Abstract
Background:
Sophora tonkinensis Gapnep. is an important medical plant in China. Early researches of S. tonkinensis were focused on rapid propagation and quality analysis of in vitro tissue culture plantlet, and still no research focuses on the plant breeding of and there were no excellent varieties for artificial cultivation of S. tonkinensis.
Objective:
To set up a method to generate and select the best varieties of S. tonkinensis by polyploid breeding after induction by colchicine treatment.
Materials and Methods:
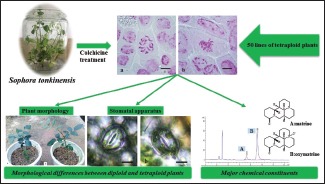
The adventitious buds were submerged in different concentrations of aqueous colchicine solution for different lengths of time to induce polyploidy in the plants, and the induced buds were identified by root-tip chromosome determination and leaf characteristics comparison. The contents of matrine and oxymatrine of radix ex rhizoma in 13 selected tetraploid lines were collected after 90 days in vitro rooting culture and were evaluated to provide evidence of good qualities of tetraploid S. tonkinensis.
Results:
The results showed that the highest percentage of tetraploid induction was 23.33% and occurred in the 0.2% (w/v) colchicine treatment for 30 h. Fifty lines of tetraploid plants were obtained and 12 of the 13 selected tetraploid lines exhibited higher productivity of total contents of matrine and oxymatrine when compared to controls.
Conclusion:
The data demonstrate that polyploidy induction can be beneficial for improving the medicinal value of S. tonkinensis.
SUMMARY
Colchicine has a good in vitro induction effect on the tetraploid plants of Sophora tonkinensis
The leaf indices and stomatal apparatus of tetraploid plants were slightly larger than diploid plants
The total content of matrine and oxymatrine of some tetraploid lines for 90-day-old in vitro rooting culture was higher than the diploid.
Abbreviations used: MS medium: Murashige and Skoog medium; BAP: 6-benzylaminopurine; NAA: A-naphthaleneacetic acid; IAA: Indole-3-acetic acid; KT: Kinetin; IBA: Indole-3-butyric acid; ABT: Rooting power.
Keywords: Colchicine, induction, matrine, oxymatrine, Sophora tonkinensis Gapnep, tetraploid
INTRODUCTION
Sophora tonkinensis Gapnep., belonging to Leguminosae family, is mainly distributed in Southern China and the north of Vietnam. It is an important medicinal plant in China and the radix ex rhizoma of S. tonkinensis is known as Shan-Dou-Gen in Chinese.[1] The active drug of S. tonkinensis derived from Guangxi Province of China possess the best curative effect for conditions such as eczema, colitis, acute pharyngolaryngeal infection, sore throat, acute dysentery, and gastrointestinal hemorrhage, and it is conventionally known as the famous authentic Chinese herbal medicine of China.[2] Pharmacological research indicated that the main active components of S. tonkinensis are alkaloids, and matrine and oxymatrine are the main active components of S. tonkinensis alkaloids,[3] both of which possess a wide range of pharmacological actions including those that are anti-inflammatory,[4] anti-arrhythmic,[5] anti-tumor,[6] and liver-protective.[7] S. tonkinensis is a perennial plant, and from seed germination to the mature plant in readiness for harvest of the drug, it can take more than 3 years. Due to it being a perennial plant and difficulties involved in its cultivation, the updating of the wild resource was very difficult. However, until now, the S. tonkinensis drug being used is mainly from the wild resource which has decreased rapidly over recent years or has even become extinct in many distribution areas.[8,9] As demand for the S. tonkinensis drug has increased rapidly, the wild drug cannot meet the need of consumption, so now the price of this drug has increased to about 25.45 dollars/kg (170 Yuan/kg), in comparison to only about 21.8 dollars/kg (about 150 Yuan/kg) in 2016. In such a situation, many researchers and ourselves have carried out numerous studies[10,11,12] and have set up protocols for artificial cultivation and seed sprouting propagation by tissue culture of S. tonkinensis.[13,14,15,16] Some seedling factories produced S. tonkinensis plantlets and many medicinal herb growers have now planted S. tonkinensis using our technology in China. However, we had found that until now, there was no research focus on the plant breeding of S. tonkinensis, and there were no excellent varieties for artificial cultivation of S. tonkinensis. This may become a major restraining factor for the sustainable development of S. tonkinensis in the future.
Hence, we have tried to set up a method to generate some excellent varieties by polyploid breeding. We report here a convenient and effective method for generating polyploid plants by colchicine treatment, whereby the adventitious buds of S. tonkinensis from tissue culture were submerged into different aqueous concentrations of colchicine solution for different times to induce mutations. The root-tip chromosome determination, leaf characteristics, and matrine and oxymatrine content of polyploid plants were used as criteria to identify and select the best varieties.
MATERIALS AND METHODS
Plant material
Seeds of S. tonkinensis (2x = 18) were collected from Jingxi County, Guangxi Zhuang Autonomous Region, China. The original plant was identified by the Guangxi Key Laboratory of Medicinal Resources Protection and Genetic Improvement at Guangxi Botanical Garden of Medicinal Plants.
Material disinfection and adventitious bud induction
Seeds of S. tonkinensis (2x = 18) collected in October were sterilized using 1% v/v sodium hypochlorite solution (containing 3–5 drops of Tween-20/l) for 10 min and washed with sterile distilled water 3–5 times, and the excess surface water removed using sterile filter paper. Then, the surface-sterilized seeds were placed onto Murashige and Skoog medium (MS medium)[17] containing 3% w/v sucrose and 0.35% w/v agar powder (gel strength: 1100 g/cm2) supplemented with 0.5 mg/L 6-benzylaminopurine (BAP) at pH 5.8 for 30 days to induce germination. The epicotyls excised from seedlings of seed germination were inoculated into MS medium containing 3% w/v sucrose and 0.35% w/v agar powder (gel strength: 1100 g/cm) supplemented with 1.5 mg/L BAP, 0.5 mg/L indole-3-acetic acid (IAA), and 0.5 mg/L kinetin (KT) according to the method established by Kun-Hua et al.[16] The inoculated epicotyl was kept in an illuminated incubator for a 16-h photoperiod under a 1200 lux light intensity and 25°C ± 1°C to induce clump buds.
Induction of polyploid plantlets
Thirty adventitious buds regenerated from the epicotyls were submerged in five different concentrations of colchicine (0, 0.1, 0.2, 0.3, and 0.4% w/v) for 24 h to select the optimum treatment concentration for polyploidy induction, and thirty adventitious buds regenerated from the epicotyls were submerged in 0.2% w/v colchicine solution for 0, 6, 12, 18, 24, 30, 36, 42, or 48 h to select the optimum treatment time of polyploidy induction. All of the treated buds were then transferred to MS media supplemented with 1.5 mg/L BAP, 0.5 mg/L IAA, and 0.5 mg/L KT and cultured in an illuminated incubator under a 16-h photoperiod of 1200 lux light intensity at 25°C for 30 days. After the survival rate counted, all of the survived materials were transferred to the MS solid medium supplemented with 1.5 mg/L BAP, 0.5 mg/L IAA, and 0.5 mg/L KT for 30 days. Then, the seedling of the subculture materials was transferred to the rooting solid MS medium at half the macronutrient concentration supplemented with 1.0 mg/L a-naphthalene acetic acid (NAA), 0.4 mg/L indole-3-butyric acid (IBA), and 0.1 mg/L rooting power (ABT) to induce root formation for chromosome determination.
Chromosome determination
As S. tonkinensis and Sophora flavescens Aiton. belong to the same genus of the same family, the chromosome determination of S. tonkinensis polyploids was carried out according to the method established by Kun-Hua et al.[18] Root tips approximately 0.5 cm in length were excised and pretreated in 0.2% w/v colchicine solution for 3 h. After pretreatment, the root tips were transferred to Carnoy's fixative (containing 3:1 ethanol and glacial acetic acid) and stored at 3°C –5°C for 2–24 h, rinsed with 95% (w/v) alcohol, 70% (w/v) alcohol, and distilled water, respectively, three times, and then macerated for 15 min with 0.2 M HCl at 60°C. After soaking in distilled water for 30 min, the fixed root tips were stained with improved carbol fuchsin (1.8 g sorbitol dissolved in 10 mL carbol fuchsin and then mixed with 90 mL of 45% v/v acetic acid). A photomicroscope (Leica DM 2000) was used for chromosome determination. A minimum of 50 cells during metaphase showing well-scattered and contracted chromosomes were counted for each plantlet. The chromosome count of each tetraploid (4x = 36) line was repeated for at least three generations.
The buds (approximately 3 cm in height) of each tetraploid line were excised and transferred to the rooting medium consisting of the solid MS medium at half the macronutrient concentration and supplemented with 1.0 mg/L NAA, 0.4 mg/L IBA, and 0.1 mg/L ABT for rooting culture for leaf characteristics estimation and for matrine and oxymatrine content determination.
Leaf characteristics estimation of polyploid plants
Leaf characteristics were obtained from diploid plants and polyploid plants of 30-day-old grown in vitro rooting culture and greenhouse transplanted for 90 days. For stomata measurements, about 0.1 cm2 on the lower epidermis of the leaf was peeled off and spread onto a glass microscope slide. A photomicroscope (Leica DM 2000) was used to measure the stomatal apparatus length and width. Four unifoliate leaves were chosen from the same part of each of five diploid plants, and each of five tetraploid plants and 20 stomatal apparatus was measured for each leaf.
Matrine and oxymatrine contents determination of polyploid plants
The radix ex rhizoma was collected from 90-day-old in vitro rooting culture of diploid lines and 13 selected tetraploid lines and was dried to determine the contents of matrine and oxymatrine according to the guidelines of the Chinese Pharmacopoeia (edition 2015).[2] 0.5 g sample of the fine-grinded powder was weighed accurately (mixture of root and rhizomes was used) and this was introduced into a flask, extracted with 50 mL of chloroform-methanol-concentrated ammonia solution (40:10:1 v/v) by ultrasonication (power 250 W, frequency 40 kHz) at room temperature for 30 min, complemented weightlessness with chloroform-methanol-concentrated ammonia solution (40:10:1 v/v), and mixed and filtered through filter paper. 10 mL of resulting filtrate was evaporated under vacuum and diluted with methanol to 10 mL, and then the solution was filtrated through a 0.45 μm filter for HPLC analysis. The HPLC conditions were as follows: Phenomenex Luna NH2 100 A column (5 μm, 150 mm × 4.6 mm) with a column temperature of 25°C and an elution solvent of acetonitrile-isopropanol-3% phosphoric acid solution (80:5:15 v/v) with a flow rate of 0.5 mL/min and the detection wavelength of 210 nm.
RESULTS
Effect of colchicine treatment on inducing tetraploid buds
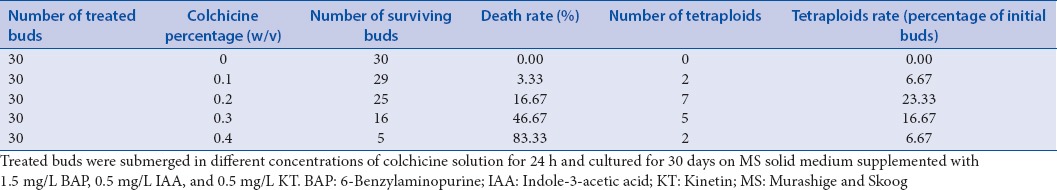
After treatment with increasing concentrations of colchicine solution for 24 h, as the number of surviving buds decreased rapidly, the death rates of treated buds were increased significantly, while all of the untreated buds survived. The results in Table 1 show that the death rate of buds was 3.33% when the colchicine concentration was 0.1% (w/v); however, as the colchicine concentration increased to 0.4% (w/v), the death rate of buds increased to 83.3%. To identify the tetraploids from the regenerated plants, the root-tip chromosome numbers of treated plants were counted using photomicroscopy. According to the chromosome count, the tetraploid induction rates were influenced by the colchicine concentration. As seen, the induction rates were increased with the first three concentrations but decreased when the colchicine concentration reached 0.3%. The highest induction rate was found at a concentration of 0.2%, and this was 23.33%. While the lowest induction rates were found at the concentration of 0.1% and 0.4%, both of these were 6.67%. These results indicated that the optimum concentration of colchicine treatment for tetraploid induction was 0.2% [Table 1].
Table 1.
The effect of different colchicine concentration on polyploid induction in Sophora tonkinensis
Based on the above experiment, the optimum concentration of colchicine treatment for tetraploid induction was 0.2%, so this concentration of colchicine was used to select the optimum treatment time for the tetraploid induction of S. tonkinensis. All of the buds were submerged in 0.2% colchicine solution for different time periods, and the effects on the tetraploid induction are shown in Table 2.
Table 2.
The effect of different treatment times of 0.2% (w/v) colchicine solution on polyploid induction in Sophora tonkinensis
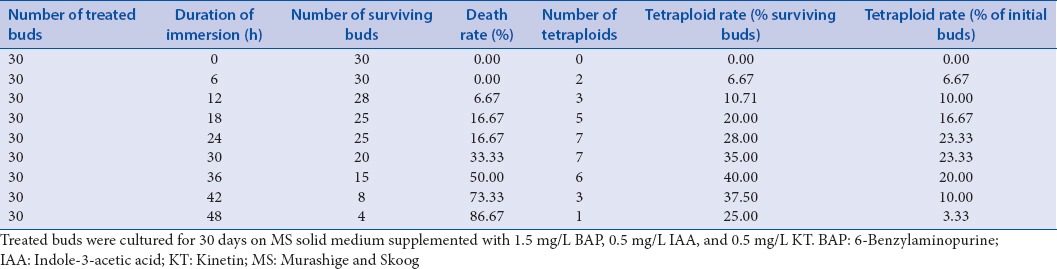
The results showed that the death rates of treated buds were increased rapidly with increasing treatment time because the number of survival buds decreased significantly. No bud died at the treatment time of 6 h, but only four buds were survival when the treatment time was increased to 48 h. According to the chromosome counts, the tetraploid induction rates were also influenced by the treatment time in 0.2% w/v colchicine solution. Immersing buds in 0.2% colchicine solution for 6, 12, 18, 24, 30, 36, 42, and 48 h could induce polyploidy in S. tonkinensis, but the induction rates were significantly different between differently treated time groups. At the shorter treatment times (no longer than 24–30 h), the induction rates increased gradually as the treated time increased. However, as the treatment time was extended, induction rates decreased gradually because of the death rates increasing. Both of the 24-h and 30-h treated groups possessed the highest polyploid induction rate of 23.33%; however, because the number of surviving buds at 30 h (20) was lower than that of the 24-h treated group (25), the induction rate in surviving buds of the 30-h treated group (35%) was higher than that of 24-h treated group (28%), so it was concluded that the optimum treatment time for tetraploid induction was 30 h when immersed in 0.2% colchicine. This is by far the highest induction ratio in our experiments.
The root-tip chromosome determination results showed that the tetraploid plantlets had 36 chromosomes (4x = 36) [Figure 1a and b] and 50 tetraploid plantlets were obtained in our experiments [Tables 1 and 2].
Figure 1.
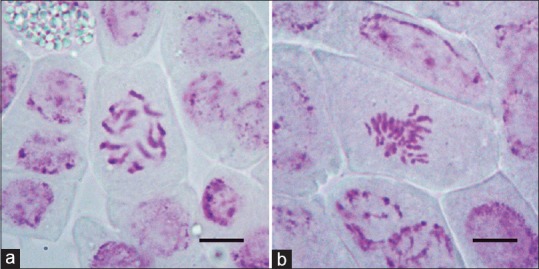
(a) The chromosomes of diploid plant, 2n = 2x = 18 (bar = 2 μm). (b) The chromosomes of tetraploid plant, 2n = 4x = 36 (bar = 2 μm)
Morphological differences between diploid and tetraploid plants
The indices (length and width) of leaf and stomatal apparatus were evaluated and compared to diploid control plants. Table 3 and Figure 2 show that the leaves of tetraploid plants appeared normal in shape when compared with diploid plants. The average leaf lengths and widths in diploid and tetraploids plants when being cultured in vitro were 2.39 and 2.44 cm and 1.85 and 1.91 cm, respectively. The leaf indices of tetraploid plants were slightly larger than diploid plants, but this was not significantly different. After transplanting to a greenhouse for 90 days, the leaves of tetraploid plants and diploid plants grew larger; the average of leaf lengths and widths of diploid plants and tetraploid plants was 2.92 and 3.12 cm and 2.12 and 2.26 cm, respectively, with the leaf indices of tetraploid plants also being larger than diploid plants. In this case, there was a significant difference between the average leaf length of tetraploid and diploid plants.
Table 3.
Leaf characteristics of diploid and tetraploid in glasshouse-grown of Sophora tonkinensis
Figure 2.

Plant morphology of (a) diploid plant and (b) tetraploid plant Sophora tonkinensis of 90-day-old grown in a glasshouse
The sizes of the stomatal apparatus of tetraploid leaves for 30-day-old in vitro cultures and 90-day-old glasshouse-grown are listed in Table 3 and Figure 3. In general, the stomata in tetraploid leaves were longer and wider than that of diploid leaves. The length and width of the stomatal apparatus of diploid leaves for 30-day-old in vitro cultures were about 24.18 μm and 21.56 μm, respectively, while the same dimensions of in vitro culture tetraploid leaves were about 24.53 μm and 22.16 μm, respectively. From Duncan's multiple range test analysis, we found that there was a significant difference between the average stomatal apparatus width of tetraploid plants and diploid plants, under in vitro culture conditions. The length and width of the stomatal apparatus in 90-day-old diploid leaves were also compared. The results showed that the length and width of the stomatal apparatus of 90-day-old greenhouse-grown diploid leaves were about 30.74 μm and 26.63 μm, respectively, while the same dimensions for 90-day-old greenhouse-grown tetraploid leaves were about 32.95 μm and 27.74 μm, respectively. The Duncan's multiple range test analysis showed that both stomatal apparatus length and width of tetraploid plants and diploid plants had significant differences when greenhouse-grown for 90 days old. From these results, we also found that there was a significant difference when the same ploidy material was grown under different conditions [Table 3]. The glasshouse-grown diploid material possessed longer and wider stomatal apparatus when compared to diploid material. The same phenomena were also found in tetraploidy.
Figure 3.
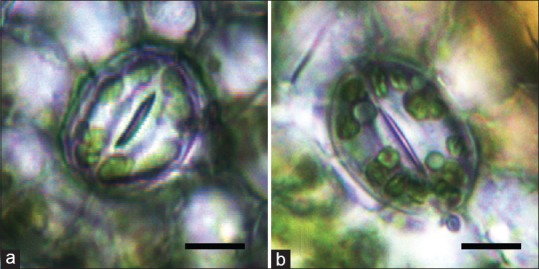
(a and b) Stomatal apparatus of diploid and tetraploid plants in Sophora tonkinensis from a glasshouse. Each stomatal apparatus was obtained from the same part of diploid (a) and tetraploid (b) unifoliate leaves in the greenhouse (bar = 10 μm)
Determination of rhizome yield and major chemical constituents
To compare the content of the effective constituents of diploid and tetraploid plants of S. tonkinensis, the radix ex rhizoma samples of each tetraploid line for 90-day-old in vitro rooting culture were extracted and analyzed by HPLC.
The content of matrine and oxymatrine in each tetraploid line is shown in Table 4. The results indicate that the matrine content of diploid and some tetraploid lines for 90-day-old in vitro rooting culture was too low to detect, and the highest matrine content was obtained from tetraploid line D-37, which was 0.021 mg/g. The highest oxymatrine content in tetraploid lines was 0.087 mg/g in line D-96, which was 107.1% higher than that of control. For the 13 tetraploid lines analyzed, 12 of them showed higher oxymatrine content than that of the control (0.042 mg/g). Only one line (E-41) showed a little lower oxymatrine content than that of the control and this was 0.037 mg/g. The results also showed that almost all of the tetraploid lines showed higher total content of matrine and oxymatrine than that of the control sample (0.042 mg/g), except line E-41, which was only 0.040 mg/g. The highest total content of matrine and oxymatrine was obtained from line A-98, which was 0.097, that is 1.31 times higher than that of than that of the control. From all the 13 tetraploid lines investigated, the total content of matrine and oxymatrine in nine lines was 0.5 times higher than that of control, and four lines of these were 1 time higher than that of the control. These lines will be further studied to select new varieties for further research work.
Table 4.
The contents of matrine and oxymatrine in diploid and tetraploid rhizomes of Sophora tonkinensis
DISCUSSION
With the modernization of traditional Chinese medicine development, there are more and more demands for the Chinese herbal medicine, which cannot be met by the wild resources. Hence, access to high quality and high yield of Chinese herbal medicine is imminent. Artificial induction of polyploids is one of the most important ways to obtain new plant type or new germplasm. Induced polyploidy breeding technology is high frequency, simple, and quick of medicinal plants; it has obtained huge nutritional organs, such as roots, stems, leaves, flowers, and fruits increased. In addition, it can enhance the disease resistance, increase the content of medicinal active ingredient, and also improve the quality and yield of medicinal plants. The polyploid breeding of medicinal plant has special application value and great yield potential.
This paper is the first to describe this methodology for efficient in vitro induction of tetraploid in S. tonkinensis by treating shoot tips with colchicine. Colchicine is the most widely used mutagens in polyploidy breeding, and many of the medicinal polyploids have been obtained by the colchicine. The method used to induction of artificial polyploidy is the advantages of economic convenience, specificity of mutagenesis, induced mutation broad spectrum, and so on. Examples of this include Pogostemon cablin.,[19] Phlox subulata L.,[20] Morus alba L.,[21] and Zingiber officinale Rosc.[22] In our research, we found that the highest percentage of tetraploid induction was 23.33% and occurred in the presence of 0.2% colchicine treatment for 30 h.
Morphological identification is a good method for primary selection of polyploid. The external morphology is changed continuously with the increase of chromosome number, being known as the “gigas” effect. If the morphology change occurrs in organs of commercial interest, which may become a valuable feature for crop improvement. In our observation, polyploids possess longer and wider stomatal apparatus and leaf sizes. Thus, the leaf characteristics are sometimes used as an indicator of the ploidy level of plants.[23,24] The method used to measure stomatal size is simple, almost nondestructive, and does not require expensive instruments. In this present study, the leaf and stomatal apparatus size of tetraploids were larger than that of the diploid plants, which indicated that the measurement of leaf characteristics could be an effective way to select tetraploid plants of S. tonkinensis.
In medicinal species, a high active compound content is important for their clinical use and industrial extraction of natural products.[18] For S. tonkinensis, increasing active compound content not only could improve the quality of medicinal materials but also could reduce the extraction cost and usage of medicinal materials, which could promote the sustainable development of S. tonkinensis resources. In our experiments, 12 lines of the 13 selected tetraploid plants of S. tonkinensis possessed higher total content of matrine and oxymatrine, and four lines of them were 1 time higher than that of the control, which proved that tetraploid plant breeding could improve the active compound content of S. tonkinensis.
Despite enormous progress in the artificial induction of polyploids, there are also some technical problems to be unanswered, and more research is needed to confirm the effects of both auto- and allo-polyploidy in plant genomes.
First, the combination of colchicine concentration and treatment times is the key factor affecting tetraploid induction efficiency. Not all Chinese herbal medicine can obtained improving yield and value by polyploid breeding technology; we might require select the obvious advantages of plant, except the use of seeds as the main breeding methods of plants, before the experiment.
Second, the result of the polyploid breeding suggests that problems with the chimerism exist. Most of the plants after induction are mostly chimeras, and it needs to screen out a complete polyploid plant in subsequent generations. At present, callus or other explants, single cell culture, suspension culture, and plant tissue culture can be very efficiently used to improve polyploid homozygote. However, how to screen out the polyploid we need from many chimeric tissues is still a hot and difficult thing worthy of our further study.
In addition, we should attach great importance not only to the production of medicinal plants but also to take into account the content of its medicinal ingredients. At present, the determination of plant active ingredient content and genetic control mechanism during the polyploid breeding of plant were studied through extensive simulations, to attain certain the breakthrough progress, the best benefits, to produce qualified Chinese herbal medicine
CONCLUSION
An efficient colchicine-mediated technique for in vitro induction of tetraploids in S. tonkinensis had been established, and this protocol is a simple, effective and is a reliable technique for producing large numbers of tetraploid plants in S. tonkinensis. Twelve lines of tetraploid with good quality of higher total content of matrine and oxymatrine were found and were selected to be used in further breeding programs aimed at increasing production of medicinal compounds from this species.
Financial support and sponsorship
This research was funded by National Public Welfare Special Project of China “Quality Guarantee system of Chinese herbal medicines” (201507002), National Natural Science Foundation of China (81460582, 81473309), Guangxi Natural Science Foundation of China (2013GXNSFBA019086) and China Agriculture Research System(CARS-21).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Dr. Dev Sooranna, Imperial College London, for helping to edit the manuscript.
REFERENCES
- 1.Wang JM, Cui Y. Research progress in chemical components, pharmacological effectiveness and toxicity of radix et Rhizoma Sophora tonkinensis. Chin J Exp Tradit Med Form. 2011;17:229–32. [Google Scholar]
- 2.Committee for the Pharmacopoeia of PR China. PR China, Beijing: People's Health Publishing House; 2015. Pharmacopoeia of PR China. Part I. [Google Scholar]
- 3.Zheng LN, Sun H, Xie YZ, Sun R. Research progress on chemical compositions of Sophora tonkinensis radix et rhizoma related to its efficacy and toxicity. Food Drug. 2011;13:205–9. [Google Scholar]
- 4.Du ZH, Liu HG, Chai CY, Luo LY, Hu CJ. Anti-inflammatory effect of Dauricine. Zhongguo Yao Li Xue Bao. 1986;7:419–22. [PubMed] [Google Scholar]
- 5.Zhou YH, Zhou JY, Wang LJ, Li JN. Modulation of matrine on sodium current induced by Ouabain. Prog Mod Biomed. 2015;15:5609–12. 5640. [Google Scholar]
- 6.Qin XG, Hua Z, Shuang W, Wang YH, Cui YD. Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteins in vitro. Pharm Biol. 2010;48:275–81. doi: 10.3109/13880200903104101. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Meng F, Li J, Sun X. Anti-apoptosis effects of oxymatrine protect the liver from warm ischemia reperfusion injury in rats. World J Surg. 2005;29:1397–401. doi: 10.1007/s00268-005-7885-y. [DOI] [PubMed] [Google Scholar]
- 8.Huang BY, Nong DX, Huang XY, Peng YD, Li Y, Lin Y, et al. Resources investigation report on Chinese materia medica Sophora tonkinensis. Modern Chin Med. 2014;16:740–4. [Google Scholar]
- 9.Shen L, Luo Y, Zhang PG, Huang RS. The progress of resource status and quality standard in Sophora tonkinensis. Da Zhong Ke Ji. 2011;5:145–6. [Google Scholar]
- 10.Li LX, Wei KH, Yao SC, Tang MQ, Wei F, Miao JH. Establishment and optimization of Sophora tonkinensis hairy root culture. Guizhou Agric Sci. 2016;44:27–31. [Google Scholar]
- 11.Qian WB, Huang BC, Huang CZ. Cultivation situation and counter measures of Guangxi Sophora tonkinensis Gagnep. Sci Farming. 2015;7:63. [Google Scholar]
- 12.Tang MQ, Wei F, Li LX, Huang YC, Qin LY, Miao JH. Study on genomic DNA extraction method for genuine medicinal material in Guangxi Sophora tonkinensis Gagnep. J Anhui Agric Sci. 2010;38:8973–4. [Google Scholar]
- 13.Li LX, Wei KH, Tang MQ, Huang YC, Miao JH. Optimized tissue culture conditions of Sophora tonkinensis by orthogonal experimentation. J Chin Med Mater. 2012;35:514–7. [Google Scholar]
- 14.Qin W, Ling Z, Xu H, Lan Z, Wu Q. Comparative analysis on effective compositions from tissue-cultured and wild Sophora tonkinensis. Zhong Yao Cai. 2004;27:552–3. [PubMed] [Google Scholar]
- 15.Qin WL, Ling ZZ, Xu HY, Lan ZZ, Wu QH, He B. Studies on tissue culture and plant regeneration of Sophora tonkinensis Gapnep. China J Chin Mater Med. 2005;30:303–4. [Google Scholar]
- 16.Kun-Hua W, Lin-Xuan L, Yong-Cai H, Mei-Ying W, Cui L, Jian-Hua M, et al. Tissue culture of Sophora tonkinensis Gapnep. and its quality evaluation. Pharmacogn Mag. 2013;9:323–30. doi: 10.4103/0973-1296.117828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant. 1962;15:473–9. [Google Scholar]
- 18.Kun-Hua W, Shan-Lin G, He-Ping H. Tissue culture and generation of autotetraploid plants of Sophora flavescens aiton. Pharmacogn Mag. 2010;6:286–92. doi: 10.4103/0973-1296.71793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan HJ, Xiong Y, Zhang HY, He ML. In vitro induction and morphological characteristics of octoploid plants in Pogostemon cablin. Breed Sci. 2016;66:169–74. doi: 10.1270/jsbbs.66.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZH, Dai HY, Xiao M. In vitro induction of tetraploids in Phlox subulata L. Euphytica. 2008;159:59–65. [Google Scholar]
- 21.Chakraborti SP, Vijayan K, Roy BN, Qadri SM. In vitro induction of tetraploidy in mulberry (Morus alba L.) Plant Cell Rep. 1998;17:799–803. doi: 10.1007/s002990050486. [DOI] [PubMed] [Google Scholar]
- 22.Kun-Hua W, Jian-Hua M, He-Ping H, Shan-Lin G. Generation of autotetraploid plant of ginger (Zingiber officinale rosc.) and its quality evaluation. Pharmacogn Mag. 2011;7:200–6. doi: 10.4103/0973-1296.84230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borrino EM, Powell W. Stomatal guard cell length as an indictor of ploidy in microspore-derived plants of barley. Genome. 1988;30:158–60. [Google Scholar]
- 24.Thomas TD, Bhatnagar AK, Bhojwani SS. Production of triploid plants of mulberry (Morus alba L) by endosperm culture. Plant Cell Rep. 2000;19:395–9. doi: 10.1007/s002990050746. [DOI] [PubMed] [Google Scholar]


