall quality of the included studies was good. A summary of the risk of bias assessment can be found in Figure 2. Selective outcome reporting was assessed but not considered an issue because IPDMA relies on IPD rather than reported outcomes.
In this IPDMA we included individual data of 3768 women with a twin pregnancy, of which 2006 women were allocated to progestogen treatment (1089 to 17Pc and 917 to vaginal progesterone) and 1762 were allocated to control. The baseline characteristics of the women administered 17Pc or placebo and vaginal progesterone or placebo are presented separately in Table 2. Baseline characteristics for the progestogen-treatment groups and their control groups were comparable, although assisted conception occurred more often in those treated with 17Pc, whereas in the vaginal progesterone studies women in the control group drank more alcohol.
Table 2.
Baseline characteristics of the participants of the IPDMA according to type of progestogen and allocation
| 17Pc | Vaginal progesterone | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristics | 17Pc n = 1089 |
Control n = 944 |
Available from how many studies |
Available for (%) |
Vaginal progesterone n = 917 |
Control n = 818 |
Available from how many studies |
Available for (%) |
| Maternal age: years (±SD) | 31.6 (5.6) | 31.4 (5.8) | 5/6 | 1976 (97) | 32.2 (5.0) | 32.2 (5.0) | 7/7 | 1714 (99) |
| Body mass index: kg/m2 (±SD) | 25.8 (6.1) | 25.7 (6.3) | 4/6 | 1688 (83) | 24.6 (4.8) | 24.6 (5.3) | 4/7 | 1033 (60) |
| Gestational age at randomisation: weeks (±SD) | 19.0 (3.0) | 19.0 (2.9) | 6/6 | 1977 (97) | 20.1 (3.1) | 20.6 (2.7) | 7/7 | 1720 (99) |
| Cervical length at randomisation: cm (±SD)* | 2.3 (1.5) | 2.5 (1.4) | 3/6 | 258 (13) | 3.8 (0.9) | 3.7 (0.9) | 5/7 | 907 (52) |
| Cervical length before 24 weeks of gestation: cm (±SD)** | 4.1 (1.1) | 4.2 (1.1) | 4/6 | 552 (27) | 3.9 (0.8) | 3.8 (0.9) | 5/7 | 890 (51) |
| Nulliparous: n (%) | 526 (48) | 449 (48) | 6/6 | 2032 (100) | 549 (60) | 488 (60) | 7/7 | 1729 (100) |
| Race | 5/6 | 1841 (91) | 5/7 | 514 (30) | ||||
| Black | 106 (11) | 103 (12) | 3 (1) | 3 (1) | ||||
| White | 773 (78) | 665 (78) | 279 (90) | 185 (90) | ||||
| Asian | 44 (4) | 20 (2) | 4 (1) | 7 (3) | ||||
| Other | 69 (7) | 61 (7) | 23 (7) | 10 (5) | ||||
| Smoking: n (%) | 95 (9) | 80 (9) | 6/6 | 2016 (99) | 117 (14) | 88 (12) | 6/7 | 1617 (93) |
| Alcohol: n (%) | 65 (7) | 45 (5) | 4/6 | 1817 (89) | 179 (37) | 177 (48) | 3/7 | 854 (49) |
| Assisted conception: n(%) | 493 (50) | 355 (42) | 4/6 | 1831 (90) | 366 (62) | 311 (59) | 5/7 | 1120 (65) |
| Monochorionic twin: n (%) | 139 (14) | 120 (14) | 5/6 | 1844 (91) | 97 (11) | 111 (15) | 6/7 | 1622 (93) |
| Previous preterm delivery: n (%) | 84 (8) | 87 (10) | 6/6 | 1933 (95) | 24 (3) | 21 (3) | 5/7 | 1630 (94) |
Data are presented as means ± standard deviations (SDs) or n (%). All numbers are based on the data as shared by the individual research groups.
Cervical length measurement is on the same day or before the moment of randomisation. Number of cervical length measurements at or before randomisation in the 17Pc studies of Lim, Senat, and Briery was 91, 165, and 2, respectively, and in the vaginal progesterone studies of Cetingoz, Serra, Rode, Fonseca, and Aboulgar was 67, 284, 445, 24, and 87, respectively.
The number of cervical length measurements before 24 weeks of gestation in the 17Pc studies of Lim, Combs, Senat, and Briery was 513, 20, 17, and 2, respectively, and in the vaginal progesterone studies of Cetingoz, Serra, Rode, Fonseca, and Aboulgar was 67, 284, 445, 13, and 81, respectively.
17Pc
Overall effects of 17Pc treatment
The overall effects of treatment with 17Pc are depicted in Appendix S5. The primary outcome, i.e. adverse perinatal outcome, occurred in 423 (20%) children in the 17Pc group and 318 (17%) children in the control group (RR 1.2; 95% CI 0.87–1.5). The rates of the individual components of the composite outcome did not differ significantly between the two groups, although it should be noted that there was a trend towards an increased risk for the majority of adverse perinatal and neonatal outcomes in those treated with 17Pc.
For IUFD or delivery before 37, 35, 32, and 28 weeks of gestation, and the other secondary outcomes no substantial differences were found between 17Pc treatment and control. All results were similar when corrected for stratified randomisation.
Heterogeneity amongst studies was low for ROP, moderate for RDS and a delivery or death before 35 weeks of gestation, and moderate to high for adverse perinatal outcome, perinatal death, sepsis, and delivery or death before 32 weeks of gestation (Appendix S5). For all other outcomes no heterogeneity was observed.
There was one study in which participants and clinicians were not blinded to treatment.33 Exclusion of all participants from this particular trial did not lead to different results (data not shown). The second sensitivity analysis focused on the analysis of IPD from studies that included 100+ participants,20,29–31,33 and showed similar results to the main IPDMA (data not shown).
Subgroup analyses in 17Pc treatment
17-Hydroxyprogesterone caproate (17Pc) had a statistically significant interaction with the following subgroups: women with a cervical length at randomisation of ≤25 mm (P = 0.0098), and those with a cervical length of ≤25 mm before 24 weeks of gestation (P = 0.0027). Stratified subgroup analyses for adverse perinatal outcome according to cervical length indicated no benefit of 17Pc over control in women with a cervical length of ≤25 mm, either at randomisation or before 24 weeks of gestation (Appendix S3). Instead, 17Pc led to an increase in adverse perinatal outcome in women with a cervical length of >25 mm at randomisation (14/78 versus 8/88; RR 2.1; 95% CI 1.9–2.2) and before 24 weeks of gestation (82/518 versus 60/512; RR 1.4; 95% CI 1.26–1.5). Cervical length measurements were available from four studies.28–30,33 17Pc did not have a significant effect on adverse perinatal outcome, time to delivery, or death when subgroup analysis was performed according to chorionicity (P = 0.32) and prior spontaneous preterm birth <37 weeks of gestation (P = 0.52).
Vaginal progesterone
Overall effects of vaginal progesterone treatment
The overall effects of vaginal progesterone treatment are shown in Appendix S6. Adverse perinatal outcome occurred in 219 (13%) children in the vaginal progesterone group and in 201 (13%) children in the control group (RR 0.96; 95% CI 0.83–1.1). The rates of the individual components of the composite outcome did not differ significantly between both groups. Women treated with vaginal progesterone had a lower gestational age at delivery when delivered before 28 weeks of gestation than those in the control group (median difference –1.6 weeks of gestation; 95% CI –2.7 to –0.43 weeks of gestation); however, the combined end point based on the proportion of delivery or death before 28 weeks of gestation and the difference in gestational age at delivery or death for those who were preterm was similar for the two groups (median difference 0.049; 95% CI –0.034 to 0.10). For delivery before 37, 35, 32, and 28 weeks of gestation, and the other secondary outcomes, no substantial differences were found between vaginal progesterone treatment and control. All results were similar when corrected for stratified randomisation.
Heterogeneity amongst studies was low for BPD, and for delivery or death before 37 and 32 weeks of gestation (Appendix S6). For all other outcomes no heterogeneity was observed.
There was one study in which participants were not blinded to the treatment.27 Exclusion of the participants of this study did not lead to different results (data not shown). An analysis on IPD from studies that included 100+ participants only did not alter the presented results (data not shown).19,22,32
Subgroup analyses in vaginal progesterone treatment
Vaginal progesterone had a statistically significant interaction with the following subgroups: women with a cervical length of ≤25 mm at randomisation (P = 0.0060); women with a cervical length of ≤25 mm before 24 weeks of gestation (P = 0.0055), and women with a prior spontaneous preterm birth <37 weeks of gestation (P = 0.0013).
Stratified subgroup analyses for adverse perinatal outcome according to cervical length indicated a benefit of vaginal progesterone over control in women with a cervical length of ≤25 mm at randomisation (15/56 versus 22/60; RR 0.57; 95% CI 0.47–0.70; NNT 10) and before 24 weeks of gestation (14/52 versus 21/56; RR 0.56; 95% CI 0.42–0.75; NNT 9.5, Appendix S4). No difference between vaginal progesterone and controls was found for women with a cervical length of >25 mm at randomisation or before 24 weeks of gestation. Cervical length measurements were available from five studies,5,9,22,27,32 of which four included women with a cervical length of ≤25 mm.5,9,22,32
Stratified subgroup analysis according to prior preterm birth <37 weeks of gestation showed no difference in adverse perinatal outcome when comparing the vaginal progesterone group with controls in those with a prior preterm birth (12/48 versus 9/42; RR 2.0; 95% CI 0.93–4.2) and in those without a previous preterm birth (187/1566 versus 160/1372; RR 1.02; 95% CI 0.96–1.09).
Vaginal progesterone did not have a significant effect on adverse perinatal outcome, time to delivery, or death when subgroup analysis was performed according to chorionicity (P = 0.46).
Discussion
Main findings
This meta-analysis based on individual participant data from 13 RCTs of progestogen treatment for the prevention of preterm birth in unselected women with an uncomplicated twin pregnancy shows that progestogen treatment, regardless of type, did not reduce the risk of adverse perinatal outcome, compared with control; however, in a subgroup of women with a short cervical length, vaginal progesterone treatment was beneficial.
Interpretation
A recent study investigating the relationship between 17Pc concentration and gestational age at delivery showed that 17Pc may reduce gestational age at delivery.36 This result could not be repeated in this study; however, given the general trend of increased incidence of adverse perinatal outcomes, the increased incidence of adverse perinatal outcomes in a subgroup of women with a cervical length >25 mm, and the ineffectiveness to prolong pregnancy, we conclude that 17Pc is contraindicated in twin pregnancies.
Two recent meta-analyses suggested that women with a twin pregnancy and a short cervical length might benefit from treatment with vaginal progesterone.12,13 In our IPDMA we found similar results. The effect estimate was similar to that previously found in an IPDMA in singletons (RR 0.57; 95% CI 0.40–0.81) and twins (RR 0.56; 95% CI 0.30–0.97) versus twin pregnancies with a cervical length of ≤25 mm at randomisation (RR 0.57; 95% CI 0.47–0.70) and before 24 weeks of gestation (RR 0.56; 95% CI 0.42–0.75).11 The narrower confidence interval in our study may be attributable to our inclusion of a more recent study that was not included in the earlier meta-analysis.22 The potential effectiveness of vaginal progesterone in twin pregnancy with short cervix may have biological plausibility, as short cervix might be an early sign of the onset of parturition. Nonetheless, these results should be interpreted with caution because they are derived from a post hoc analysis of relatively small subgroups, which can lead to spurious conclusions. These findings should stimulate further research on progesterone in twin pregnancies with a short cervix.
Two individual studies previously reported a non-significant trend towards a benefit from vaginal progesterone in monochorionic twin pregnancies.19,32 One study showed that vaginal progesterone reduced the number of IUFDs or deliveries before 34 weeks of gestation in monochorionic twins by 48%, whereas these were increased by 73% in dichorionic twins, both compared with the placebo group.19 We did not find a significant interaction between vaginal progesterone treatment and chorionicity. We conclude that progestogens are not effective in a subgroup of women based on chorionicity.
Strengths and limitations
An IPDMA has several distinct advantages over ADMA. IPDMA involves the synthesis of individual-level data from the individual trials, and therefore allows for the verification of published results. As IPD are available, an IPDMA allows for more flexibility regarding the inclusion and exclusion of individuals,14 and the choice of end points and subgroups,15,37 compared with ADMA. Furthermore, an IPDMA allows for more options to perform subgroup analyses and time-to-event analysis, as it can take account of the time between the initiation of treatment and the outcome of interest.38 This is important because most published trials have reported a non-significant trend towards a shorter duration of pregnancy after the use of progestogens in women with twin pregnancies.19,20 A final advantage of IPDMA is that IPD from unpublished studies can be included in the analysis. It is, however, important to contact the primary investigators and ask for the study protocol in order to assess the risk of bias, which is normally assessed using the published article. Although the results found in this study are similar to two previous ADMAs,12,13 we can be more confident in our findings given our rigorous approach using IPD.
Other strengths of the study are that this is the largest meta-analysis so far conducted on the effects of progestogens in twins and that it includes all currently published studies on this topic, in contrast to previously published meta-analyses.11–13 Furthermore, our study is strengthened by the low influence of publication bias. Indeed, publication bias is a potential problem in meta-analysis, and can be investigated using funnel plots. Given the low number of included studies we were not able to reliably construct funnel plots. Instead, studies were identified through trial registries, from which we were able to identify continuing as well as terminated studies. Moreover, most of the trials included in this IPDMA failed to show a benefit of progestogens, but were included in a formal trials register, and published according to good practice advice.
Heterogeneity in the primary outcome between the six 17Pc studies was moderate to high (I2 64%; 95% CI 14–85%). This heterogeneity is likely to result from the different directions of the effect of 17Pc in the individual studies. In the seven vaginal progesterone studies no heterogeneity was observed for the primary outcome (I2 0%; 95% CI 0–65%).
The sensitivity analyses performed in blinded studies, and in studies that included 100+ participants, did not lead to different results than are presented here. This shows the robustness of the results of our study.
The number of women with missing primary outcome data was very low (0.0–6.7%). Consequently, it was not likely that these missing values would influence the final conclusions. Therefore, a complete case analysis was considered appropriate.
There are several deviations from our published protocol that must be discussed.17 First, the cut-off of cervical length was changed from <25 mm to ≤25 mm to allow for a better comparison with another meta-analysis.11 Second, periventricular leucomalacia and retinopathy of prematurity were excluded from the composite outcome because this information was not registered in the majority of studies. We would like to emphasise that both changes were made before we investigated treatment effects. Furthermore, the conclusions of our study were similar when using the cut-off of cervical length as previously published in the protocol of this IPDMA.
Conclusion
In conclusion, this IPDMA has shown that in unselected women with uncomplicated twin pregnancies, treatment with progestogens does not prolong pregnancy or improve perinatal outcome. In a subgroup of women with a short cervical length vaginal progesterone treatment did improve perinatal outcome.
Supplementary Material
Acknowledgments
Funding
KGMM is funded by the Netherlands Organization for Scientific Research (grants 918.10.615 and 9120.8004).
Footnotes
Disclosure of interests
None of the authors has any financial, personal, political, academic, or other relationships that could lead to a conflict of interest relevant to this article.
Contribution to authorship
ES, ACL, and BWJM were involved in the concept and the design of the study. ES performed the analyses in collaboration with NPAZ, RHHG, and BWJM. ES, SS, LR, DJR, ACL, JEN, AHN, VS, CAC, CV, SW, EÇ, CMB, EBF, EAT, SNC, KK, AT, JA, IMU, AP, JM, KM, TG, SR, CC, AKa, JCM, EFM, KHN, RHHG, KGMM, AKw, and BWJM participated in face-to-face meetings, teleconferences and/or e-mail conversations to discuss the article, the design of the meta-analysis, the choice of outcome measures, and analysis strategies. SS, LR, DJR, ACL, JEN, AHN, VS, CAC, CV, MMA, SW, EÇ, CMB, EBF, EAT, SNC, KK, AT, JA, IMU, AP, JM, KM, TG, MAA, YMA, SR, CC, AKa, JCM, EFM, KHN, and BWJM were involved in the construction and design of one of the trials included in the meta-analysis. ES wrote the initial article, with significant contributions by RHHG, AK, KGMM, and BWJM. All authors critically reviewed the subsequent versions of the article and approved the final version.
Details of ethics approval
All studies had institutional review board approval and informed consent from all participants.
References
- 1.EURO-PERISTAT. Better statistics for better health for pregnant women and their babies [Internet] [Last accessed 29 March 2010];European perinatal health report. 2008 [ www.europeristat.com]
- 2.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, et al. Births: final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 3.Ananth CV, Joseph KK, Smulian JC. Trends in twin neonatal mortality rates in the United States, 1989 through 1999: influence of birth registration and obstetric intervention. Am J Obstet Gynecol. 2004;190(5):1313–1321. doi: 10.1016/j.ajog.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Schaaf JM, Mol BW, bu-Hanna A, Ravelli AC. Trends in preterm birth: singleton and multiple pregnancies in the Netherlands, 2000–2007. BJOG. 2011;118(10):1196–1204. doi: 10.1111/j.1471-0528.2011.03010.x. [DOI] [PubMed] [Google Scholar]
- 5.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283(3):423–429. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 6.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 7.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30(5):687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357(5):462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 10.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206(2):124–19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Likis FE, Edwards DR, Andrews JC, Woodworth AL, Jerome RN, Fonnesbeck CJ, et al. Progestogens for preterm birth prevention: a systematic review and meta-analysis. Obstet Gynecol. 2012;120(4):897–907. doi: 10.1097/AOG.0b013e3182699a15. [DOI] [PubMed] [Google Scholar]
- 13.Sotiriadis A, Papatheodorou S, Makrydimas G. Perinatal outcome in women treated with progesterone for the prevention of preterm birth: a meta-analysis. Ultrasound Obstet Gynecol. 2012;40(3):257–266. doi: 10.1002/uog.11178. [DOI] [PubMed] [Google Scholar]
- 14.Riley RD, Lambert PC, bo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2(3):209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuit E, Stock S, Groenwold RH, Maurel K, Combs CA, Garite T, et al. Progestogens to prevent preterm birth in twin pregnancies: an individual participant data meta-analysis of randomized trials. BMC Pregnancy Childbirth. 2012;12:13. doi: 10.1186/1471-2393-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. 2011 [Google Scholar]
- 19.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034–2040. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 20.Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, Spong CY, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 21.Romero R. Progesterone to prevent preterm birth in twin gestations: what is the next step forward? BJOG. 2013;120(1):1–4. doi: 10.1111/1471-0528.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serra V, Perales A, Meseguer J, Parrilla J, Lara C, Bellver J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double-blind multicentre trial. BJOG. 2013;120(1):50–7. doi: 10.1111/j.1471-0528.2012.03448.x. [DOI] [PubMed] [Google Scholar]
- 23.Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? BJOG. 2004;111(3):213–219. doi: 10.1111/j.1471-0528.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan N, anning WG, orris CN, ewhouse JP. A comparison of alternative models for the demand for medical care. J Bus Econ Stat. 1983;1:115–126. [Google Scholar]
- 26.Geraci M, Bottai M. Linear quantile mixed models. Statistics and Computing. 2014;24(3):461–479. [Google Scholar]
- 27.Aboulghar MM, Aboulghar MA, Amin YM, Al-Inany HG, Mansour RT, Serour GI. The use of vaginal natural progesterone for prevention of preterm birth in IVF/ICSI pregnancies. Reprod Biomed Online. 2012;25(2):133–138. doi: 10.1016/j.rbmo.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Briery CM, Veillon EW, Klauser CK, Martin RW, Chauhan SP, Magann EF, et al. Progesterone does not prevent preterm births in women with twins. South Med J. 2009;102(9):900–904. doi: 10.1097/SMJ.0b013e3181afee12. [DOI] [PubMed] [Google Scholar]
- 29.Combs CA, Garite T, Maurel K, Das A, Porto M. 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2011;204(3):221–228. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 30.Lim AC, Schuit E, Bloemenkamp K, Bernardus RE, Duvekot JJ, Erwich JJ, et al. 17alpha-hydroxyprogesterone caproate for the prevention of adverse neonatal outcome in multiple pregnancies: a randomized controlled trial. Obstet Gynecol. 2011;118(3):513–520. doi: 10.1097/AOG.0b013e31822ad6aa. [DOI] [PubMed] [Google Scholar]
- 31.Nassar AH, Awwad J, Usta IM. Prevention of Preterm Delivery in Twin Pregnancies by 17 Alpha-hydroxyprogesterone Caproate. BJOG. 2014 In press. [Google Scholar]
- 32.Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound Obstet Gynecol. 2011;38(3):272–280. doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 33.Senat M, Porcher R, Winer N, Vayssière C, Deruelle P, Capelle M, et al. Prevention of preterm delivery by 17 alpha-hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. Am J Obstet Gynecol. 2013;208(3):194, e1–8. doi: 10.1016/j.ajog.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 34.Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal Progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. J Perinat Med. 2012 doi: 10.1515/jpm-2012-0057. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Brizot M, Liao A. Prophylactic Administration of Natural Progesterone in the Prevention of Preterm Delivery in Twin Pregnancies. 2012 ClinicalTrials gov Identifier: NCT01031017.
- 36.Caritis SN, Simhan HN, Zhao Y, Rouse DJ, Peaceman AM, Sciscione A, et al. Relationship between 17-hydroxyprogesterone caproate concentrations and gestational age at delivery in twin gestation. Am J Obstet Gynecol. 2012;207(5):396, e1–8. doi: 10.1016/j.ajog.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit? Lancet. 2005;365(9456):341–346. doi: 10.1016/S0140-6736(05)17790-3. [DOI] [PubMed] [Google Scholar]
- 38.Clarke MJ. Individual patient data meta-analyses. Best Pract Res Clin Obstet Gynaecol. 2005;19(1):47–55. doi: 10.1016/j.bpobgyn.2004.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.