Abstract
Background:
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a cytokine that activates apoptosis through death receptors on the cell surface and is regarded as a potential anticancer agent. However, many cancer cells are resistant to TRAIL-induced apoptosis.
Objective:
The aim is to identify the herbal medicines that could help overcome resistance in TRAIL-resistant lung cancer cells.
Materials and Methods;
TRAIL-resistant A549 cells and 13 herbal medicines with known apoptosis-related anticancer effects were used in this study: Clematidis Radix, Corydalis Tuber Rhizoma, Paeoniae Radix Rubra, Corni Fructus, Curcumae longae Rhizoma (CLR), Moutan Cortex, Salviae miltiorrhizae Radix, Phellodendri Cortex, Farfarae Flos, Paeoniae Radix Alba, Angelicae gigantis Radix, Coptidis Rhizoma (CR), and Taraxaci Herba. Cytotoxic effects were investigated after a 48-h incubation, using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, to identify the herbal medicines with the most potent synergistic effects with TRAIL.
Results:
The majority of the 13 medicines exhibited concentration-dependent cytotoxicity against A549 cells. Among them, CR and CLR showed the most potent cytotoxic effects, based on the IC50. We then investigated the use of these two medicines in combination with TRAIL and identified synergistic cytotoxic effects against TRAIL-resistant A549 cells.
Conclusion:
Synergistic cytotoxic effects of the combination of TRAIL and herbal medicines, in particular, CR and CLR, were confirmed in A549 cells. Therefore, CR and CLR showed potential to be used as candidates to overcome TRAIL resistance. Future studies to identify their underlying mechanism of action are required.
SUMMARY
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an attractive anticancer agent which can induce apoptosis in tumor cells without causing cytotoxicity to normal cells
However, resistance to TRAIL is often observed in some tumor cells, including nonsmall cell lung cancers, which may limit its cytotoxic efficacy in cancer treatment
The combination treatment of TRAIL and herbal medicines, particularly Coptidis Rhizoma (CR) and Curcumae longae Rhizoma (CLR), can induce the synergistic cytotoxic effects against TRAIL-resistant A549 cells, indicating that TRAIL resistance was reduced by combination therapy.
Abbreviations used: TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand; CLR: Curcumae longae Rhizoma; CR: Coptidis Rhizoma; NSCLC: non-small cell lung cancer.
Keywords: A549 nonsmall cell lung cancers cells, herbal medicine, resistance, synergism, tumor necrosis factor-related apoptosis-inducing ligand
INTRODUCTION
Apoptosis, a genetically programmed and active cell suicide process, plays a crucial role in homeostasis and eliminating unwanted cells. Evasion of programmed cell death is one of the important genetic changes in malignant transformation. Therefore, apoptosis induction is a potential therapeutic goal of cancer treatment.[1]
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has long been regarded as an effective target for cancer therapy. It is a small molecule, known to induce apoptosis selectively in cancer cells through interaction with the death receptors DR4 and DR5, while not damaging normal cells.[2] There have been several single-agent clinical trials, including phase 1 and 2 trials, evaluating the efficacy of various TRAIL receptor agonists. However, because of resistance, their anticancer effects have proven to be insufficient.[3] Therefore, much effort has focused on overcoming TRAIL resistance in cancer cells through the use of various natural products.[4,5]
In traditional Korean medicine, herbal medicines have been used in the treatment of many diseases, including cancer, for centuries, because of their safety, efficacy, and inexpensiveness. The efficacy of a number of herbal medicines in the treatment of lung cancer has been well documented.[6] Thus, for the present study, we obtained water extracts of herbal medicines with demonstrated apoptosis-related antitumor activities and screened them based on their anti-proliferative effects. We then evaluated their TRAIL-sensitizing effects in A549 TRAIL-resistant lung cancer cells.
MATERIALS AND METHODS
Preparation of herbal aqueous extract
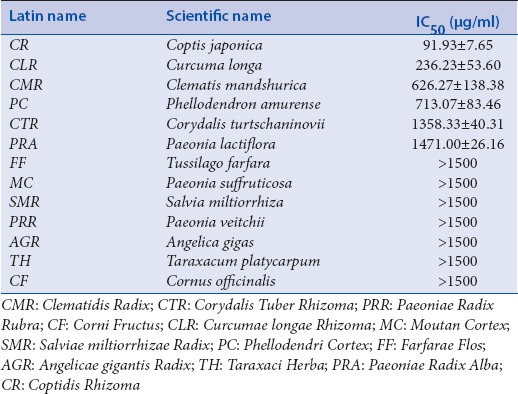
Thirteen herbal medicines, Clematidis Radix (CMR), Corydalis Tuber Rhizoma (CTR), Paeoniae Radix Rubra (PRR), Corni Fructus (CF), Curcumae longae Rhizoma (CLR), Moutan Cortex (MC), Salviae miltiorrhizae Radix (SMR), Phellodendri Cortex (PC), Farfarae Flos (FF), Paeoniae Radix Alba (PRA), Angelicae gigantis Radix (AGR), Coptidis Rhizoma (CR), and Taraxaci Herba (TH), were purchased from the Kyung Hee Hanyak Company (Seoul, Korea), and extracts were prepared by boiling the flowers in water at 100°C for 2 h. Herbal water extracts were obtained by evaporating and freeze-drying. The yield of each herbal water extract was 3.06, 3.44, 7.44, 9.71, 2.48, 6.19, 4.50, 1.83, 5.10, 5.00, 3.41, 9.61, and 3.33% of CMR, CTR, PRR, CF, CLR, MC, SMR, PC, FF, PRA, AGR, CR, and TH, respectively.
Cell viability
To evaluate the cytotoxic effect of the herbal extracts, cell viability was assessed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma Chemical Co., St. Louis, MO) assay. Briefly, the cells were seeded in 96-well plates at a density of 1 × 104 cells per well and incubated for 48 h. They were then treated with various concentration of herbal extracts (0, 0.01, 0.1, 10, 100, 1000, and 10000 μg/ml). After incubation for a further 48 h, 200 ml of MTT (0.5 mg/ml) was added to each well. Followed by 4-h incubation, absorbance was measured using an ELISA reader. Absorbance was measured using an ELISA reader (Molecular Devices, Sunnyvale, CA, USA) at 540 nm. MTT assays were carried out in triplicate. The IC50 was defined as the concentration of herbal medicine water extract that inhibited cell growth by 50% relative to the untreated control.
Cell viability was again investigated using MTT assay to evaluate the synergistic effects of combinations of herbal extracts and TRAIL on the proliferation of A549 cells. The cells were seeded at a density of 1 × 104 cells per well and incubated for 48 h and were then treated with 0, 100, 200, or 400 μg/ml of herbal extracts and 40 ng/ml of TRAIL concurrently. MTT assays were then used to assess cell viability.
Combination index calculation
The combination index (CI) for the treatment combining herbal extracts (CLR and CR) and TRAIL was calculated using CompuSyn software and Chou–Talalay median-effect analyses.[7] The efficacy of each drug combination was represented by its CI value, with CI <1 indicating synergism, CI = 1 indicating an additive effect, and CI >1 indicating antagonism.
RESULTS
Cytotoxic activities of aqueous herbal extracts on A549 cells
To investigate the cytotoxic activity of whole-plant water extracts, A549 cells were incubated with different herbal water extract concentrations for 48 h. Each herbal water extract exhibited some degree of inhibition on the proliferation of A549 cell in a concentration-dependent manner and IC50 values were calculated [Table 1]. The water extracts of CR and CLR were determined to have the most potent inhibitory effects. Therefore, we selected CR and CLR as candidate drugs for maximum synergy with TRAIL against A549 cells.
Table 1.
Cytotoxic activities of herbal aqueous extracts
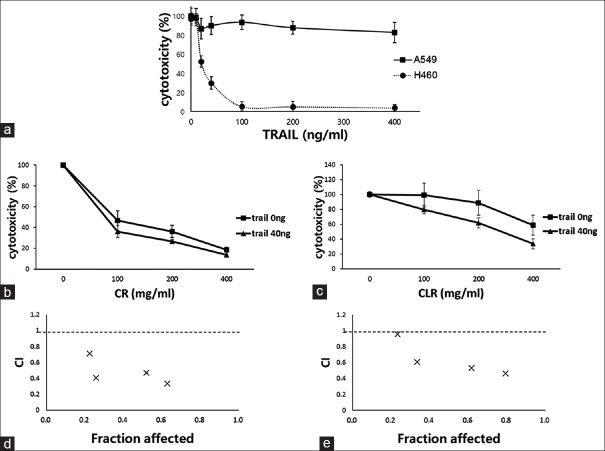
Identification of tumor necrosis factor-related apoptosis-inducing ligand resistance in A549 cells and the synergistic effect of cotreatment with tumor necrosis factor-related apoptosis-inducing ligand and aqueous herbal extracts
Resistance to TRAIL was confirmed by evaluating its cytotoxicity against A549 cells at different concentrations [Figure 1a]. In addition, to identify synergistic effects of TRAIL and aqueous herbal extracts, the cytotoxicity of CR and CLR at different doses were again investigated, and their cytotoxicity in combination with a fixed concentration of 40 ng/ml TRAIL, which had been confirmed to have no cytotoxic effect on A549 cells, were determined. The observed cytotoxicity for each single and combination therapy was then compared. The results of this analysis indicated that single-agent treatment inhibited the proliferation of A549 cells in a dose-dependent manner, while combination treatment further inhibited proliferation at several concentrations in a synergistic manner [Figure 1b and c].
Figure 1.
Combination effect of tumor necrosis factor-related apoptosis-inducing ligand and Coptidis Rhizoma or Curcumae longae Rhizoma in A549 cells. (a) Dose-dependent effect of tumor necrosis factor-related apoptosis-inducing ligand in A549 and H460 cells. A549 cells showed resistance to tumor necrosis factor-related apoptosis-inducing ligand. (b and c) Synergistic effect of tumor necrosis factor-related apoptosis-inducing ligand and CR or CLR, respectively, in A549 cells. A549 cells were incubated with tumor necrosis factor-related apoptosis-inducing ligand (40 ng/ml) and various concentrations of Coptidis Rhizoma or Curcumae longae Rhizoma (0, 100, 200, and 400 μg/ml) for 48 h, and cytotoxicity was measured using MTT assay. (d and e) Combination index of tumor necrosis factor-related apoptosis-inducing ligand and Coptidis Rhizoma or Curcumae longae Rhizoma, respectively. Combination index <1 indicates synergistic effects of drug combinations
Combination index of tumor necrosis factor-related apoptosis-inducing ligand and herbal medicines
Combination effects were assessed using the Chou–Talalay method, which is based on the median effect equation. From the affected fraction (Fa)–CI plot, the efficacy of each combination was determined. Both CR and CLR combined with TRAIL had CI values <1, suggesting synergism against A549 cell lines, and implying the overcoming of resistance to TRAIL [Figure 1d and e].
DISCUSSION
Lung cancer is a major cause of cancer-related deaths worldwide and is divided into small cell and nonsmall cell lung cancers (NSCLC). The latter are less sensitive to conventional chemotherapeutic agents. Therefore, the development of novel therapeutic strategies, including induction of apoptosis, is necessary.[1] TRAIL is regarded as an attractive therapeutic agent because it directly induces apoptosis that selectively eliminates tumor cells without harming normal cells.[2] However, TRAIL resistance has been observed in several types of NSCLC, and clinical trials using TRAIL therapies have shown disappointingly weak therapeutic effects, especially when TRAIL agonists are used as a monotherapy.[1] Thus, it is important to find novel drugs that act synergistically with TRAIL and can also overcome this resistance.[3]
There have been some studies on overcoming TRAIL resistance in various cancers, including lung cancer, using herbal medicines, and natural compounds.[4,8,9] To the best of our knowledge, none of the studies has examined TRAIL-resistant NSCLC. Therefore, in this study, we investigated the antitumor effects of 13 frequently used herbal medicines containing compounds associated with or related to apoptosis and assessed their ability to overcome TRAIL resistance in A549 cells.
It is well documented that CR and CLR have antitumor effects against various cancers.[10] Specifically, berberine, from CR, has been shown to enhance TRAIL-induced apoptosis in breast cancer.[11] In addition, curcumin, from CLR, is known to have excellent antitumor effects and to reduce TRAIL resistance in prostate cancer.[12] However, clinically, herbal medicines are most commonly used as decocted aqueous extracts,[13] and to date, no study has investigated the effects of total-herb aqueous extracts of CR and CLR on overcoming TRAIL resistance in NSCLC. In this study, CR and CLR aqueous extracts have been shown to not only act synergistically with TRAIL but also to overcome TRAIL resistance. Animal experiments and studies on the molecular mechanisms of action of these extracts should be conducted in the near future to establish a foundation for clinical trials.
CONCLUSION
In the present study, we confirmed CR and CLR to be the most potent of the 13 anticancer herbal medicines known to induce apoptosis. We further investigated whether these two medicines could reduce resistance to TRAIL, an anticancer cytokine causing apoptosis, and examined the synergistic effect of combinations of TRAIL and these herbal medicines. We found that combination treatment with TRAIL and CR or CLR resulted in greater cytotoxicity than single-agent treatment in A549 TRAIL-resistant cells, indicating that TRAIL resistance was reduced by combination therapy.
Financial support and sponsorship
This research was financially supported by Kyung Hee university.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by a grant from Kyung Hee University in 2015.(KHU-20150848).
REFERENCES
- 1.Stegehuis JH, de Wilt LH, de Vries EG, Groen HJ, de Jong S, Kruyt FA, et al. TRAIL receptor targeting therapies for non-small cell lung cancer: Current status and perspectives. Drug Resist Updat. 2010;13:2–15. doi: 10.1016/j.drup.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–98. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 3.Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL, et al. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2013;32:1341–50. doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed F, Ishibashi M. Bio-active natural products with TRAIL-resistance overcoming activity. Chem Pharm Bull (Tokyo) 2016;64:119–27. doi: 10.1248/cpb.c15-00732. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi M, Ohtsuki T. Studies on search for bioactive natural products targeting TRAIL signaling leading to tumor cell apoptosis. Med Res Rev. 2008;28:688–714. doi: 10.1002/med.20123. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Zhu J, Zhang W. Antitumor effect of traditional chinese herbal medicines against lung cancer. Anticancer Drugs. 2014;25:983–91. doi: 10.1097/CAD.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 7.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 8.Abdelhamed S, Yokoyama S, Hafiyani L, Kalauni SK, Hayakawa Y, Awale S, et al. Identification of plant extracts sensitizing breast cancer cells to TRAIL. Oncol Rep. 2013;29:1991–8. doi: 10.3892/or.2013.2293. [DOI] [PubMed] [Google Scholar]
- 9.Dai X, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, et al. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood) 2015;240:760–73. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y, et al. Berberine and coptidis rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J Ethnopharmacol. 2009;126:5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Refaat A, Abdelhamed S, Yagita H, Inoue H, Yokoyama S, Hayakawa Y, et al. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol Lett. 2013;6:840–4. doi: 10.3892/ol.2013.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta HJ, Patel V, Sadikot RT. Curcumin and lung cancer – A review. Target Oncol. 2014;9:295–310. doi: 10.1007/s11523-014-0321-1. [DOI] [PubMed] [Google Scholar]
- 13.Jang S, Kim KH, Sun SH, Go HY, Lee EK, Jang BH, et al. Characteristics of herbal medicine users and adverse events experienced in South Korea: A Survey study. Evid Based Complement Alternat Med. 2017;2017:4089019. doi: 10.1155/2017/4089019. [DOI] [PMC free article] [PubMed] [Google Scholar]