Abstract
Magnetic resonance imaging (MRI) of the breast is primarily used as a supplemental tool to breast screening with mammography or ultrasound. A breast MRI is mainly used for women who have been diagnosed with breast cancer, to help measure the size of the cancer, look for other tumors in the breast, and to check for tumors in the opposite breast. For certain women at high risk for breast cancer, a screening MRI is recommended along with a yearly mammogram. MRI is known to give some false positive results which mean more test and/or biopsies for the patient. Thus, although breast MRI is useful for women at high risk, it is rarely recommended as a screening test for women at average risk of breast cancer. Also, breast MRI does not show calcium deposits, known as micro-calcifications which can be a sign of breast cancer.
Keywords: Breast imaging, high-risk screening, identify nonresponders, neo-adjuvant setting, X-ray mammogram
Introduction
The use of contrast-enhanced magnetic resonance imaging is based on neo angiogenesis. Tumour associated blood vessels have increased vascular permeability which is responsible for the uptake and washout of gadolinium after its administration. The morphology of the lesions, the enhancement and washout kinetics help distinguish breast cancers from benign lesions. The sensitivity of breast MRI is reported to be very high (over 90%) but the specificity is still low to moderate (72%) making the discrimination between benign and malignant lesions challenging.
Since 2000, breast MRI has been extensively used and has become an important modality in high risk screening, diagnosis, staging and follow up of breast cancer [Figure 1a]. Breast MRI has proven value in high risk screening, evaluation of unknown primary, evaluating local extent of disease, multicentricity and bilaterally especially in dense breasts, differentiating a scar from local recurrence in women who had breast conserving surgery, evaluation of response to neoadjuvant chemotherapy and in evaluating the integrity of implants [Figure 1b]. However, breast MRI has its share of controversies.
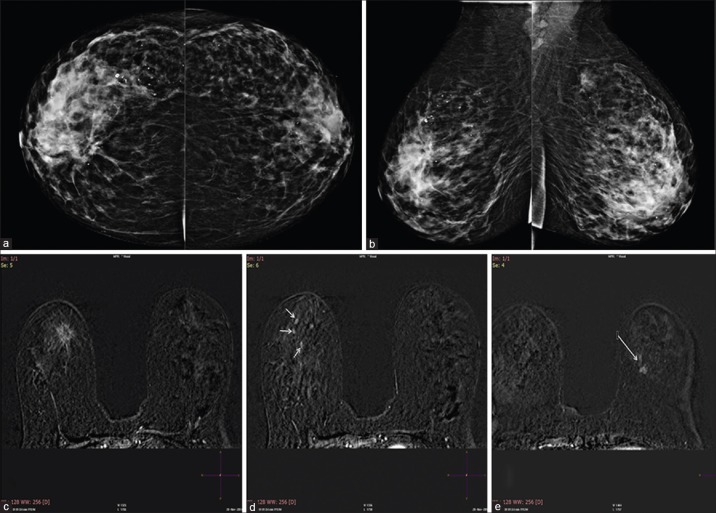
Figure 1.
(a) Cranio-caudal view of both breasts. (b) Medio-lateral view of both breasts. Heterogeneously dense breast. Architectural distortion is seen in the right breast. There is focal asymmetry in the left breast upper outer quadrant seen in MLO view. (c) Postcontrast axial view showing a contrast enhancing spiculated lesion in the right breast corresponding to the mammographic image. HPE confirmed a tubular carcinoma. (d) In addition, there were other areas of contrast enhancement in the right breast. Histopathology of these lesions confirmed an invasive lobular carcinoma. (e) The left breast enhancing lesion was reported to be an invasive ductal carcinoma
Discussion
It is estimated that 5% of cancers are due to inherited genetic mutation. BRCA 1 and 2 mutations are the commonest, in addition to mutations in P53, PTEN, CHEK2, ATM and a host of other mutations that are unknown at present. Another small but clinically significant group of women at high risk of breast cancer are those who have been treated with mantle radiation therapy (typically for Hodgkin's lymphoma), between 8 and 30 years of age.
The controversy surrounding high risk screening is now limited. American Cancer society, European society of breast Cancer Specialists (EUSOMA), European society of breast imaging (EUSOBI) have all included breast MRI in the recommendations for high risk screening. The German EVA trial and the Italian HIBCRIT -1 trial have raised the possibility of moving away from mammography and breast ultrasound for high risk screening.[1,2]
MRI for screening has not been very popular in women with average risk due to concerns about the low specificity leading to additional biopsies, time and cost of technology. It is also evident that mammography with its lower sensitivity is limited in women with dense breasts. Mammography is also accused of picking up the more indolent cancers. Breast MRI uses intravenous contrast administration (Gadolinium) but not limited by breast density and preferentially detects the higher-grade lesions. Christiane Kuhls's study published recently did not report any interval cancers, and the negative predictive value of MRI is high, thereby allowing a longer screening interval. The abbreviated protocol for MRI screening developed by Christiane Kuhl, promises to reduce the time taken for the study and interpretation, and the cost with a high negative predictive value in breast cancer screening.[3] There is potential to expand the role of MRI in breast cancer screening in women with average or moderate risk.
Breast magnetic resonance imaging and DCIS
DCIS on a mammogram is usually identified by the presence of microcalcifications. The tumour within the terminal ductal units and the ducts outgrows its blood supply, undergoes necrosis and calcifies. MRI does not pick up these calcifications. However, the non-mass enhancement that is seen in DCIS id probably because the gadolinium permeating into the ducts through the leaky basement due to protease activity produced by tumour cells. Thus MRI might actually detect the more clinically relevant high grade lesions. The low grade DCIS readily picked up by the x ray mammogram may be missed on MRI. On the other hand 10-15% of DCIS present as non-calcifying DCIS are missed X ray mammogram but detected on MRI.
Non-mass enhancement in a ductal or segmental distribution with clumped or stippled morphological appearance is the typical presentation of DCIS in about 70 -80% of cases. The remaining 20-30% of various enhancement patterns such as focus or a mass in a focal area or regional distribution is seen. The kinetic are variable and contribute less to the diagnosis of DCIS.
The role of breast magnetic resonance imaging in preoperative evaluation
The use of breast MRI in the preoperative setting for women with a recent breast cancer diagnosis is controversial, with wide variations in practice. Preoperative MRI is likely to detect multifocal and multicentric lesions and evaluate the contralateral breast, especially in lobular cancers and in dense breast. A systematic review that included 3 RCT's and 16 comparative studies were included in the meta-analysis was performed to identify studies reporting quantitative data on pre-operative MRI and surgical outcomes. This review concluded that pre-operative MRI is associated with increased odds of receiving ipsilateral mastectomy and contralateral prophylactic mastectomy as surgical treatment in newly diagnosed breast cancers [Figure 1c–e].[4]
MRI is also said to define the size and extent of the tumour better for planning surgery. While this is expected to reduce re-excision rates along with a decrease in the local recurrence rates and overall survival rates, this has actually not borne out in reality. It however leads to increase in additional biopsies, patient anxiety, cost, delay the onset of treatment and possibly increase in mastectomy rates.
The COMICE study and MONET trials evaluated the role of preoperative MRI with regard to reducing re excision rates. The COMICE study failed to show significant differences in reduction of re-excision rates. MONET trial evaluated the role of preoperative MRI in non-palpable lesions that included benign and malignant lesions. Addition of MRI to routine clinical care in patients with non-palpable breast cancer was paradoxically associated with an increased re-excision rate. Therefore they recommended that breast MRI should not be used routinely for preoperative work-up of patients with non-palpable breast cancers.[5,6]
The adequacy of the margins has been discussed extensively with wide variation in practice. The SSO-ASTRO consensus guidelines in 2014 made clear recommendations on the adequacy of margins which for an invasive cancer is no ink on tumour and 2 mm for ductal carcinoma in situ.[7] The re-excision rates have decreased since then and the role of routine preoperative MRI to reduce re excisions rate has probably become redundant.
Pre-operative MRI is probably not warranted routinely in patients who can be adequately analyzed by mammography and ultrasound examination. It certainly may be valuable in women with dense breasts and in patients with lobular cancer.
Role of magnetic resonance imaging in assessment of response to neoadjuvant therapy
Neoadjuvant chemotherapy has been used successfully to downstage tumours to bring them within the scope of surgery. Neoadjuvant chemotherapy is now increasingly used to conserve the breast in large operable lesions. Breast MRI provides the best imaging correlation with pathology and many studies have shown the MRI is superior to clinical assessment, mammogram and ultrasound.[8,9] Breast MRI is useful to monitor response to neoadjuvant chemotherapy to identify non responders early and to delineate the residual tumour after NACT to determine appropriate extent of surgical excision.[10,11]
In most centres, a clip is placed after a core biopsy in the centre of the tumour with additional clips to mark the extent of the lesion. Prior to NACT, a pretreatment MRI is performed and compared with MRI done after 1 or 2 cycles of NACT. Non responders are identified early on MRI using a combination of size and kinetic changes with interpretation facilitated by CAD systems offering volumetric analysis and parametric colour mapping.
Contrast enhancement on MRI correlates with viable tumour. However, the estimation of tumour size by measuring the extent of enhancement may not be accurate with possible underestimation and over estimation. Tumour necrosis may lead to reparative changes, and result in granulation tissue, which may also enhance with contrast leading to overestimation. Chemotherapeutic agents like taxanes may have anti angiogenic action without corresponding tumour necrosis resulting in lack of enhancement and thereby over estimating the response to NACT. Scattered focal areas of enhancement (Swiss cheese like appearance) may have scattered residual tumour nests across the original extent of the tumour necessitating a mastectomy.
Conclusion
MRI is superior to x-ray mammogram in high-risk breast cancer screening. In women with low to average risk of breast cancer, the role of MRI remains controversial. The use of pre-operative MRI continues to be controversial with wide variations in practice. In a neo-adjuvant setting, MRI breast is useful to identify the non-responders early. In those who respond to chemotherapy, it is helpful in planning conservation where feasible.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Santoro F, Podo F, Sardanelli F. MRI screening of women with hereditary predisposition to breast cancer: Diagnostic performance and survival analysis. Breast Cancer Res Treat. 2014;147:685–7. doi: 10.1007/s10549-014-3097-1. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: The EVA trial. J Clin Oncol. 2010;28:1450–7. doi: 10.1200/JCO.2009.23.0839. [DOI] [PubMed] [Google Scholar]
- 3.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB, et al. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–10. doi: 10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 4.Houssami N, Turner RM, Morrow M. Meta-analysis of pre-operative magnetic resonance imaging (MRI) and surgical treatment for breast cancer. Breast Cancer Res Treat. 2017;165:273–83. doi: 10.1007/s10549-017-4324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: A randomised controlled trial. Lancet. 2010;375:563–71. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 6.Peters NH, van Esser S, van den Bosch MA, Storm RK, Plaisier PW, van Dalen T, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: The MONET – Randomised controlled trial. Eur J Cancer. 2011;47:879–86. doi: 10.1016/j.ejca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Harness JK, Giuliano AE, Pockaj BA, Downs-Kelly E. Margins: A status report from the annual meeting of the american society of breast surgeons. Ann Surg Oncol. 2014;21:3192–7. doi: 10.1245/s10434-014-3957-2. [DOI] [PubMed] [Google Scholar]
- 8.Martincich L, Montemurro F, De Rosa G, Marra V, Ponzone R, Cirillo S, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83:67–76. doi: 10.1023/B:BREA.0000010700.11092.f4. [DOI] [PubMed] [Google Scholar]
- 9.Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kühn T, et al. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002;12:1711–9. doi: 10.1007/s00330-001-1233-x. [DOI] [PubMed] [Google Scholar]
- 10.Stucky CC, McLaughlin SA, Dueck AC, Gray RJ, Giurescu ME, Carpenter SG, et al. Does magnetic resonance imaging accurately predict residual disease in breast cancer? Am J Surg. 2009;198:547–52. doi: 10.1016/j.amjsurg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Thibault F, Nos C, Meunier M, El Khoury C, Ollivier L, Sigal-Zafrani B, et al. MRI for surgical planning in patients with breast cancer who undergo preoperative chemotherapy. AJR Am J Roentgenol. 2004;183:1159–68. doi: 10.2214/ajr.183.4.1831159. [DOI] [PubMed] [Google Scholar]