Abstract
While dose dependencies in pharmacokinetics and clearance are often observed in clinically used small molecules, very few studies have been dedicated to the understandings of potential dose-dependent in vivo transport of nanomedicines. Here we report that the pharmacokinetics and clearance of renal clearable gold nanoparticles (GS-AuNPs) are strongly dose-dependent once injection doses are above 15 mg/kg: high dose expedited the renal excretion and shortened the blood retention. As a result, the no-observed-adverse-effect-level (NOAEL) of GS-AuNPs was >1000 mg/kg in CD-1 mice. The efficient renal clearance and high compatibility can be translated to the non-human primates: no adverse effects were observed within 90 days after intravenous injection of 250 mg/kg GS-AuNPs. These fundamental understandings of dose effect on the in vivo transport of ultrasmall AuNPs open up a pathway to maximize their biomedical potentials and minimize their toxicity in the future clinical translation.
Keywords: biocompatibility, dose dependencies, nanoparticles, non-human primates, renal clearance
Graphical abstract
Faster renal clearance at higher doses renders renal clearable gold nanoparticles high biocompatibility in both mouse and non-human primate models.
Fundamental understandings of dose-dependencies in pharmacokinetics (PK) and clearance of imaging agents[1] and therapeutic drugs[2] are key to maximizing their potential and minimizing their toxicity in the clinics.[3] Many small molecules have shown strong dose-dependent PK and clearance in both animal models and patients.[4] For example, increasing dose of mezlocillin from 20 mg/kg to 200 mg/kg resulted in a 50% decrease in plasma clearance due to saturation of both biliary and renal excretion pathway.[4b] In contrast, MK-826, another small-molecule drug, exhibited 5-times faster plasma clearance when dose increased from 10 to 180 mg/kg because of concentration-dependent protein binding.[4a] Dose-dependent clearance was also observed from large PEGylated liposomal doxorubicin (Doxil): an 8-fold enhancement in dose from 2.5 to 20 mg/kg led to a 15-fold increase in plasma concentration, suggesting prolonged blood circulation at high doses.[5] Although dose-dependencies have been well recognized in small drug molecules, they are still largely unknown for engineered nanoparticles (NPs), which are partially because engineered NPs often severely accumulate in the reticuloendothelial system, limiting their clinical translation.[5–6] However, with the emergence of more and more renal clearable NPs that can behave like small drug molecules and be rapidly removed from the body through the urinary system[6c, 7], systematical investigations of whether and how injection doses affect their pharmacokinetics and clearance become extremely critical to both fundamental understanding of their in vivo transport and future clinical translation.
Unlike many renal-clearable engineered NPs such as quantum dots[6c] and silica NPs[7b], which are rapidly eliminated out of the body within a few hours, renal clearable gold nanoparticles (AuNPs) exhibit relatively slower elimination and have much longer blood retention. Within 24 h, only 50% of the 2 nm glutathione-coated AuNPs (GS-AuNPs) were excreted in the urine.[7c] The slow clearance of the AuNPs fundamentally originates from the density-dependent margination of engineered NPs in the laminar blood flow: the high-density AuNPs tend to marginate toward the blood vessel walls more quickly and be transported in the laminar blood flow more slowly than similar-sized low-density NPs.[8] While the slow clearance renders the AuNPs unique capabilities of passive tumor targeting through the enhanced permeability and retention (EPR) effect[9] and noninvasive fluorescence imaging of kidney dysfunction for a long period of time,[10] some critical questions regarding their future clinical translation are also naturally raised: (1) whether margination effect of the renal clearable AuNPs will result in any dose-dependent PK and clearance in the laminar blood flow; (2) whether the prolonged blood retention will induce any toxicity, in particular, any damage to the kidneys at high doses; (3) whether efficient renal clearance of the AuNPs observed in mice can be translated to the non-human primates, the first step towards the clinical translation of renal clearable AuNPs.
Herein, we report that strong dose-dependent PK and renal clearance of GS-AuNPs were observed once the doses is above 15 mg/kg: the higher dose resulted in more rapid renal clearance. Owing to the unique dose-dependent transport and clearance, no damages was observed on the glomerular filtration barrier of CD-1 mice even at a dose of 1059 mg/kg, higher than the maximal tolerated dose (MTD) of the highly biocompatible silica NPs (450 mg/kg, core size of 115 nm).[11] Furthermore, the efficient renal clearance and high biocompatibility of GS-AuNPs observed in mice can also be translated to monkeys, indicating a great potential in translating renal clearable AuNPs into the clinics.
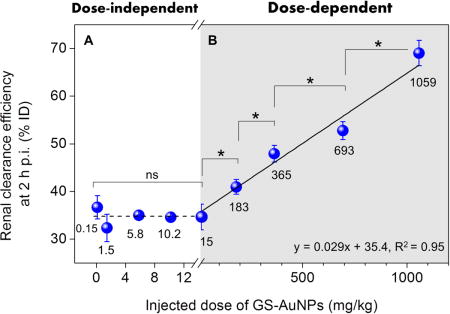
Near-infrared-emitting 2.5 nm GS-AuNPs were chosen for this study because of their great potential in cancer imaging[8–9, 12] and kidney functional imaging.[10, 13] The synthesis and characterization of GS-AuNPs has been reported in our previous work.[9] We first intravenously injected GS-AuNPs into the CD-1 mice at nine doses ranging from 0.15 to 1059 mg/kg and measured the renal clearance efficiency in the early elimination stage (2 h p.i.). The renal clearance of GS-AuNPs was dose-independent at low doses from 0.15 to 15 mg/kg: the 2-h renal clearance efficiency remains constant at ~35 %ID (Figure 1A&S1). However, with a dose increase from 15 to 183, 365, 693 and 1059 mg/kg, the 2-h renal clearance became linearly increased from 34.6 to 41.0, 47.9, 52.7 and 69.0 %ID (Figure 1B). The 24-h renal clearance showed the same trend as the results obtained at 2 h p.i. (Figure S2).
Figure 1.

Dose effect on renal clearance of 2.5 nm glutathione-coated gold nanoparticles (GS-AuNPs) after intravenous injection in CD-1 mice. (A) The renal clearance efficiency at 2 h post injection was independent of injected dose at a low-dose range from 0.15 to 15 mg/kg, (B) but it linearly increased from 34.6 to 69.0 percentage of injected dose (%ID) as dose increased from 15 to 1059 mg/kg (200 μL PBS solutions containing different amounts of NPs were injected; N = 3 for each group; *P < 0.05; ns, no significant difference, P > 0.05). For the injection doses at 5.8 and 10.2 mg/kg, the standard deviations of renal clearance efficiencies are too small to be seen clearly.The dose range of 0.15~1059 mg/kg was selected based on the minimum detection limit of ICP-MS and maximum solubility of GS-AuNPs in PBS. This figure is shown in a logarithmic scale in Figure S1.
To unravel whether the enhanced clearance is due to kidney damage, we investigated kidney function and kidney structure of the mice after being exposure to GS-AuNPs. We monitored the two renal function markers, blood urea nitrogen (BUN) and serum creatinine (sCr), following 14 days after intravenously injecting 15-1059 mg/kg GS-AuNPs into the mice. No significant changes in BUN and sCr levels were found from Day 1 to Day 14, compared to control mice injected with phosphate buffer saline (PBS). The sCr levels were normal for almost all the doses, except for a slight decrease in the group of 365 mg/kg at Day 1 (Figure 2A; P<0.05). Although transient decrease was also observed in BUN for doses at 15, 183, and 693 mg/kg at Day 7 (Figure 2B; P<0.05), BUN levels were normal at Day 1, 4, 10 and 14.
Figure 2.
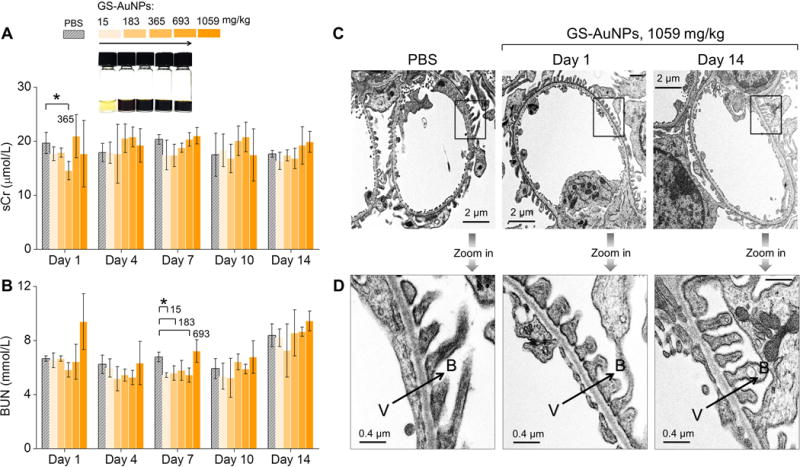
Evaluation of renal function and structure of kidney filtration barrier of CD-1 mice after intravenous injection of renal clearable GS-AuNPs at doses from 15 to 183, 365, 693 and 1059 mg/kg. (A) Levels of serum creatinine (sCr) and (B) blood urea nitrogen (BUN) were monitored within 14 days after NP injection. Mice receiving PBS served as control. N = 4 for each group. *P < 0.05. (C, D) Representative transmission electron microscopy (TEM) images of (C) glomeruli and (D) glomerular filtration membranes were taken from mice injected with 1059 mg/kg GS-AuNPs at Day 1 and Day 14. V, vascular lumen; B, Bowman’s space; arrows point the direction of filtration.
Using 70 kD dextran, of which the size is slightly larger than the kidney filtration threshold (~6 nm) as a marker[14], we found that there was no leakage of 70 kD dextran into urine after the mice receiving 1059 mg/kg GS-AuNPs (Figure S3), indicating that the glomerular barrier remains intact. In addition, as shown by transmission electron microscopy (TEM) imaging, the glomeruli (Figure 2C) and glomerular filtration membranes (Figure 2D) remained normal at Day 1 and Day 14 after injection of 1059 mg/kg GS-AuNPs (Figure S4). Moreover, no structural changes were found in both renal glomeruli and tubules at the histological level at Day 1, while some AuNPs accumulated in the lumen of renal tubule (Figure S5). At Day 14, the glomeruli were still normal; no tubular damage was found in two mice. For other two mice, only 5-10% of kidney tissue showed minor interstitial fibrosis and inflammation, tubular atrophy with few intratubular casts with entrapped NPs (Figure S6). These results clearly indicate that the observed enhancement in renal clearance of GS-AuNPs at high dose is a normal physiological response of the body rather than caused by the damages of the kidneys.
To further unravel the origin of the dose-dependent renal clearance of GS-AuNPs, we investigated the pharmacokinetics of GS-AuNPs at the three doses (15, 183 to 1059 mg/kg) (Figure 3A). The analysis of pharmacokinetics parameters revealed that plasma clearance (CL) of GS-AuNPs was increased from 1.31, 3.11 to 3.87 mL/h when the dose increased, corresponding to a decline of the area under the curve (AUC) from 76.5, 32.2 to 25.5 %ID•h/g (Figure 3B & S7; Table S1). These results suggested an enhanced plasma clearance of GS-AuNPs as dose increased, which was on contrary to Doxil and mezlocillin that shows decreased plasma clearance as dose increases.[4b, 5] It would be interesting to investigate renal clearance of GS-AuNPs at an even higher dose (>1059 mg/kg); however, limited by the solubility of GS-AuNPs in PBS, it is hard to study their renal clearance at even higher doses, which demands the development of more water-soluble renal clearable engineered nanoparticles in the future.
Figure 3.
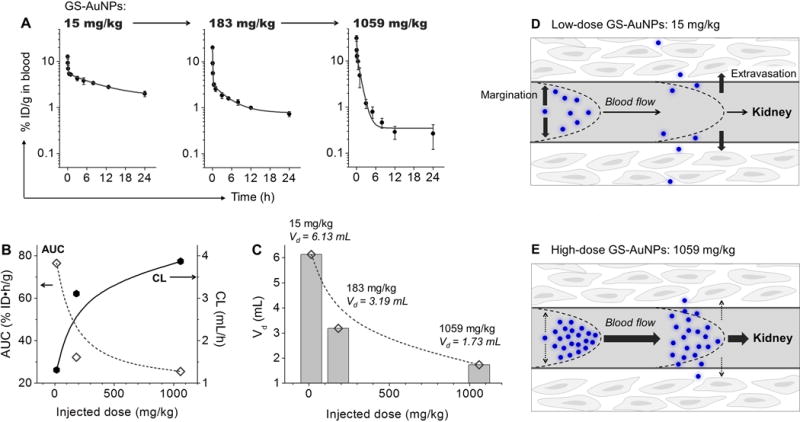
Dose dependency in pharmacokinetics of renal clearable GS-AuNPs after intravenous injection. (A) Blood pharmacokinetics at doses of 15, 183 and 1059 mg/kg. N = 3 for each group. (B, C) Changes of pharmacokinetics parameters with dose increased from 15 to 183 and 1059 mg/kg. AUC, the area under curve; CL, plasma clearance; Vd, volume of distribution. (D, E) Scheme of dose-dependent transport of GS-AuNPs in blood flow that affects blood pharmacokinetics and renal clearance. (D) At low doses, GS-AuNPs could easily marginate toward and then cross the blood vessel wall and enter into the extravascular space, resulting in large Vd and relatively slow clearance. (E) At high doses, rather than marginating toward the blood vessel wall and diffusing into the background tissues, GS-AuNPs tend to be confined in the blood vessels (small Vd); as a result, they were transported in the blood flow more rapidly and more efficiently eliminated through the kidney.
The enhanced plasma clearance of GS-AuNPs at higher doses originated from dose-dependent transport of NPs in blood flow. The volume of distribution (Vd) was decreased from 6.13, 3.19 to 1.73 mL as dose increased from 15, 183 to 1059 mg/kg (Figure 3C). The Vd of 6.13 mL (at 15 mg/kg) is comparable to the extracellular fluid volume of mice (~6 mL),[15] and the Vd of 1.73 mL (at 1059 mg/kg) becomes as small as the total plasma volume of mice (~1.56 mL; Table S2).[16] These data suggest that at low doses, GS-AuNPs could easily marginate and cross the blood vessel wall and enter into the extravascular space (large Vd), thereby dramatically reducing the blood concentration and slowing down the renal clearance process (Figure 3D). At high doses more GS-AuNPs were confined in the blood vessels (small Vd) rather than marginating to the blood vessel wall and diffusing into the background tissues; as a result, they were transported in the blood flow more rapidly and more efficiently eliminated through the kidney (Figure 3E).
Such unique dose-dependent transport and clearance of renal clearable AuNPs significantly reduced their toxicity in mice. The 24-h biodistribution study showed that the accumulation of GS-AuNPs in the kidney was 2-5 %ID per gram of tissue (%ID/g) at all doses; less than 2 %ID/g remained in other vital organs (liver, spleen, bone, lungs, heart and brain) (Figure S8). Histological analysis of liver, spleen, heart and lungs at Day 1 and Day 14 revealed no abnormalities after injection of 1059 mg/kg NPs (Figure S5&S6). Total accumulation of Au in major organs was less than 5 %ID at 14 days p.i. for all the five doses from 15 to 1059 mg/kg (Figure S9). During 1~14 days post injection, body weights of mice injected with 15~1059 mg/kg GS-AuNPs were similar to the control mice that received PBS (Figure S10). All these results suggest that the no-observed-adverse-effect-level (NOAEL) of GS-AuNPs is larger than 1000 mg/kg in CD-1 mice, 2~25 times higher than the maximal tolerated doses (MTDs) of the highly biocompatible silica NPs in CD-1 mice (30~450 mg/kg).[11]
The NOAEL of GS-AuNPs at >1000 mg/kg in mice suggests a NOAEL value of >250 mg/kg for monkeys (to convert mouse dose to monkey dose, the mouse dose is divided by 4.0).[17] We chose cynomolgus monkeys, a species often used in toxicity assessments of nanomedicines,[18] to study renal clearance and biocompatibility of GS-AuNPs. Three monkeys received a single injection of GS-AuNPs at 250 mg/kg body weight through intravenous drip. Consistent with the results in mice, we observed two-compartment blood pharmacokinetics of GS-AuNPs in monkeys after intravenous injection (Figure 4A). By measuring the amount of gold in urine and feces with ICP-AES and ICP-MS, we found that only 1.1 %ID was excreted in feces at 24 h p.i., and urine contained 48.6~65.4 %ID (varied among the three monkeys, Figure 4B), comparable to the renal clearance efficiency in CD-1 mice (43.3~71.3 %ID for 365~1059 mg/kg, 24 h p.i.). After circulating in the body and being excreted into the urine, GS-AuNPs maintained the same core size (2.5 ± 0.4 nm) as pre-injection samples (2.3 ± 0.3 nm; Figure S11), and Fourier transform infrared spectroscopy confirmed that glutathione remained on the particle surface (Figure S12). The GS-AuNPs excreted into urine also exhibited little changes in the absorption, excitation and emission spectra except for a slightly increase of 600 nm emission (Figure S13), indicating the surface ligand orientation might be slightly altered during the circulation in the physiological environment.[19]
Figure 4.
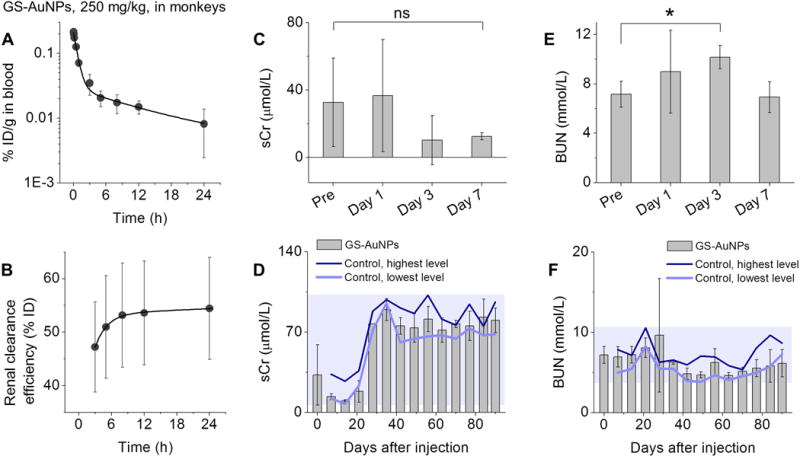
(A) Blood pharmacokinetics and (B) renal clearance of intravenously injected GS-AuNPs (250 mg/kg) in cynomolgus monkeys. Renal function markers, (C, D) serum creatinine (sCr) and (E, F) blood urea nitrogen (BUN) were monitored for 90 days after intravenous injection of GS-AuNPs (250 mg/kg). Monkeys without any treatment served as control. N = 3 for each group. *P < 0.05.
We then conducted a preliminary 90-day safety assessment of GS-AuNPs by performing serum biochemistry analysis, urinalysis, complete blood count and blood clotting tests every week. The results were compared with those of three monkeys without any treatment. No evidence of kidney damage was observed after exposure to 250 mg/kg GS-AuNPs. In the case of renal function biomarker sCr, we found no difference between NP-injected group and pre-injection level of the same monkeys at Day1, 3 and 7 (Figure 4C) as well as control group during 7-90 days (Figure 4D). The relatively large variation in sCr levels at pre-injection, Day 1 and Day 3 could be due to that the sCr is also affected by many other factors such as food intake and muscle mass.[20] While BUN transiently increased at Day 3 relative to pre-injection value (P<0.05; Figure 4E), it was normal at Day 1 and returned to a level comparable to pre-injection value at Day 7 (Figure 4E) and those of control group during 7~90 days (Figure 4F). No abnormalities were detected in urinalysis parameters (urine specific gravity, urine pH, urine protein and number of blood cells) during 90 days (Figure S14).
The liver function biomarkers—alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), triglycerides (TG), cholesterol (CHOL), total protein (TP), albumin (ALB), total protein (TP) and albumin (ALB)—were also in a normal range after NP injection relative to control group during 90 days p.i. (Figure S15&S16). While a lower level of alkaline phosphatase (ALP) was found in NP-injected monkeys during 7-90 days p.i., ALP is a less specific indicator of liver function[18b] and elevated ALP level is often observed after injection of NPs.[21] Parameters of complete blood count remained in a normal range, suggesting that GS-AuNPs did not induce systemic inflammation (Figure S17). Results of blood clotting tests confirmed that GS-AuNPs did not affect the coagulation system (Figure S18). Body weights and body temperatures were also monitored every week and no differences were found between NP-injected group and the control, implying minimal systemic toxicity of 250 mg/kg GS-AuNPs (Figure S19). No abnormal behaviors were observed after receiving GS-AuNPs. All these results indicated that GS-AuNPs were well tolerated by monkeys and did not induce adverse effects within 90 days following the single injection at 250 mg/kg. Therefore, the no-observed-adverse-effect-level (NOAEL) of GS-AuNPs shouldbe larger than 250 mg/kg in cynomolgus monkeys.
In summary, using CD-1 mice as model, we studied the dose effect on pharmacokinetics and clearance of renal clearable AuNPs in the range of 0.15-1059 mg/kg. The renal clearance efficiency remained at a constant level (~35 %ID) at doses ranging from 0.15 to 15 mg/kg. Taking advantage of this dose-independent renal clearance at low doses, we ruled out the dose effect when studying how particle size impacted the glomerular filtration of gold nanoclusters (100 μL, 100 μmol/L, 2~6 mg/kg) in a sub-nanometer regime.[7d] On the other hand, we observed a strong dose dependencies in PK and renal clearance at doses from 15-1059 mg/kg: As dose increased, renal clearance efficiency at 2 h p.i. linearly increased from 34.6 to 69.0 %ID, since more AuNPs were confined in the blood vessels and rapidly eliminated into the urine. The unique responses of the body to high doses of renal clearable AuNPs significantly reduce their toxicity: no structural changes in renal glomeruli and tubules of mice as well as low systematic toxicity were observed even at the dose of 1059 mg/kg AuNPs, which are higher than MTD value of the highly biocompatible silica NPs (450 mg/kg). This efficient renal clearance and high biocompatibility of the GS-AuNPs were also confirmed in non-human primates. These results significantly advance our fundamental understanding of in vivo transport of NPs at the different doses, lay down a foundation for optimizing the injected dose to maximize their potential in the future clinical practices.
Supplementary Material
Acknowledgments
The mouse studies were supported in part by the NIH (R01DK103363), CPRIT (140544 and 160866) and the start-up fund from the University of Texas at Dallas. The monkey studies were supported by the start-up fund from the University of Texas at Dallas. J.Z. would also like to thank Prof. Hua Shen, associate director of Kunming Institute of Zoology, Chinese Academy of Sciences and Prof. Shuo Shi, associate director of National Astronomical Observatories, Chinese Academy of Sciences for their help on the monkey studies.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a) Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, Wolf KJ, Weinmann HJ, Lange L. Radiology. 1995;195:785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]; b) Shamsi K, Balzer T, Saini S, Ros PR, Nelson RC, Carter EC, Tollerfield S, Niendorf HP. Radiology. 1998;206:365–371. doi: 10.1148/radiology.206.2.9457187. [DOI] [PubMed] [Google Scholar]
- 2.a) Reidenberg MM, Levy M, Warner H, Coutinho CB, Schwartz MA, Yu G, Cheripko J. Clin Pharmacol Ther. 1978;23:371–374. doi: 10.1002/cpt1978234371. [DOI] [PubMed] [Google Scholar]; b) Johnson HJ, Swan SK, Heim-Duthoy KL, Nicholls AJ, Tsina I, Tarnowski T. Clin Pharmacol Ther. 1998;63:512–518. doi: 10.1016/S0009-9236(98)90102-3. [DOI] [PubMed] [Google Scholar]
- 3.a) Tallarida R, Jacob LS. The Dose—Response Relation in Pharmacology. Springer Science & Business Media; 2012. [Google Scholar]; b) Katzung BG, Masters SB, Trevor AJ. Basic & clinical pharmacology. (12) 2004 [Google Scholar]; c) Nicholas H, Holford G, Sheiner LB. Clin Pharmacokinet. 1981;6:429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- 4.a) Wong BK, Bruhin PJ, Lin JH. J Pharm Sci. 1999;88:277–280. doi: 10.1021/js980232k. [DOI] [PubMed] [Google Scholar]; b) Jungbluth GL, Jusko WJ. Antimicrob Agents Chemother. 1989;33:839–843. doi: 10.1128/aac.33.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bernus I, Dickinson RG, Hooper WD, Eadie MJ. Epilepsy research. 1996;24:163–172. doi: 10.1016/0920-1211(96)00011-3. [DOI] [PubMed] [Google Scholar]; d) Cleveland PA, Teller S, Kachevsky V, Pinili E, Evans R, Modi MW. Drug Metab Dispos. 1991;19:245–250. [PubMed] [Google Scholar]
- 5.Gabizon A, Tzemach D, Mak L, Bronstein M, Horowitz AT. J Drug Target. 2002;10:539–548. doi: 10.1080/1061186021000072447. [DOI] [PubMed] [Google Scholar]
- 6.a) Baek M, Chung HE, Yu J, Lee JA, Kim TH, Oh JM, Lee WJ, Paek SM, Lee JK, Jeong J. Int J Nanomed. 2012;7:3081–3097. doi: 10.2147/IJN.S32593. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shinohara N, Oshima Y, Kobayashi T, Imatanaka N, Nakai M, Ichinose T, Sasaki T, Zhang G, Fukui H, Gamo M. Toxicology. 2014;325:1–11. doi: 10.1016/j.tox.2014.08.003. [DOI] [PubMed] [Google Scholar]; c) Choi H Soo, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Nat Biotech. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yu M, Zheng J. ACS Nano. 2015;9:6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Longmire M, Choyke PL, Kobayashi H. Nanomedicine (Lond) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Kang H, Gravier J, Bao K, Wada H, Lee JH, Baek Y, El Fakhri G, Gioux S, Rubin BP, Coll J-L, Choi HS. Adv Mater. 2016;28:8162–8168. doi: 10.1002/adma.201601101. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Nano Lett. 2008;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhou C, Long M, Qin Y, Sun X, Zheng J. Angew Chem Int Ed. 2011;50:3168–3172. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2011;123:3226–3230. [Google Scholar]; d) Du B, Jiang X, Das A, Zhou Q, Yu M, Jin R, Zheng J. Nat Nanotechnol. 2017;12:1096–1102. doi: 10.1038/nnano.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang S, Peng C, Xu J, Du B, Wang Q, Vinluan RD, Yu M, Kim MJ, Zheng J. Angew Chem Int Ed. 2016;55:16039–16043. doi: 10.1002/anie.201609043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:16273–16277. [Google Scholar]
- 9.Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J. J Am Chem Soc. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Yu M, Liu J, Ning X, Zheng J. Angew Chem Int Ed. 2015;54:15434–15438. doi: 10.1002/anie.201507868. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2015;127:15654–15658. [Google Scholar]; b) Yu M, Zhou J, Du B, Ning X, Authement C, Gandee L, Kapur P, Hsieh JT, Zheng J. Angew Chem Int Ed. 2016;55:2787–2791. doi: 10.1002/anie.201511148. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:2837–2841. [Google Scholar]
- 11.Yu T, Greish K, McGill LD, Ray A, Ghandehari H. ACS Nano. 2012;6:2289–2301. doi: 10.1021/nn2043803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Yu M, Zhou C, Liu L, Zhang S, Sun S, Hankins JD, Sun X, Zheng J. Angew Chem Int Ed. 2017;56:4314–4319. doi: 10.1002/anie.201612647. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2017;129:4378–4383. [Google Scholar]; b) Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J. Angew Chem Int Ed. 2013;52:12572–12576. doi: 10.1002/anie.201304465. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2013;125:12804–12808. [Google Scholar]; c) Peng C, Gao X, Xu J, Du B, Ning X, Tang S, Bachoo RM, Yu M, Ge WP, Zheng J. Nano Res. 2017;10:1366–1376. doi: 10.1007/s12274-017-1472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Yu M, Carter P, Hernandez E, Dang A, Kapur P, Hsieh J-T, Zheng J. Angew Chem Int Ed. 2017;56:13356–13360. doi: 10.1002/anie.201707819. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2017;129:13541–13545. [Google Scholar]
- 14.Long YS, Zheng S, Kralik PM, Benz FW, Epstein PN. J Diabetes Res. 2016;2016 doi: 10.1155/2016/8749417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman ME, Hu L, Plato CF, Kohan DE. Am J Physiol Renal Physiol. 2010;299:F280–F283. doi: 10.1152/ajprenal.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riches AC, Sharp JG, Thomas DB, Smith SV. J Physiol. 1973;228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair AB, Jacob S. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Kotb S, Piraquive J, Lamberton F, Lux F, Verset M, Di Cataldo V, Contamin H, Tillement O, Canet-Soulas E, Sancey L. Sci Rep. 2016;6:35053. doi: 10.1038/srep35053. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Moore L, Yang J, Lan TTH, Osawa E, Lee DK, Johnson WD, Xi J, Chow EKH, Ho D. ACS Nano. 2016;10:7385–7400. doi: 10.1021/acsnano.6b00839. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Duchesne PN, Yu M, Jiang X, Ning X, Vinluan RD, Zhang P, Zheng J. Angew Chem Int Ed. 2016;55:8894–8898. doi: 10.1002/anie.201602795. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:9040–9044. [Google Scholar]
- 20.Dharnidharka VR, Kwon C, Stevens G. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 21.a) Parivar K, Malekvand Fard F, Bayat M, Alavian SM, Motavaf M. Iran Red Crescent Med J l. 2016;18:e28939. doi: 10.5812/ircmj.28939. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang J, Wang L, Fan Y. Int l J Mol Sci. 2016;17:798. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


