Abstract
The ndh genes encoding for the subunits of NAD(P)H dehydrogenase complex represent the largest family of plastid genes without a clearly defined function. Tobacco (Nicotiana tabacum) plastid transformants were produced in which the ndhB gene was inactivated by replacing it with a mutant version possessing translational stops in the coding region. Western-blot analysis indicated that no functional NAD(P)H dehydrogenase complex can be assembled in the plastid transformants. Chlorophyll fluorescence measurements showed that dark reduction of the plastoquinone pool by stromal reductants was impaired in ndhB-inactivated plants. Both the phenotype and photosynthetic performance of the plastid transformants was completely normal under favorable conditions. However, an enhanced growth retardation of ndhB-inactivated plants was revealed under humidity stress conditions causing a moderate decline in photosynthesis via stomatal closure. This distinctive phenotype was mimicked under normal humidity by spraying plants with abscisic acid. Measurements of CO2 fixation demonstrated an enhanced decline in photosynthesis in the mutant plants under humidity stress, which could be restored to wild-type levels by elevating the external CO2 concentration. These results suggest that the plastid NAD(P)H:plastoquinone oxidoreductase in tobacco performs a significant physiological role by facilitating photosynthesis at moderate CO2 limitation.
Comparative analyses of the completely sequenced plastid genomes of such taxonomically distant plant species as liverwort (Ohyama et al., 1986), tobacco (Nicotiana tabacum; Shinozaki et al., 1986), and rice (Hiratsuka et al., 1989) has revealed a set of genes showing a surprising homology to subunits of the mitochondrial NADH dehydrogenase complex. This set of ndh genes proved to contain at least 11 members (Fearnley et al., 1989; Videira et al., 1990; Dupuis et al., 1991; Masui et al., 1991; Pilkington et al., 1991; Arizmendi et al., 1992), which are represented in all vascular plant divisions (Meng et al., 1986; Maier et al., 1995; Neyland and Urbatsch, 1996). The deduced amino acid sequence of the subunits of this plastid NAD(P)H dehydrogenase (NDH) complex shows significant homology with that of the corresponding subunits of the bacterial proton-pumping NADH:ubiquinone oxidoreductase and with the appropriate subunits of mammalian, fungal, and plant mitochondrial complex I (Fearnley and Walker, 1992; Weidner et al., 1993; Rasmusson et al., 1998). Subunits forming the highly conserved NADH-binding unit of complex I are apparently absent in the plastid NDH complex (Friedrich et al., 1995). However, this module might correspond to the additional, still uncharacterized (and presumably nuclear-encoded) subunits detected in the plastid NDH complex by biochemical methods (Quiles and Cuello, 1998; Sazanov et al., 1998b).
Plastid ndh genes are transcribed (Matsubayashi et al., 1987; Kanno and Hirai, 1993), the mRNAs are edited (Freyer et al., 1995; Maier et al., 1995), and the protein products of these genes are located in the stromal thylakoid membranes (Nixon et al., 1989; Berger et al., 1993; Kubicki et al., 1996). Expression of the various genes under different developmental and environmental conditions has been studied primarily in monocotyledonous plants (Kubicki et al., 1996; Martín et al., 1996; Catalá et al., 1997; Fischer et al., 1997). On the basis of western-blot analyses these investigators suggested that NDH proteins are primarily expressed in tissues of limited photosynthetic capacity. However, the expression of the ndhD gene in tobacco, measured as the extent of RNA editing creating the start codon, was restricted to chloroplasts and was highest in young, illuminated, photosynthetically active leaves (Hirose and Sugiura, 1997). Light activation of the thylakoidal NDH activity has also been recently demonstrated (Teicher and Scheller, 1998).
The longstanding question of the function of this putative NAD(P)H:plastoquinone (PQ) oxidoreductase in plastids has recently been tackled by targeted inactivation of several of the plastid-encoded ndh genes (ndhA, B, C, H, I, J, and K) in tobacco (Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998). A common feature of these NDH-inactivated plastid transformant plants is the absence of a transient increase in postillumination chlorophyll fluorescence, the presence of which is interpreted as a dark reduction of the PQ pool (Groom et al., 1993; Feild et al., 1998). On the basis of these results it was concluded that the NDH complex is functional in tobacco chloroplasts, mediating donation of electrons from a stromal reductant to the PQ pool in the dark (Burrows et al., 1998; Endo et al., 1998; Kofer et al., 1998; Sazanov et al., 1998a; Shikanai et al., 1998). These plastid transformant plants showed the normal characteristics of steady-state photosynthesis and, although water stress seemed to delay non-photochemical fluo-rescence quenching during induction of photosynthesis in certain mutants (Burrows et al., 1998), no NDH-specific phenotype was observed under normal or various stress conditions (Burrows et al., 1998; Sazanov et al., 1998a; Shikanai et al., 1998). In one laboratory the primary regenerants showed various abnormalities but neither linkage with the inactivated ndh genes nor cytoplasmic inheritance of these traits was demonstrated (Kofer et al., 1998). Therefore, the role of the NDH complex in the light reactions of photosynthesis and its physiological role in higher plants has remained hypothetical, and the various controversial conclusions are a matter of extensive discussion (Koop et al., 1998; Maliga and Nixon, 1998; Nixon and Maliga 1999; Roldán, 1999).
In the present study we have produced tobacco plastid transformants in which the ndhB gene was translationally inactivated. Preliminary data obtained on chlorophyll fluorescence transients under illumination showed marked differences between ndhB-inactivated and wild-type plants under anaerobic conditions (Cournac et al., 1998; Joët et al., 1998). Whereas this observation demonstrated the functioning of NDH complex during photosynthesis, since it was based on anaerobic conditions lacking both CO2 and O2, it was difficult to predict what natural physiological conditions might reveal a role for NDH. We show that under conditions that do not block but moderately inhibit photosynthesis by CO2 limitation, the lack of NDH activity results in an enhanced growth retardation of ndhB-inactivated tobacco plants in comparison with the wild type.
RESULTS
Targeted Inactivation of the Plastid ndhB Gene in Tobacco
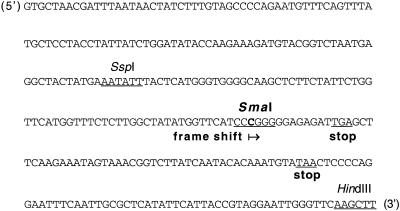
The ndhB is the only gene of the plastid ndh family located in the inverted repeat (IRA and IRB) region of the tobacco plastid genome (Shinozaki et al., 1986) and is most probably a part of the rps12(3′)-rps7-ndhB-trnL transcription unit (Matsubayashi et al., 1987; Kanno and Hirai, 1993). Inactivation of ndhB was accomplished by creating translational stop codons in the coding region of the gene. The pSSH1 plastid transformation plasmid contains a nightshade (Solanum nigrum L.) plastid DNA fragment and possesses mutations conferring spectinomycin and streptomycin insensitivity (Kavanagh et al., 1994, 1999). In the 7.8-kb inverted repeat region covered by the insert there is a 2.4% nucleotide sequence divergence between tobacco and nightshade plastid DNA (Kavanagh et al., 1999). The insert spans the first 732 nucleotides of ndhB (Wakasugi et al., 1998), which are identical in tobacco and nightshade. A single C was introduced into codon 206 of ndhB by oligonucleotide-directed mutagenesis of the plasmid in a region of the gene showing no editing site in tobacco (Freyer et al., 1995). This additional nucleotide generated a SmaI site and, in addition to a frame shift, stop codons (Fig. 1).
Figure 1.
Translational inactivation of the ndhB gene in the pSSH1 plastid transformation plasmid. The pSSH1 plasmid insert spans the first 732 bp of the ndhB gene. An additional C-G bp was introduced into codon 206 of ndhB by oligonucleotide-directed mutagenesis. The resulting plasmid was called pSSH1M. This additional nucleotide generated a diagnostic SmaI site and, in addition to a frame shift, all three stop codons (only two of which are shown). A 300-bp portion of the ndhB coding region (identical in tobacco and nightshade) adjacent to the HindIII cloning site is shown as it appears in IRA (5′–3′ direction, strand A), from position 143,798 in the tobacco plastid genome. Selected restriction enzyme sites are also shown.
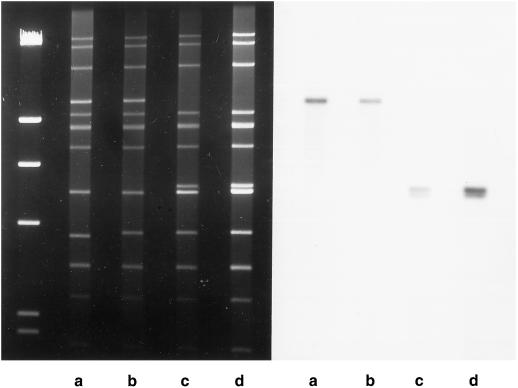
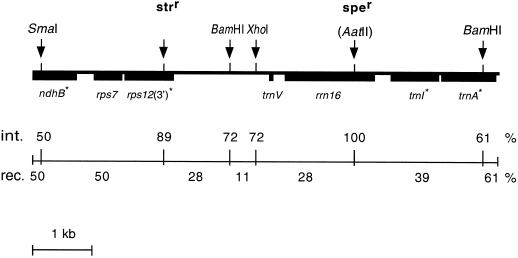
The mutant plasmid (pSSH1M) was introduced into tobacco protoplasts by polyethylene glycol treatment (O'Neill et al., 1993). Putative plastid transformant colonies were selected on the basis of their green color in a medium containing spectinomycin. The distinction between transformed and non-transformed tobacco plastids was facilitated, in addition to their insensitivity to streptomycin, by diagnostic RFLP differences in respect of the restriction enzymes SmaI, BamHI, XhoI, and AatII (Kavanagh et al., 1999). The selected lines comprised only 20% spontaneous spectinomycin-resistant mutants. On average one plastid transformant callus was selected in 104 viable colonies (or in 105 protoplasts treated). Each primary regenerant and its seed progeny was completely homoplasmic for the resistance markers. The polymorphic DNA regions (revealed as RFLPs between the donor and the recipient plastid DNA) were shown to be homoplasmic in all but one of the transformants demonstrating complete intraorganellar plastid DNA segregation after transformation (for representative SmaI patterns, see Fig. 3). A compilation of the RFLP and genetic markers in all of the 18 transformants revealed a high-frequency co-integration of the non-selected markers (Fig. 2). Nevertheless, the integration of the homeologous nightshade plastid DNA was mediated by multiple recombination events (Fig. 2). A schematic interpretation of the postulated recombination events following transformation of tobacco plastids with the pSSH1M plasmid in the individual transformants has been published elsewhere (Kavanagh et al., 1999). Because of the remarkably high recombination frequency in the 113-bp homologous peripheral region located between the SmaI site and the pUC19 vector (Fig. 2), one-half of the plastid transformants possessed the mutated (and putatively inactivated) ndhB gene.
Figure 3.
Site-specific inactivation of the ndhB gene in the plastid transformants. Gel electrophoresis of SmaI-digested plastid DNA of wild-type tobacco (a) and several plastid transformants (b–d) distinguishes a noninactivated transformant (b) from those possessing the inactivated ndhB gene (c–d). The smaller of the new, inactivation-specific fragments (5.68 and 5.45 kb) comigrates with the unchanged fragment number 9. On the left a HindIII digest of λ DNA is also shown (fragment sizes: 23.13, 9.42, 6.56, 4.36, 2.32, and 2.03 kb). Southern hybridization with a plastid DNA probe spanning the region containing the diagnostic restriction site in the 11.13-kb SmaI fragment number 4 reveals both the site-specificity and homoplasmy of the introduced mutation.
Figure 2.
Distribution of co-integration and recombination frequencies in the targeted region following transformation of tobacco plastids with the pSSH1M plasmid. The 7.8-kb donor insert of the pSSH1M plasmid is shown at the top of the figure. Arrows mark the location of the specific resistance and RFLP sites scored (brackets indicate the absence of the wild-type restriction enzyme site). Asterisks mark intron-containing genes. At the lower part of the figure the line is sectioned to show the major intervals between the donor-type marker sites investigated in the plastid transformants. The frequency of co-integration (int.) of the individual non-selected markers with the selected spectinomycin resistance locus is shown above the line. A 100% value represents the total number of spectinomycin-resistant transformants possessing a donor marker. The observed recombination frequency (rec.) in the individual internal sections, calculated as a percentage of the transformants recombined in the particular interval, is shown below the line. A 100% value represents the total number of transformants possessing a recombination event.
Molecular and Biochemical Analysis of the Inactivation of the NDH Complex
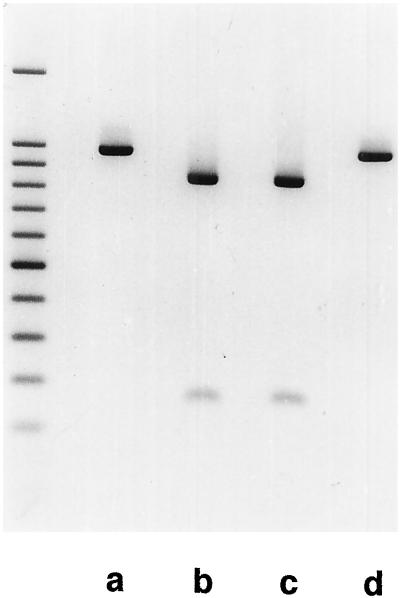
Homoplasmy of selected transformants for the mutation inactivating the ndhB gene was also verified by Southern hybridization and PCR analysis. Probing of SmaI-digested plastid DNA by a plastid DNA probe covering the diagnostic SmaI site revealed both the site-specificity and homoplasmy of the introduced mutation (Fig. 3). This was further verified by SmaI digestion of PCR products generated using isolated plastid DNA as template and primers flanking the diagnostic SmaI site on the plastid genome (Fig. 4). In the subsequent investigations, unless stated otherwise, both wild-type tobacco and a double-resistant tobacco plastid transformant (possessing the full nightshade insert of the original pSSH1 plasmid) were used as controls. Furthermore, in most of the investigations two types of ndhB-inactivated transformant were used: number 1.2 was resistant to both streptomycin and spectinomycin, whereas number 3.3 contained only the spectinomycin-insensitivity mutation.
Figure 4.
Homoplasmy of the plastid DNA population in the ndhB-inactivated transformants. Gel electrophoresis of SmaI-digested PCR product of wild-type tobacco (a), two ndhB-inactivated transformants (b and c), and a noninactivated transformant (d). The priming sites were located to cover the 5′ end of ndhB and flank the diagnostic SmaI site. The primer located inside the ndhB gene is outside the targeted plastid DNA region. The primers amplify a product of 966 bp, which is cut into 814- and 152-bp fragments by SmaI if the mutation introduced into ndhB is present. On the left a 100-bp DNA ladder is also shown (fragment sizes: 1,500 and 1,000–100 bp). The complete and correct cleavage of the PCR product in the ndhB-inactivated transformants reveals both the homoplasmy and site-specificity of the introduced mutation.
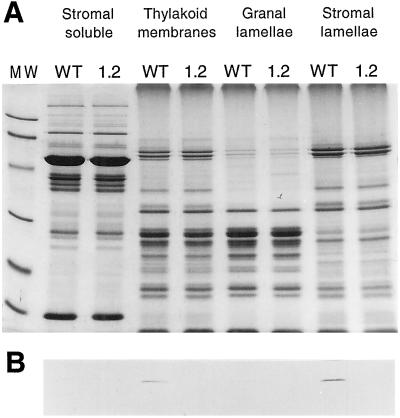
The fate of the NDH complex in the ndhB-inactivated plants was investigated by protein analysis. Fractioned chloroplast protein extracts were analyzed by PAGE and Coomassie Blue staining. No obvious difference was observed between the protein patterns of control (noninactivated transformant) and ndhB− plants (Fig. 5). As has been previously demonstrated (Burrows et al., 1998; Sazanov et al., 1998b) the NDH complex has a very low abundance in the thylakoid membrane. Attempts to produce a recombinant NDH-B polypeptide were not successful probably due to the high overall hydrophobicity of the NDH-B protein (Fearnley and Walker, 1992). Therefore, antibodies raised against NDH-H were used for western-blot analysis of chloroplast proteins. Immunostaining with the NDH-H antiserum confirmed the localization of NDH proteins in the stroma lamellae of thylakoid membranes (Nixon et al., 1989; Berger et al., 1993; Kubicki et al., 1996) in control plants (Fig. 5). However, the western-blot analysis detected no NDH-H protein in the ndhB− plastid transformant investigated (Fig. 5), indicating that no functional NDH complex can be assembled. This conclusion is supported by similar observations in other tobacco plastid transformants where the inactivation of one of the ndh genes resulted in disappearance of the other NDH subunits investigated, even if they were encoded by separate transcription units (Burrows et al., 1998; Kofer et al., 1998).
Figure 5.
Absence of the NDH-H subunit in the ndhB− plastid transformants. Separation of protein fractions (20 mg of protein per lane) derived from purified chloroplasts by fully denaturating PAGE reveals no obvious difference after Coomassie Brilliant Blue staining between wild-type (WT) and ndhB− (1.2) tobacco plants. Western hybridization of the separated protein fractions electrotransferred onto nitrocellulose membranes by an anti-NDH-H antibody detects the protein in total thylakoid membranes and the stroma lamellae of the wild type. The absence of detectable NDH-H protein in the ndhB− mutant indicates that no functional NDH complex can be assembled. Molecular mass markers: 96, 66.2, 45, 31, 21.5, and 14.4 kD.
Growth and Photosynthetic Performance of the NDH-Inactivated Transformants under Normal Conditions
Plastid transformants with a wild-type or an inactivated ndhB gene showed no visible NDH-specific phenotype under standard growth conditions either when cultured in vitro (autotrophically or on sugar-containing medium) or grown in soil in the greenhouse. Several of the primary regenerants showed morphological abnormalities (e.g. slow growth, distorted leaves, and poor pollen production) typical of chromosomal aneuploidy detected regularly in a certain percentage of tobacco plants regenerated from cell culture (Thanh et al., 1988). These morphological deviations did not show maternal inheritance and were not observed in the progeny obtained after pollination with wild-type tobacco. Transformant plants derived from three crosses with wild-type tobacco were analyzed in detail. No developmental deviation was observed in ndhB− plants compared with wild type from seed germination to seed set, including aging.
Measurements of photosynthetic CO2 fixation or O2 evolution in plants grown either in vitro or in soil showed no significant difference in steady-state photosynthetic rates between controls and ndhB− mutants. The in vivo light dependence curves of photosynthetic oxygen evolution were similar in control and ndhB− plants, indicating that neither the maximum efficiency nor the light-saturated capacity were affected in intact leaves under normal conditions (data not shown). Chlorophyll fluorescence measurements performed during dark-light-dark transitions indicated no difference in photochemical and non-photochemical quenching processes between control and ndhB− plants (Cournac et al., 1998). In these fluorescence measurements, following light extinction, a transitory and slow increase in the fluorescence level, before reaching the F0 level, was observed in control plants. This phenomenon, generally ascribed to the dark reduction of the PQ pool by stromal reductants like NADPH or NADH (Groom et al., 1993), was not observed in ndhB− transformants (Cournac et al., 1998), indicating that stromal reductants in the dark do not reduce the PQ pool in the absence of the ndhB gene product. This impaired non-photochemical PQ reduction is apparently a standard feature of ndh gene-inactivated mutants (Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998) and is the primary basis for the conclusion that the NDH complex is functional in chloroplasts (see the introduction). However the absence of any obvious phenotype in our ndhB− mutants, as was reported for other ndhB− mutants (Shikanai et al., 1998), supports the conclusion that under favorable growth conditions NDH function is dispensable. All these data prompted an extensive search for potential NDH function-specific stress conditions.
A Decrease in Air Humidity Generates a Discriminating Phenotype in NDH-Inactivated Transformants
We have investigated the effect of various stress conditions that have been proposed to stimulate cyclic electron transport activity (Heber and Walker, 1992; Fork and Herbert, 1993). The effect of a moderately long light stress (5 h, 700 μmol m−2 s−1) on light dependence curves of photosynthetic O2 evolution were measured in in vitro-grown ndhB-inactivated and noninactivated transformants. Neither the maximum efficiency nor the light-saturated capacity of photosynthetic O2 evolution were differentially affected in intact leaves (data not shown). The effect of light stress on soil-grown plants was investigated in growth chambers. No significant difference was observed in photosynthesis performance (measured as whole-plant CO2 fixation) of ndhB-inactivated and noninactivated transformants grown under an illumination of 1,200 μmol m−2 s−1 (16-h day) for 2 weeks (data not shown).
The effect of water stress (by severely limiting or withholding water) was also investigated on control and ndhB− plants grown under normal phytotron conditions. Under such stress conditions both types of plants showed a similarly strong (gradual or immediate) inhibition of vegetative development, in addition to a similar degree of wilting, yellowing, and withering of the older leaves. However, natural drought typically involves not only a soil water deficiency but also a dry atmosphere. Therefore, in another experiment we investigated the effect of low air humidity (30 relative %) on well-watered plants in a growth chamber. A few days of growth under these conditions surprisingly resulted in a remarkable growth difference between wild-type and ndhB− plants (for a representative pair of plants, see Fig. 6). After a 5-d-long growth period in low air humidity both fresh and dry weights of ndhB− plants (19.04 ± 2.05 and 1.38 ± 0.10 g, respectively) were almost 20% lower than those of wild-type plants (22.83 ± 1.16 and 1.70 ± 0.03 g, respectively). The enhanced growth retardation of ndhB− plants was visible primarily as a reduced growth of the young, expanding leaves (Fig. 6). No other phenotypic difference between the mutant and wild-type plants was detected in this experiment. The increased sensitivity to air humidity of mutants with a non-functional NDH complex prompted an analysis of gas exchange under humidity stress.
Figure 6.
Enhanced growth delay of the ndhB− plastid transformants under humidity stress. Well-watered wild-type (left) and ndhB− (right) plants were grown in low air humidity (30% and 40% relative humidity during the day and the night, respectively), following a month of growth under normal conditions (60% relative humidity). A visible growth difference was developed in less than a week under humidity stress. The development of freshly expanding leaves was specifically hindered in ndhB− plants.
Enhanced Reduction of Photosynthesis under Conditions Generating Moderate Stomatal Closure in NDH-Inactivated Transformants
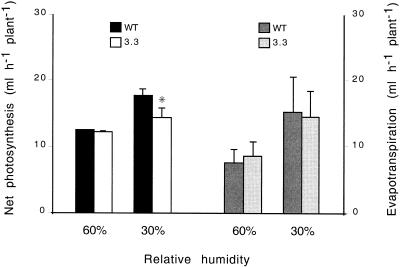
Measurement of total CO2 exchange by intact soil-grown plants was recorded continuously in computer-controlled growth chambers. The vigorous development of young wild-type tobacco plantlets in normal air humidity (60 relative %) was manifested as a steady day-by-day increase in the levels of whole-plant CO2 fixation, whereas under conditions of low air humidity (30 relative %) photosynthetic development was considerably delayed (data not shown). Under normal growth conditions both wild-type and ndhB− plants displayed similar photosynthetic activity. In contrast, after a transition to low air humidity ndhB− plants showed a reduced photosynthesis that was up to 20% lower than that of wild-type plants (Fig. 7). These results indicated a primary role of humidity stress in triggering the cascade of events leading to a differential decline in photosynthesis and, concomitantly, growth. Therefore, the direct role of stomatal closure in generating the mutant-specific phenotype was investigated. Stomatal closure was induced in well-watered plants grown in normal air humidity by spraying with abscisic acid (ABA). A moderate treatment (spraying with a 10-μm ABA solution every 2nd d) resulted in a clearly visible growth difference between wild-type and mutant plants under normal phytotron conditions (Fig. 8). After a 2-week-long ABA treatment both fresh and dry weights of ndhB− plants (18.40 ± 1.20 and 0.93 ± 0.11 g, respectively) were almost 25% lower than those of wild-type plants (23.84 ± 3.60 and 1.25 ± 0.27 g, respectively). Similar to the effect of humidity stress, the differential phenotype appeared primarily as a reduced growth of the young, expanding leaves of ndhB− plants (Fig. 8). It was notable that a strong ABA treatment (daily spraying with 20 μm ABA) resulted in a strong but non-differential growth inhibition of both wild-type and mutant plants, similar to that caused by water stress (data not shown). These results suggested that a moderate increase in stomatal resistance is an important intermediary in the process leading to a decline in photosynthesis in low air humidity. Therefore, we measured stomatal conductance changes occurring during the transition from high (75 relative %) to low (30 relative %) air humidity on the basis of gas exchange measurements on attached leaves. These investigations demonstrated a moderate and similar decline in leaf conductance in ndhB− mutants (from 22.73 ± 7.29 to 16.05 ± 6.13 mmol m−2 s−1) and the wild type (from 23.61 ± 7.30 to 17.08 ± 5.58 mmol m−2 s−1). In line with these results whole-plant evapotranspiration rates of ndhB− and wild-type plants measured in growth chambers showed a similar increase after a transition to low air humidity (Fig. 7). We have concluded from these observations that reduced CO2 availability, due to the (non-differential) stomatal response to low air humidity, is the principle factor generating the mutant-specific phenotype.
Figure 7.
Differential reduction in photosynthesis of wild-type and NDH-inactivated plants grown in low air humidity. Photosynthesis and evapotranspiration of 5-week-old wild-type and ndhB− plants are shown in normal (60 relative %) and low (30 relative %) air humidity on the day preceding and following the humidity transition, respectively. The whole-plant photosynthesis and evapotranspiration values recorded in one experiment were the sum of four to eight plants of the same type grown in one computer-controlled growth chamber recording CO2 consumption and water vapor condensation. On the left net photosynthesis calculated for one plant is displayed as the mean ± sd of four independent experiments. On the right evapotranspiration calculated for one plant is displayed as the mean ± sd of four independent experiments. Asterisk indicates significant differences from controls (P < 0.05). Low air humidity generated a difference in photosynthesis of ndhB− and wild-type plants, demonstrating an enhanced sensitivity of the ndhB− transformants to humidity stress. Evapotranspiration of ndhB− and wild-type plants showed a similar response, indicating that their stomata responded non-differentially to low air humidity.
Figure 8.
ABA treatment provokes the mutant-specific stress phenotype under normal growth conditions. Wild-type (left) and ndhB− (right) plants grown for a month under normal phytotron conditions were subsequently sprayed with 10 μm ABA solution. The growth difference was developed during 2 weeks of spraying of the leaves every 2nd d. The development of freshly expanding leaves was specifically hindered in ndhB− plants.
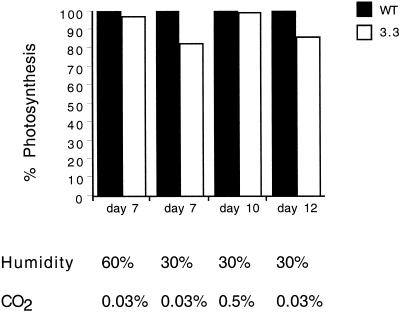
Our conclusion that a decline in internal CO2 concentration is the key element in the cascade of events leading to the differential inhibition of photosynthesis was further investigated by photosynthesis measurements on plants simultaneously grown in low air humidity and elevated external CO2 concentration. Low air humidity triggered a greater decrease in the photosynthetic capacity of ndhB− plants in comparison with that of wild-type plants. However, increasing the ambient CO2 level to 0.5% resulted in the disappearance of the difference in photosynthesis levels while markedly increasing overall levels in both types of plant (Fig. 9). When the ambient CO2 concentration was returned to normal, the differential effect of low air humidity on photosynthesis levels re-appeared. The above data strongly support the view that the differential effect of low air humidity on NDH-inactivated plants is implemented by a differential sensitivity of photosynthesis to limiting CO2 availability. Our observations also demonstrate that the lack of a functional NDH complex is primarily manifested at a level of CO2 limitation that does not strongly inhibit the growth and photosynthetic development of wild-type plants.
Figure 9.
Elevated external CO2 concentration complements the differential photosynthesis reduction generated by humidity stress. Five-week-old wild-type and ndhB− plantlets were grown under normal conditions in computer-controlled growth chambers recording CO2 exchange. The effect of humidity stress (caused by decreasing the relative air humidity from 60% to 30%) and the additional effect of elevated CO2 concentration (from ambient to 0.5%) was tested. Whole-plant photosynthesis in one chamber during illumination was recorded as the CO2 consumption of six plants of the same type. Relative photosynthesis values are displayed on the y axis. For ease of comparison, photosynthesis of wild-type plants was taken to be 100%. The mean wild-type absolute photosynthesis values (from left to right) were the following: 15.55, 18.06, 54.26, and 31.51 mL−1 h−1 plant−1. The relative decrease in whole-plant photosynthesis in NDH-inactivated plants was fully compensated during the transitory elevation of the CO2 level. This result pinpoints limitation of CO2 availability as a direct cause of the differential reduction in photosynthesis in wild-type and ndhB− transformant plants under humidity stress conditions.
DISCUSSION
The low abundance of the chloroplast NDH complex (Burrows et al., 1998; Sazanov et al., 1998b) has hampered investigations into its molecular and physiological role. However, the recent application of plastid transformation techniques, which permit targeted inactivation of individual ndh genes, has greatly facilitated these investigations (see the introduction). In these experiments insertional mutagenesis or deletion of the gene was achieved via site-specific integration of a dominant selectable marker gene (Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998). Our experiments demonstrate that the ndhB gene can also be efficiently inactivated using a different strategy: translational inactivation by replacement of the wild-type plastid ndhB gene with a frame-shifted mutant produced by site-directed mutagenesis. In this approach a single nucleotide change was introduced into a cloned copy of the ndhB gene, which was located on the same DNA fragment several kilobase pair distant from a binding-type antibiotic insensitivity mutation in the rrn16 gene. The latter gene was then used to select for plastid transformants in which both mutant genes had replaced their wild-type counterparts on the plastid genome. In our experiments the mutated ndhB gene was located close to the end of the transforming DNA (0.1 kb from the junction with vector DNA) and at a distance of 5 kb from the spectinomycin insensitivity mutation used for selection purposes. Nevertheless, in 50% of the transformants the unselected mutation was co-integrated with the selectable marker and yielded a homozygous population of ndhB-inactivated plastid DNA, indicating high local recombination frequencies near the vector-insert junction. In homeologous plastid transformation experiments in Nicotiana sp. this phenomenon routinely results in recombination/integration frequencies up to 10 times higher than expected at the ends of the plastid DNA insert (Kavanagh et al., 1999). An additional remarkable observation was that our experiments revealed transformation efficiencies typical of those found in homologous plastid DNA transformations (Golds et al., 1993; O'Neill et al., 1993), despite the 2.4% nucleotide sequence divergence between tobacco and nightshade plastid DNA in the transformed region. This observation indicates the prevalence of a RecA-type homeologous recombination mechanism in higher plant plastids (for discussion, see Kavanagh et al., 1999) and suggests that plastid transformation vectors directed to this region do not need to be species specific, at least for species that show a similarly low degree of nucleotide sequence divergence.
The absence of any obvious specific phenotype in the ndhB-inactivated transformants when grown under favorable conditions supports earlier conclusions concerning the dispensability of NDH function (Burrows et al., 1998; Shikanai et al., 1998). In other experiments various abnormalities of ndh-inactivated primary regenerants were detected (Kofer et al., 1998), but since their linkage with the inactivated gene was not demonstrated, tissue culture effects cannot be excluded as a plausible explanation of the morphological deviations (Maliga and Nixon, 1998). We have shown that under conditions where air humidity is decreased or when plants are sprayed with ABA, photosynthesis is more significantly reduced in ndhB-inactivated transformants than in wild-type plants, and this effect on photosynthesis causes a corresponding reduction in biomass. This phenotypic difference was suppressed when ambient CO2 concentration was increased, thus showing that it is likely mediated by stomatal closure triggered either by low air humidity or by ABA treatment (Downton et al., 1988; Robinson et al., 1988; Willmer and Fricker, 1996). Since no differences in transpiration rates were observed in either whole plants or leaves of controls and ndhB− mutants in response to changes in air humidity, we conclude that stomatal regulation was not affected in the mutant plants. Therefore, low internal CO2 concentration resulting from partial stomatal closure is likely responsible for the observed phenotypic difference. In previous investigations ndh− mutants have been reported to display a reduced non-photochemical quenching of fluo-rescence during photosynthesis induction under water stress conditions (Burrows et al., 1998). However, no distinctive visible phenotype was observed by the authors in response to water stress (Burrows et al., 1998). We also observed that in conditions where stomatal closure is pronounced, which occurred in response either to a severe limitation in water supply or to spraying with a high ABA concentration, there was no phenotypic difference. This can be explained by the fact that under such conditions net photosynthesis and growth are so strongly inhibited in both types of plants that the presence or absence of a functional NDH complex has no discernible effect. This is in line with the observations that water stress decreases photosynthetic assimilation of CO2 by metabolic inhibition (Tezara et al., 1999) and that fully functional photosynthesis is required for the humidity dependence of CO2 assimilation to be manifested (Stitt et al., 1991). We conclude from our experiments that moderate inhibition of photosynthesis by CO2 limitation can trigger a phenotypic difference between wild-type and ndh-inactivated plants.
Several processes are part of the photosynthetic controls that coordinate the synthesis of ATP and NADPH with their rate of use in carbon metabolism (Foyer et al., 1990; Heber and Walker, 1992). Metabolic demands can often require that light-dependent ATP production be increased relative to NADP reduction. Inhibition of the linear electron flow can occur if there is an imbalance between the stoichiometry of ATP/NADPH production and consumption. A common feature of the NDH-inactivated tobacco plants is the disappearance of a dark transient increase in fluorescence after illumination (Burrows et al., 1998; Cournac et al., 1998; Kofer et al., 1998; Shikanai et al., 1998). This phenomenon has been ascribed to a dark reduction of the PQ pool by stromal reductants (Groom et al., 1993) and has been considered to be an after-effect of a light-dependent process, i.e. cyclic electron transport around photosystem I (Burrows et al., 1998; Shikanai et al., 1998). This auxiliary electron flow may modulate the ATP to NAD(P)H ratio by participating in the redox control of the PQ pool. However, different pathways of cyclic electron flow around photosystem I have been suggested to occur in chloroplasts, which are considered likely to involve the NDH complex or a ferredoxin:PQ oxidoreductase activity (Ravenel et al., 1994; Endo et al., 1997). Also, the recovery of the postillumination fluorescence increase in NDH-inactivated plants under certain stress or developmental conditions (Sazanov et al., 1998a; Shikanai et al., 1998) supports the existence of alternative pathways involved in non-photochemical PQ reduction. The absence of a phenotypic difference between wild-type and ndhB-inactivated plants when grown under normal conditions suggests that the alternative mechanisms can generate sufficient extra ATP. In contrast, under conditions where CO2 availability decreases due to moderate stomatal closure, a differential phenotype is observed. Under such conditions photorespiratory activity is increased due to competition between CO2 and O2 at the Rubisco catalytic site. It has been reported that the requirement for ATP is increased during photorespiration (Osmond, 1981). Therefore, we propose that the alternative pathways involved in the production of extra ATP are not efficient enough to fulfill the higher ATP demand of active photosynthesis when photorespiration is operating at a high rate. This would explain why the phenotypic differences between wild-type and NDH-inactivated plants are observed only under conditions that result in moderate CO2 limitation.
Field-grown plants typically experience extensive periods during which the evaporative demand exceeds the water supply. These conditions can occur in the absence of severe soil water deficiency, e.g. because of fluctuating water inputs from rainfall or irrigation in a dry atmosphere. Under such conditions, the sensitive response of stomata to humidity as the environmental evaporative demand changes provides an efficient means by which tissue water deficits can be avoided. As a consequence, photosynthetic tissues will be subjected to partial stomatal closure for extensive time periods. Under these conditions, in which photosynthesis is limited by CO2 availability, extra ATP production through an NAD(P)H:PQ oxidoreductase-dependent pathway may confer a selective advantage of sufficient magnitude to explain the conservation of plastid ndh genes during the course of evolution.
MATERIALS AND METHODS
Plasmid Construction
The pSSH1 plasmid (Kavanagh et al., 1999) contains a 7.8-kb HindIII fragment cloned from a black nightshade (Solanum nigrum) plastid double mutant (McCabe et al., 1989; Kavanagh et al., 1994). The pSSH1 mutations confer spectinomycin and streptomycin insensitivity and are located in the rrn16 and the rps12(3′) genes, respectively. A 732-bp initial portion of the ndhB gene is located at one end of the cloned cpDNA insert. The nucleotide sequence of this region of ndhB is identical in both tobacco (Nicotiana tabacum) (Shinozaki et al., 1986; GenBank accession no. Z00044) and nightshade (Kavanagh et al., 1999; EMBL accession no. Y18934). Oligonucleotide-directed mutagenesis of the ndhB gene was performed using the in vitro mutagenesis system (Altered Sites II, Promega, Madison, WI). The HindIII fragment from pSSH1 was cloned into the pAlter-1 vector and was mutagenized using the following mutagenic oligonucleotide: 5′-AATCTCTCCCCCGGGATGAACCATA-3′.
Plastid Transformation
Tobacco (N. tabacum L. cv Petit Havana) was maintained as shoot cultures on agar-solidified Murashige and Skoog medium (Murashige and Skoog, 1962) in the light (50 μmol m−2 s−1, 16-h day, 25°C). Polyethylene glycol-mediated plastid transformation was performed as described (O'Neill et al., 1993). The selective medium contained 1,000 mg L−1 spectinomycin dihydrochloride. Plants were regenerated from the resistant colonies, and leaf calli and seedlings were tested for their resistance as described (Cséplő, 1994; Medgyesy, 1994). In the resistance tests spectinomycin dihydrochloride and streptomycin sulfate were used separately at 1,000 mg L−1 each.
Plastid DNA Analysis
Chloroplasts were isolated from aseptically grown plants according to Bookjans et al. (1984). Lysis of chloroplasts, the purification of DNA, and the RFLP analysis followed standard protocols (Sambrook et al., 1989). Non-radioactive Southern hybridization was performed using the DIG DNA Labeling and Detection Kit (Boehringer Mannheim, Mannheim, Germany). SmaI-digested plastid DNA separated by horizontal agarose slab-gel electrophoresis and visualized by ethidium bromide staining was denatured and bound to positively charged nylon membranes (Hybond N+, Amersham, Arlington Heights, IL) according to standard protocols. The probe was a 483-bp NcoI-BsrGI fragment of tobacco plastid DNA, which covered the diagnostic SmaI site. The nucleotide sequence of oligonucleotide primers that were used for PCR analysis of plastid transformants together with their position in IRB within the tobacco plastid genome is as follows: 5′-ACGTCAGGAGTCCATTGATGA-3′ (98,495–98,515) and 5′-CGAAACAAACGAAAAGGAAAG-3′ (99,459–99,439). Efficient amplification was achieved using approximately 20 ng of plastid DNA in a 20-μL reaction using the PCR System of Fermentas (Vilnius, Lithuania) and the following cycle parameters: 94°C, 30 s; 55°C, 30 s; 72°C, 30 s; 25 cycles.
Preparation of Antibody against NDH-H
The tobacco ndhH gene that extends from nucleotide 123,672 to 124,910 (Shinozaki et al., 1986; GenBank accession no. Z00044) was PCR-amplified using Pfu polymerase (Stratagene, La Jolla, CA) and cloned by blunt-end ligation into the SmaI site of pGEX4-T3 (Pharmacia Biotech, Uppsala). The recombinant plasmid was transformed into the Escherichia coli strain DH5α (Gibco-BRL, Cergy Pontoise, France). The resulting clones were sequenced to ensure in-frame fusion of ndhH with the glutathione-S-transferase gene and to avoid clones that contained PCR-generated mutations. Overexpression of the GST−NDH-H fusion protein was induced with 50 μm isopropylthio-β-galactoside for 5 h at 37°C. Bacterial cultures were pelleted by centrifugation (2,000g, 10 min) and resuspended in a buffer containing 50 mm Tris-HCl (pH 8.0), 1 mm Na2-EDTA, and 100 mm NaCl. Fusion protein was extracted from inclusion bodies by standard procedure (Sambrook et al., 1989) and separated by SDS-PAGE. The relevant band was excised from the gel and the protein was electroeluted. Antiserum was raised against the fusion protein in rabbit (Bioenvirotech, Marseille, France).
Preparation of Thylakoid Membranes
Intact chloroplasts were isolated and purified from leaves using discontinuous Percoll (Pharmacia Biotech) gradients as described (Rumeau et al., 1996). Chloroplasts were osmotically lysed in MNM solution containing 20 mm MES (2-[N-morpholino]-ethanesulfonic acid), pH 6.0, 15 mm NaCl, and 5 mm MgCl2, and centrifuged for 20 min at 35,000g. The supernatant fraction comprised stromal soluble proteins. Stromal and grana lamellae were separated following a stacking step carried out as described by Sazanov et al. (1998b). Briefly, thylakoid membranes were allowed to stack for 1 h and then solubilized by adding n-dodecyl-β-d-maltoside dropwise to 1% (w/v) with constant stirring. After incubation (30 min) insoluble material was removed by centrifugation at 1,000g for 2 min, and the different fractions were recovered by differential centrifugation. Grana thylakoids were recovered by centrifugation at 10,000g for 30 min and stroma thylakoids by centrifugation at 150,000g for 1 h.
PAGE and Immunodetection
Denaturing SDS-PAGE was performed as described by Laemmli (1970) using 13% (w/v) acrylamide gels. Proteins were either stained with Coomassie Brilliant Blue or electrotransferred onto 0.45-μm nitrocellulose membranes (Schleicher & Schuell, Keene, NH) and probed with NDH-H antibodies. Immunocomplexes were detected using alkaline phosphatase-conjugated antibodies.
O2 Evolution Measurements in Plants Cultured in Vitro
Seedling-derived plants were grown in vitro as described (Peter et al., 1999). The leaf discs were collected from plantlets grown under 60 μmol m−2 s−1 photon flux density (PFD), illuminated by cool-white fluorescence tubes. The effect of light stress was analyzed by exposing the culture vessels to a PFD of approximately 700 μmol m−2 s−1. The light was provided by low-voltage (12-V/50-W) multi-mirror halogen lamps (Precise, General Electric, Fairfield, CT). Three heat filters (Tempax, Schott, Cologne, Germany) and a water filter (10-cm height, 13°C) were placed between the culture vessel and the light source to minimize any temperature increase in the vessel. Light dependence curves of net oxygen evolution were measured at 25°C, approximately 2% (v/v) CO2 (carbonate/bicarbonate buffer), with a leaf disc oxygen electrode (LD2, Hansatech, King's Lynn, UK) and a pulse-amplitude-modulation fluorometer (PAM, Walz, Effeltrich, Germany). The leaf discs were exposed to alternating light periods (7.5 min) and dark periods (5 min), and the PFD was raised at each light period up to 400 μmol m−2 s−2. Gross photosynthesis was calculated from the difference of O2 evolution rate in the light and in the consecutive dark period.
Leaf Conductance Measurements in Soil-Grown Plants
CO2 and H2O exchange of attached leaves of soil-grown plants under humidity transition was measured in an air-tight chamber. Relative humidity (75% and 30%) was set by generating moist air using a portable dew point generator (LI-610, LI-COR, Lincoln, NE) at a flow rate of 2 mL s−1. The moist air was drawn into a leaf clip (PLC model, Ppsystem, Hotchin, UK), equipped with leaf ventillation, thermistor air temperature measurement, and infrared sensor leaf temperature measurement. A gas mixer (SEMY Engineering, Montpellier, France) was used to generate gas mixture with a defined CO2 and O2 concentration. CO2 and O2 concentrations were measured using an infrared gas analyzer (LI-6262, LI-COR, Lincoln, NE) and an oxygen analyzer (OXOR 6N, Maihak, Hamburg, Germany). CO2 and H2O exchange was measured by monitoring air humidity and CO2 concentration changes in air between the inlet and outlet of the chamber. Standard calculations were used to determine stomatal conductance (Farquhar and Sharkey, 1982).
Whole-Plant Photosynthesis and Growth Measurements
Three-week-old in vitro-grown seedlings were potted into soil and grown for an additional 2 weeks in a phytotron or a growth chamber before using a stress condition. The standard conditions in the phytotron were 16-h light (250–350 μmol m−2 s−1 PFD, Osram HQI-T/DV lamps, 30°C–32°C), 8-h dark (22°C), 50% to 60% relative humidity. The plants were supplied six times a day by nutrient solution (one-half-diluted Hoagland salts; Hoagland and Arnon, 1950) in an excess amount resulting in over-dipping from the soil. ABA treatment was performed by spraying 15 to 25 μL of ABA solution (10 μm) on the lower surface of all leaves of a plant every 2nd d. The computer-controlled C23A system (Fabreguettes et al., 1994) consists of air-tight twin growth chambers suitable for comparative investigation of two sets of plants. The continuously adjusted and recorded parameters in the chambers (the standard values are indicated in brackets) were the following: CO2 concentration (0.034%), O2 concentration (16%), air humidity (60 relative %), evapotranspiration, temperature (day/night: 30°C/25°C). Illumination (16-h day) was provided by metal halogen lamps (Powerstar HQI-T/D, Osram, Munich), offering 250 and 350 μmol m−2 s−1 PFD at the level of upper leaves of young and mature (non-flowering) plants, respectively. Water was supplied normally four times a day as nutrient solution (one-half-diluted Hoagland salts; Hoagland and Arnon, 1950) in controlled amounts 20% to 50% more than the daily water loss by evapotranspiration. In the case of extended water stress by limited watering, after some days pure water was used to avoid salt accumulation. Humidity stress was achieved by specifying 30% and 40% relative humidity during day and night, respectively. The measurements of net photosynthesis and dark respiration were based on the quantitative balance of CO2 injection and trapping, respectively, maintaining a constant CO2 concentration (measured by an infrared gas analyzer) in the chambers (Fabreguettes et al., 1994). Air humidity was measured using, in addition to the in-built humidity detector, a portable humidity detector (Hydrodig 2010, Tecnic Instruments, Marseilles, France) at the level of leaves. Evapotranspiration was measured by weighing the collected condensed water vapor. Six plants normally were grown in each chamber. The use of large growth chambers for whole-plant photosynthesis measurements does not easily allow statistical evaluation of individual plants, therefore the experiments were repeated to test their reproducibility.
ACKNOWLEDGMENTS
The authors thank Gabriella Végh and Stéphan Cuiné for their technical assistance and Dr. Michel Pean for the management of the computer-controlled growth chambers.
Footnotes
This work was supported by the Országos Műszaki Fejlesztési Bizottság (no. EU–98–D8–11), the Országos Tudományos Kutatási Alap (nos. T016995 and T019759), the Volkswagen-Stiftung (no. I70961), the French-Hungarian Intergovernmental S&T Cooperation (no. F/8–95), and the European Community Biotechnology Program (no. Bio–4–97–2245). S.O.P. was the recipient of a fellowship from the Daimler-Benz-Stiftung.
LITERATURE CITED
- Arizmendi JM, Runswick MJ, Skehel JM, Walker JE. NADH:ubiquinone oxidoreductase from bovine heart mitochondria: a fourth nuclear encoded subunit with a homologue encoded in chloroplast genomes. FEBS Lett. 1992;301:237–242. doi: 10.1016/0014-5793(92)80248-f. [DOI] [PubMed] [Google Scholar]
- Berger S, Ellersiek U, Westhoff P, Steinmüller K. Studies on the expression of NDH-H, a subunit of the NAD(P) H-plastoquinone-oxidoreductase of higher-plant chloroplasts. Planta. 1993;190:25–31. [Google Scholar]
- Bookjans G, Stummann BM, Henningsen KW. Preparation of chloroplast DNA from pea plastids isolated in a medium of high ionic strength. Anal Biochem. 1984;141:244–247. doi: 10.1016/0003-2697(84)90452-4. [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 1998;17:868–876. doi: 10.1093/emboj/17.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá R, Sabater B, Guéra A. Expression of the plastid ndhF gene product in photosynthetic and non-photosynthetic tissues of developing barley seedlings. Plant Cell Physiol. 1997;38:1382–1388. doi: 10.1093/oxfordjournals.pcp.a029133. [DOI] [PubMed] [Google Scholar]
- Cournac L, Guedeney G, Joët T, Rumeau D, Latouche G, Cerovic Z, Redding K, Horváth EM, Medgyesy P, Peltier G. Garab G, ed, Photosynthesis: Mechanism and Effects. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. Non-photochemical reduction of intersystem electron carriers in chloroplasts of higher plants and algae; pp. 1877–1882. [Google Scholar]
- Cséplő A. Transfer of lincomycin resistance through somatic and sexual cybridization in Nicotiana. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry. 27, Somatic Hybridization in Crop Improvement I. Berlin: Springer-Verlag; 1994. pp. 394–404. [Google Scholar]
- Downton WJS, Loveys BR, Grant WJR. Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytol. 1988;108:263–266. doi: 10.1111/j.1469-8137.1988.tb04161.x. [DOI] [PubMed] [Google Scholar]
- Dupuis A, Skehel JM, Walker JE. A homologue of a nuclear-coded iron-sulfur protein subunit of bovine mitochondrial complex I is encoded in chloroplast genomes. Biochemistry. 1991;30:2954–2960. doi: 10.1021/bi00225a032. [DOI] [PubMed] [Google Scholar]
- Endo T, Mi H, Shikanai T, Asada K. Donation of electrons to plastoquinone by NAD(P) H dehydrogenase and by ferredoxin-quinone reductase in spinach chloroplasts. Plant Cell Physiol. 1997;38:1272–1277. [Google Scholar]
- Endo T, Shikanai T, Sato F, Asada K. NAD(P) H dehydrogenase-dependent, antimycin A-sensitive electron donation to plastoquinone in tobacco chloroplasts. Plant Cell Physiol. 1998;39:1226–1231. [Google Scholar]
- Fabreguettes V, Gibiat F, Pintena J, Vidal D, André M. SAE Technical Paper Series 941544. PA: Warrendale; 1994. The C23A system: a tool for global control of plant environment and exchange measurements. [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:317–345. [Google Scholar]
- Fearnley IM, Runswick MJ, Walker JE. A homologue of the nuclear coded 49 kD subunit of bovine mitochondrial NADH-ubiquinone reductase is coded in chloroplast DNA. EMBO J. 1989;8:665–672. doi: 10.1002/j.1460-2075.1989.tb03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley IM, Walker JE. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim Biophys Acta. 1992;1140:105–134. doi: 10.1016/0005-2728(92)90001-i. [DOI] [PubMed] [Google Scholar]
- Feild TS, Nedbal L, Ort DR. Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. Plant Physiol. 1998;116:1209–1218. doi: 10.1104/pp.116.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Funk E, Steinmüller K. The expression of subunits of the mitochondrial complex I-homologous NAD(P) H-plastoquinone-oxidoreductase during plastid development. Z Naturforsch. 1997;52c:481–486. [Google Scholar]
- Fork DC, Herbert SK. Electron transport and photophosphorylation by photosystem I in vivo in plants and cyanobacteria. Photosynth Res. 1993;36:149–168. doi: 10.1007/BF00033035. [DOI] [PubMed] [Google Scholar]
- Foyer C, Furbank R, Harbinson J, Horton P. The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res. 1990;25:83–100. doi: 10.1007/BF00035457. [DOI] [PubMed] [Google Scholar]
- Freyer R, López C, Maier RM, Martín M, Sabater B, Kössel H. Editing of the chloroplast ndhB encoded transcript shows divergence between closely related members of the grass family (Poaceae) Plant Mol Biol. 1995;29:679–684. doi: 10.1007/BF00041158. [DOI] [PubMed] [Google Scholar]
- Friedrich T, Steinmüller K, Weiss H. The proton-pumping respiratory complex I of bacteria and mitochondria and its homologue in chloroplasts. FEBS Lett. 1995;367:107–111. doi: 10.1016/0014-5793(95)00548-n. [DOI] [PubMed] [Google Scholar]
- Golds T, Maliga P, Koop H-U. Stable plastid transformation in PEG-treated protoplasts of Nicotiana tabacum. Biotechnology. 1993;11:95–97. [Google Scholar]
- Groom QJ, Kramer DM, Crofts AR, Ort DR. The non-photochemical reduction of plastoquinone in leaves. Photosynth Res. 1993;36:205–215. doi: 10.1007/BF00033039. [DOI] [PubMed] [Google Scholar]
- Heber U, Walker D. Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol. 1992;100:1621–1626. doi: 10.1104/pp.100.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka J, Shimada H, Whittier R, Ishibashi T, Sakamoto M, Mori M, Kondo C, Honji Y, Sun C-R, Meng B-Y, Li Y-Q, Kanno A, Nishizawa Y, Hirai A, Shinozaki K, Sugiura M. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989;217:185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Hirose T, Sugiura M. Both RNA editing and RNA cleavage are required for translation of tobacco chloroplast ndhD mRNA: a possible regulatory mechanism for the expression of a chloroplast operon consisting of functionally unrelated genes. EMBO J. 1997;16:6804–6811. doi: 10.1093/emboj/16.22.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agric Exp Stn Circ. 1950;347:1–32. [Google Scholar]
- Joët T, Cerovic Z, Rumeau D, Cournac L, Guedeney G, Horváth EM, Medgyesy P, Peltier G. Increased sensitivity of photosynthesis to anaerobic conditions induced by targeted inactivation of the chloroplast ndhB gene. In: Garab G, editor. Photosynthesis: Mechanism and Effects. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1967–1970. [Google Scholar]
- Kanno A, Hirai A. A transcription map of the chloroplast genome from rice (Oryza sativa) Curr Genet. 1993;23:166–174. doi: 10.1007/BF00352017. [DOI] [PubMed] [Google Scholar]
- Kavanagh TA, O'Driscoll KM, McCabe PF, Dix PJ. Mutations conferring lincomycin, spectinomycin, and streptomycin resistance in Solanum nigrum are located in three different chloroplast genes. Mol Gen Genet. 1994;242:675–680. doi: 10.1007/BF00283422. [DOI] [PubMed] [Google Scholar]
- Kavanagh TA, Thanh ND, Lao NT, McGrath N, Peter SO, Horváth EM, Dix PJ, Medgyesy P. Homeologous plastid DNA transformation in tobacco is mediated by multiple recombination events. Genetics. 1999;152:1111–1122. doi: 10.1093/genetics/152.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofer W, Koop H-U, Wanner G, Steinmüller K. Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P)H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet. 1998;258:166–173. doi: 10.1007/s004380050719. [DOI] [PubMed] [Google Scholar]
- Koop H-U, Kofer W, Steinmüller K. Reply. Trends Plant Sci. 1998;3:377. [Google Scholar]
- Kubicki A, Funk E, Westhoff P, Steinmüller K. Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that the complex I-homologous NAD(P) H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta. 1996;199:276–281. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maier RM, Neckermann K, Igloi GL, Kössel H. Complete sequence of the maize chloroplast genome: gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J Mol Biol. 1995;251:614–628. doi: 10.1006/jmbi.1995.0460. [DOI] [PubMed] [Google Scholar]
- Maliga P, Nixon PJ. Judging the homoplastomic state of plastid transformants. Trends Plant Sci. 1998;3:376–377. [Google Scholar]
- Martín M, Casano LM, Sabater B. Identification of the product of ndhA gene as a thylakoid protein synthesized in response to photooxidative treatment. Plant Cell Physiol. 1996;37:293–298. doi: 10.1093/oxfordjournals.pcp.a028945. [DOI] [PubMed] [Google Scholar]
- Masui R, Wakabayashi S, Matsubara H, Hatefi Y. The amino acid sequence of the 9 kD polypeptide and partial amino acid sequence of the 20 kD polypeptide of mitochondrial NADH:ubiquinone oxidoreductase. J Biochem. 1991;110:575–582. doi: 10.1093/oxfordjournals.jbchem.a123622. [DOI] [PubMed] [Google Scholar]
- Matsubayashi T, Wakasugi T, Shinozaki K, Yamaguchi-Shinozaki K, Zaita N, Hidaka T, Meng BY, Ohto C, Tanaka M, Kato A, Maruyama T, Sugiura M. Six chloroplast genes (ndhA-F) homologous to human mitochondrial genes encoding components of the respiratory chain NADH dehydrogenase are actively expressed: determination of the splice sites in ndhA and ndhB pre-mRNAs. Mol Gen Genet. 1987;210:385–393. doi: 10.1007/BF00327187. [DOI] [PubMed] [Google Scholar]
- McCabe PF, Timmons AM, Dix PJ. A simple procedure for the isolation of streptomycin-resistant plants in Solanaceae. Mol Gen Genet. 1989;216:132–137. [Google Scholar]
- Medgyesy P. Cybrids: transfer of chloroplast traits through protoplast fusion between sexually incompatible Solanaceae species. In: Bajaj YPS, editor. Biotechnology in Agriculture and Forestry. 27, Somatic Hybridization in Crop Improvement I. Berlin: Springer-Verlag; 1994. pp. 72–85. [Google Scholar]
- Meng BY, Matsubayashi T, Wakasugi T, Shinozaki K, Sugiura M, Hirai A, Mikami T, Kishima Y, Kinoshita T. Ubiquity of the genes for components of an NADH dehydrogenase in higher plant chloroplast genomes. Plant Sci. 1986;47:181–184. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Neyland R, Urbatsch LE. The ndhF chloroplast gene detected in all vascular plant divisions. Planta. 1996;200:273–277. doi: 10.1007/BF00208318. [DOI] [PubMed] [Google Scholar]
- Nixon PJ, Gounaris K, Coomber SA, Hunter CN, Dyer TA, Barber J. psbG is not a photosystem two gene but may be an ndh gene. J Biol Chem. 1989;264:14129–14135. [PubMed] [Google Scholar]
- Nixon PJ, Maliga P. Reply. Chlororespiration: only half a story. Trends Plant Sci. 1999;4:51. doi: 10.1016/s1360-1385(98)01373-9. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H. Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature. 1986;322:572–574. [Google Scholar]
- O'Neill C, Horváth GV, Horváth E, Dix PJ, Medgyesy P. Chloroplast transformation in plants: polyethylene glycol (PEG) treatment of protoplasts is an alternative to biolistic delivery systems. Plant J. 1993;3:729–738. [PubMed] [Google Scholar]
- Osmond CB. Photorespiration and photoinhibition: some implications for the energetics of photosynthesis. Biochim Biophys Acta. 1981;639:77–98. [Google Scholar]
- Peter S, Spang O, Medgyesy P, Schäfer C. Consequences of intergeneric chloroplast transfers on photosynthesis and sensitivity to high light. Aust J Plant Physiol. 1999;26:171–177. [Google Scholar]
- Pilkington SJ, Skehel JM, Walker JE. The 30-kilodalton subunit of bovine mitochondrial complex I is homologous to a protein coded in chloroplast DNA. Biochemistry. 1991;30:1901–1908. doi: 10.1021/bi00221a024. [DOI] [PubMed] [Google Scholar]
- Quiles MJ, Cuello J. Association of ferredoxin-NADP oxidoreductase with the chloroplastic pyridine nucleotide dehydrogenase complex in barley leaves. Plant Physiol. 1998;117:235–244. doi: 10.1104/pp.117.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AG, Heiser V, Zabaleta E, Brennicke A, Grohmann L. Physiological, biochemical and molecular aspects of mitochondrial complex I in plants. Biochim Biophys Acta. 1998;1364:101–111. doi: 10.1016/s0005-2728(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Ravenel J, Peltier G, Havaux M. The cyclic electron pathways around photosystem I in Chlamydomonas reinhardtii as determined in vivo by photoacoustic measurements of energy storage. Planta. 1994;193:251–259. [Google Scholar]
- Robinson SP, Grant WJR, Loveys BR. Stomatal limitation of photosynthesis in abscisic acid-treated and in water-stressed leaves measured at elevated CO2. Aust J Plant Physiol. 1988;15:495–503. [Google Scholar]
- Roldán M. Can chlororespiration in plants help to explain the controversial phenotype of ndh mutants? Trends Plant Sci. 1999;4:50. doi: 10.1016/s1360-1385(98)01368-5. [DOI] [PubMed] [Google Scholar]
- Rumeau D, Cuiné S, Fina L, Gault N, Nicole M, Peltier G. Subcellular distribution of carbonic anhydrase in Solanum tuberosum L. leaves: characterization of two compartment-specific isoforms. Planta. 1996;199:79–88. doi: 10.1007/BF00196884. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sazanov LA, Burrows PA, Nixon PJ. The chloroplast Ndh complex mediates the dark reduction of the plastoquinone pool in response to heat stress in tobacco leaves. FEBS Lett. 1998a;429:115–118. doi: 10.1016/s0014-5793(98)00573-0. [DOI] [PubMed] [Google Scholar]
- Sazanov LA, Burrows PA, Nixon PJ. The plastid ndh genes code for an NADH-specific dehydrogenase: isolation of a complex I analogue from pea thylakoid membranes. Proc Natl Acad Sci USA. 1998b;95:1319–1324. doi: 10.1073/pnas.95.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA. 1998;95:9705–9709. doi: 10.1073/pnas.95.16.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Shinozaki KY, Ohto C, Torazawa K, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986;5:2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Quick WP, Schurr U, Schulze E-D, Rodermel SR, Bogorad L. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with 'antisense' rbcS: II. Flux-control coefficients for photosynthesis in varying light, CO2, and air humidity. Planta. 1991;183:555–566. doi: 10.1007/BF00194277. [DOI] [PubMed] [Google Scholar]
- Teicher HB, Scheller HV. The NAD(P)H dehydrogenase in barley thylakoids is photoactivable and uses NADPH as well as NADH. Plant Physiol. 1998;117:525–532. doi: 10.1104/pp.117.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature. 1999;401:914–917. [Google Scholar]
- Thanh ND, Páy A, Smith MA, Medgyesy P, Márton L. Intertribal chloroplast transfer by protoplast fusion between Nicotiana tabacum and Salpiglossis sinuata. Mol Gen Genet. 1988;213:186–190. [Google Scholar]
- Videira A, Tropschug M, Werner S. Primary structure and expression of a nuclear-coded subunit of complex I homologous to proteins specified by the chloroplast genome. Biochem Biophys Res Commun. 1990;171:1168–1174. doi: 10.1016/0006-291x(90)90807-y. [DOI] [PubMed] [Google Scholar]
- Wakasugi T, Sugita M, Tsudzuki T, Sugiura M. Updated gene map of tobacco chloroplast DNA. Plant Mol Biol Rep. 1998;16:231–241. [Google Scholar]
- Weidner U, Geier S, Ptock A, Friedrich T, Leif H, Weiss H. The gene locus of the proton-translocating NADH:ubiquinone oxidoreductase in Escherichia coli: organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J Mol Biol. 1993;233:109–122. doi: 10.1006/jmbi.1993.1488. [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker MD. Stomata. London: Chapman & Hall; 1996. [Google Scholar]