Abstract
Background:
Diabetes mellitus (DM) is a metabolic disorder that occurs as a result of absolute or relative insufficiency of insulin release and/or insulin effect due to impairment of carbohydrate, fat and protein metabolism, and it is characterized by hyperglycemia and leads to various complications.
Objective:
In this study, it was aimed to investigate the effects of hesperidin (HP) and quercetin, which are natural flavonoids, on serum malondialdehyde (MDA), glutathione (GSH), tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) levels in rats with streptozotocin (STZ)-induced diabetes.
Materials and Methods:
The experimental animals were divided into four groups, each group comprising ten rats designated as follows: Group 1 served as control rats (C); Group 2 served as diabetic rats (DM); Group 3 served as diabetic rats administered HP (DM + HP) (100 mg/kg b. w.); and Group 4 served as diabetic rats administered quercetin (DM + Q) (100 mg/kg b. w.).
Results:
Serum MDA and GSH levels were significantly higher in STZ-induced DM group than control group (P < 0.05). In DM + HP and DM + Q groups, MDA levels were significantly decreased compared to DM groups (P < 0.05), but there was no significant difference GSH levels between DM, DM + HP, and DM + Q groups (P > 0.05). TNF-α levels in STZ-induced DM group were significantly decreased compared to control group (P < 0.05), and groups of DM + HP and DM + Q had higher serum TNF-α levels than STZ-induced DM group (P < 0.05). In STZ-induced DM group, serum IL-6 levels were decreased compared to control group (P < 0.05).
Conclusion:
As a result, in this study, we determined that HP and quercetin may play an effective role in regulating insulin metabolism metabolism in diabetes. However, considering the incompatibility of various results in the literature as well as our own results, we think that the actual role of cytokines in the pathogenesis of diabetes is one of the issues that need to be clarified in further studies.
SUMMARY
Hesperidin (HP) and quercetin reduced the insulin, total cholesterol, triglyceride, low-density lipoprotein cholesterol, and malondialdehyde (MDA) serum levels and raised the glutathione (GSH) levels compared to diabetes mellitus (DM) group
SZT-induced DM increased the MDA serum levels and decreased the GSH levels compared to control group
HP and quercetin-treated rats showed higher interleukin-6 and tumor necrosis factor alpha cytokine levels than DM group
HP and quercetin may play an effective role in regulating insulin metabolism in diabetes.
Abbreviations used: DM: Diabetes mellitus, MDA: Malondialdehyde, GSH: Glutathione; IL-6: Interleukin-6, TNF-α: Tumor necrosis factor alpha, HP: Hesperidin, Q; Quercetin, STZ: Streptozotocin, TC: Total cholesterol, TG: Triglyceride, HDL-C: High density lipoprotein cholesterol, LDL-C: Low density lipoprotein cholesterol, VLDL-C: Very-low-density lipoprotein cholesterol.
Keywords: Diabetes mellitus, hesperidin, interleukin-6, quercetin, tumor necrosis factor-alpha
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder that occurs as a result of absolute or relative insufficiency of insulin release and/or insulin effect due to impairment of carbohydrate, fat, and protein metabolism, and it is characterized by hyperglycemia and leads to various complications.[1,2] Formation of free radicals is increased in diabetes, as a result of protein glycation and glucose autoxidation. Increased formation of free radicals and consequently decreased production of antioxidants was observed in diabetic patients. Therefore, the increased free radical concentration is considered to be one of the major complications of diabetes.[3,4] Peroxidation of membrane lipids is one of the most important mechanisms of cell damage due to free radicals. Accordingly, structural and functional damage can occur in the cell membranes. The unsaturated bonds of cholesterol and fatty acids located in the membranes react with free radicals to cause peroxidation.[5] Membrane damage due to lipid peroxidation is irreversible and quite detrimental. Malondialdehyde (MDA), one of the end products of lipid peroxidation, causes cross-linking and polymerization of membrane components, and this causes deformation and changes in the membrane properties such as ion transportation, enzyme activity, and aggregation of cell surface components. Many studies have demonstrated the relationship between diabetic complications and lipid peroxidation. Therefore, controlling lipid peroxidation is critical.[6,7] Under physiological conditions, the harmful effects of free radicals are reduced by the antioxidant defense system. Antioxidants inhibit lipid peroxidation by suppressing the peroxidation chain reaction and/or by collecting reactive oxygen species. Glutathione (GSH) is an endogenous antioxidant, and it is the primary mechanism that reduces intracellular hydroperoxides by reacting with free radicals.[8,9] Numerous genetic and environmental factors play a role in the development of diabetic complications. Studies indicate a relationship between the occurrence and development of diabetic complications and the inflammation caused by increased production and release of cytokines. Nonetheless, the exact mechanism of chronic inflammation in DM could not be clarified, yet it is known that proinflammatory factors are synthesized and released from fat tissue.[10,11] Cytokines are hormone-like mediators in the form of peptides or glycoproteins, that are produced by various cell types, and transmit intracellular signals and exhibit biological functions through specific cell surface receptors.[12] Tumor necrosis factor-alpha (TNF-α) is a potent cytokine, which is among type 1 proinflammatory cytokines released from macrophages an T lymphocytes and plays a dominant role in the development of insulin resistance. TNF-α has important functions in DM and its microvascular complications such as growth stimulation, cytotoxicity, and angiogenesis.[12,13] Interleukin-6 (IL-6) is a cytokine that plays a key role in various cells that are not of immune system as well as in immune regulation. IL-6 is produced in most cell types of the immune system, mainly in endothelial cells, skeletal and smooth muscle cells, adipocytes, and islet β-cells. Induction of adhesion molecules is thought to be involved in the development of atherogenesis and vascular inflammation because of their possible role in damage-related inflammation and the relationship between monocytes and endothelium.[14,15] The reason why DM is a popular research topic is because of its high incidence and high cost of treatment, and the fact that the definitive treatment is not developed yet. For this reason, many studies are being conducted on the treatment of this disease and new treatment methods are sought. Some researchers have focused on surgical procedures for the treatment of this disease while others have tried to develop new treatment options by testing some plant extracts rich in antioxidants.[16] Nutrients with antioxidant potential and various antioxidant compounds in these nutrients are capable of scavenging reactive oxygen derivatives as well as repairing or preventing damage from these compounds.[17] Quercetin (Q), a major flavonoid in human diet, exhibits maximal antiradical properties against hydroxyl radical, peroxyl, and superoxide anion, with respect to other flavonoids. As an effective antioxidant, quercetin is known to have cardioprotective, antiulcerative, anti-inflammatory, antiallergic, antiviral, and antibacterial properties.[18,19,20] Hesperidin (HP), a citrus bioflavonoid, exhibits such biological and pharmacological properties as anti-inflammatory, anticarcinogenic, antioxidative, and lipid-lowering activities.[21,22] In this study, it was aimed to investigate the effects of HP and quercetin, which are natural flavonoids, on serum MDA, GSH, TNF-α, and IL-6 levels in rats with streptozotocin (STZ)-induced diabetes.
MATERIALS AND METHODS
Chemicals
HP, quercetin, and STZ were purchased from Sigma Chemicals (Sigma Chemicals Co., St. Louis, MO, USA), stored at 2°C–4°C and protected from sunlight. All other chemicals were of analytical grade and were obtained from standard commercial supplies.
Experimental animals
This study was carried out in the Experimental Animal Laboratory of Karadeniz Technical University. It was supported by the Scientific Research Projects Department of the Artvin Coruh University with the ethical committee decision of date April 12, 2016, and the project number 2015.M80.02.05. Male Wistar albino rats weighing about 200–250 g and 4–6 weeks of age were used in the experiments. The animals were monitored under standard laboratory conditions of 22°C–25°C and controlled photoperiod of 12:12 h light: Dark in the Animal Laboratory of the Experimental Research Unit of Karadeniz Technical University. During the experiment, the animals were fed with standard laboratory diet and tap water.
Forming experimental groups
The experimental animals were divided into four groups, each group comprising ten rats designated as follows: Group 1 served as control rats (C); Group 2 served as diabetic rats (DM); Group 3 served as diabetic rats administered HP (DM + HP) (100 mg/kg b. w.)[23] in aqueous suspension orally for 15 days; and Group 4 served as diabetic rats administered quercetin (DM + Q) (100 mg/kg b. w.)[24] in aqueous suspension orally for 15 days.
The experimental diabetes model was administered as single-dose intraperitoneal injection of STZ at a dose of 60 mg/kg dissolved in a 0.4 mL (0.1M) sodium citrate buffer (pH: 4.5) using a 26 gauge insulin injector. After 72 h, blood was taken from the tail vein of the rats and measured by a glucometer.[25] Rats with fasting blood glucose over 200 mg/dl were considered as diabetic.[26] Blood samples were taken around 09.00–10.00 in the morning after 8–10 h of fasting to determine fasting blood glucose levels in rats.
Preparation of serum and tissue samples
At the end of the experiment, blood samples were taken by cardiac route to obtain sera samples of the rats which were administered ketamine (60 mg/kg) and xylazine (12 mg/kg) intraperitoneally and afterward cervical dislocations were performed and pancreatic tissues were removed. Blood samples were allowed for 20 min in the separation tubes then centrifuged for 10 min at 3500 rpm; and the supernatant was kept in the Eppendorf tubes at-80°C in the freezer until being analyzed for insulin, triglyceride (TG), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low density lipoprotein (VLDL-C), MDA, GSH, TNF-α, and IL-6.
Biochemical analysis
The level of MDA, a measure of lipid peroxidation, was measured spectrophotometrically by the method described by Okhawa et al.[27] Then, 0.5 mL of 8.1% sodium dodecyl sulfate, 0.5 mL of 0.8% thiobarbituric acid, 1.0 mL of 10% of trichloroacetic acid (TCA), 1.0 mL of 2% glacial acetic acid/sodium hydroxide (pH = 3,5) and 50 μL of 2% butylhydroxytoluene were added to the serum samples (1.0 mL) and this mixture was thoroughly mixed and kept in a water-bath at 95°C for 60 min. After the tubes were chilled, a mixture of 4.0 mL of butanol/pyridine (1:15) was added, and the tubes were centrifuged at 4000 × g at + 4°C for 10 min. After centrifugation, the upper organic phase was removed and the absorbance was read at 532 nm for all samples. The MDA results were stated as nmol/ml.
The GSH level was measured according to the method described by Elman.[28] Accordingly, 1.0 mL of 10% TCA reagent was added to 0.5 mL of serum samples and then centrifuged at 2790 × g for 10 min to precipitate the pellet. The supernatant was transferred to another tube, mixed with 1.0 mL of 5,5 “dithiobis 2-nitrobenzoic acid (DTNB) solution, prepared by dissolving 30 mg DTNB in 1% sodium citrate, and 0.3 M sodium phosphate dibasic (Na2 HPO4) solution was added. When the yellow color appeared, the absorbance values of the samples were read at a wavelength of 412 nm. The GSH results were stated as mg/dl.
The serum TNF-α and IL-6 levels were measured by means of enzyme-linked Immunosorbent Assay (ELISA) technique, using Rat ELISA kit (R and D Systems, USA) with the test procedure suggested by the producer. The TNF-α and IL-6 results were stated as pg/ml.
Histologic analysis
After all, animals were sacrificed; the pancreas tissues were collected and put into 10% neutral formaldehyde solution for fixation. After fixation for 72 h, the pancreas tissues were dehydrated in graded alcohol series, embedded in paraffin, and sectioned into 5 mm thicknesses using a microtome (Leica RM2125RT, Germany). The sections were used for histopathologic analysis. For that analysis, the sections were stained with Crossman modified Mallory's triple staining, and a photograph was taken using a light microscope with a camera attachment (Nikon Eclipse i50, Tokyo, Japan).
Statistical analysis
The SPSS 15.0 (SPSS 13.0 for Windows/SPSS® Inc., Chicago, USA) package program was used to anayze the data and the results were expressed as mean ± standard error. Kolmogorov–Smirnov test was done to evaluate the distribution of variables. Continuous normally distributed measurements were compared across the groups using one-way analysis of variance with the Tukey method and the Student-Newman–Keuls method multiple comparisons. P < 0.05 was accepted as statistically significant.
RESULTS
A total of 40 rats, 10 rats in each group, were included in the study. Initial and final body weights of the rats are shown as the arithmetic mean ± standard error on Table 1. There was no statistically significant difference between the initial body weights of the groups (P > 0.05). At the end of the experiment, the rats in the control group gained weight while the rats in DM, DM + HP, and DM + Q groups lost weight. Body weights in STZ-induced DM, DM + HP, and DM + Q groups were significantly decreased compared to control group (P < 0.05), but there was no significant difference in body weights between DM, DM + Q, and DM + HP groups (P > 0.05).
Table 1.
Mean body weights of experimantal animals

Biochemical results
The biochemical characteristics of the groups are summarized in Table 2. Serum total cholesterol, TG, LDL-C, VLDL-C, MDA, and GSH levels were significantly higher in STZ-induced DM group than control group (P < 0.05), but there was no significant difference HDL-C levels between groups (P > 0.05). In DM + HP and DM + Q groups, serum total cholesterol, TG, LDL, VLDL, and MDA levels were significantly decreased compared to DM groups (P < 0.05). Administration of quercetin and HP caused an increase in GSH levels more than those subjects in DM group, but this increase was not statistically significant (P > 0.05). Insulin concentrations were significantly decreased compared to control group (P < 0.05), and groups of DM + HP and DM + Q had higher insulin concentrations than STZ-induced DM group (P < 0.05). TNF-α levels in STZ-induced DM group were significantly decreased compared to control group (P < 0.05), and groups of DM + HP and DM + Q had higher serum TNF-α levels than STZ-induced DM group (P < 0.05) [Figure 1]. In STZ-induced DM group, serum IL-6 levels were decreased compared to control group (P < 0.05). There was no significant difference in serum IL-6 levels between DM, DM + Q and DM + HP groups (P > 0.05) [Figure 2].
Table 2.
Biochemical paramaters of the groups
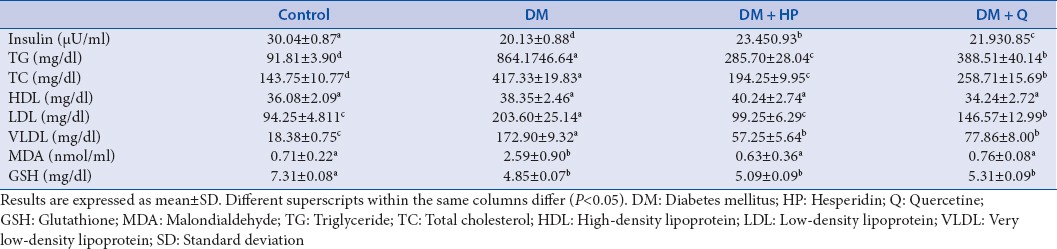
Figure 1.
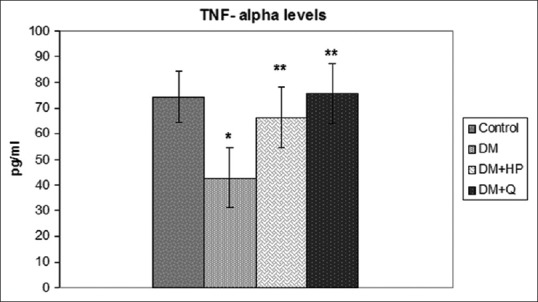
Serum tumor necrosis factor alpha levels in experimental groups. *Significantly different when compared with control group, (P < 0.05), **Significantly different when compared with diabetes mellitus group, (P < 0.05)
Figure 2.
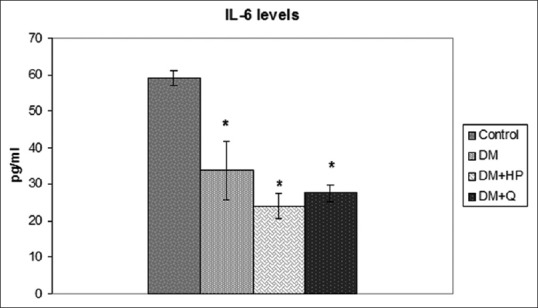
Serum interleukin-6 levels in experimental groups. *Significantly different when compared with control group, (P < 0.05)
Histologic results
In analysis of all groups, islets of Langerhans of control group were seen normal histologic structure as ellipsoids to round in shape and composed specifically of beta cells located at the central zone [Figure 3]. Other endocrine cells in pancreas were generally placed in the peripheral region of Langerhans islets. However, in untreated diabetic group, many cells were characterized by the presence of cytoplasmic degranulation, pyknosis, and vacuolization. On the other hand, treatment with HP of diabetic rats led to a decrease in degenerated islet cells as compared with untreated diabetic group. Moreover, in quercetin group, a few of degenerated cells were observed as compared to those on untreated diabetic group [Figure 3].
Figure 3.
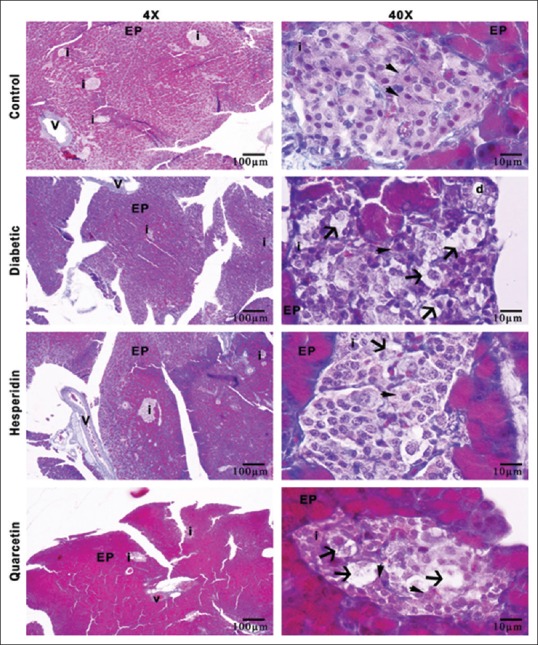
Illustration of histologic structure of rat pancreas tissues for all groups, i: Langerhans islets, EP: Exocrine pancreas, v: Vessel of pancreas, d: Ductus of pancreas, arrowhead; beta cells arrow; degenerated beta cells, Crossman-modified triple staining
DISCUSSION
DM is a chronic disease with high mortality and morbidity that is rapidly increasing worldwide due to changing living conditions, and it is an endocrine disease as well as a metabolic disease characterized by impaired metabolism of carbohydrate, lipid, and protein.[29,30] Recently, there has been increased interest in natural antioxidants in plants. Scientific reports and laboratory studies showed that the plants contain substances with a wide variety of antioxidant properties. Although numerous drugs have been developed and used for the treatment of diabetes, it is mainly because of their toxicity effect to the liver and kidneys, as well as their failure to respond to patient expectations, today we observe that alternative treatment options are also gaining importance. Indeed, about 800 kinds of plants, having the potential of clinical application are used in the treatment of diabetes today.[31,32] In our study, we determined the effect of quercetin and HP, which are also natural flavonoids obtained from plants, on insulin, HDL-C, LDL-C, TG, MDA, GSH, TNF-α, and IL-6 in experimental diabetes-induced rats.
In their study on rats with STZ-induced DM, Pawelczyk et al.[33] observed a significant decrease in plasma insulin levels and an increase in glucose levels. In another study, Sanders et al.[34] reported significantly elevated serum glucose levels in the rats of STZ-induced DM groups while the results in the control and quercetin-administered rat groups were close to each other. In the serum samples obtained in our study, TG and LDL-C levels increased significantly in the diabetic rats compared to the control group, and insulin levels decreased in the DM group compared to the control group. We observed no statistically significant difference in HDL-C levels between the groups in our study.
In rats, flavonoids were reported to protect the Langerhans islets in pancreas, after diabetes was induced with STZ; however, regeneration was reported in these cells.[35] STZ leads to insulin insufficiency by causing insulin deficiency in ß cells where insulin production occurs.[31] Quercetin was also reported to have antifibrotic, anticoagulative, antibacterial, antiatherogenic, anticancerogenic, antihypertensive, and antiproliferative properties.[36,37] In many studies, quercetin supplements were shown to have positive effects on decreasing insulin levels in diabetic rats.[32,35,38] In accordance with the previous studies, quercetin administration reduced blood TG and LDL-C levels and increased insulin levels of diabetic rats compared with DM group in this study.[32,34] In their study investigating antidiabetic effects of quercetin in STZ-induced diabetic rats, Vessal et al.[39] reported that plasma glucose levels significantly decreased and insulin levels increased in the diabetic group treated with quercetin with respect to the STZ-induced diabetic group. Abdelmoaty et al.[35] also reported in their study that, quercetin administration reduced blood glucose levels while increased insulin levels in those rats that diabetes was induced, suggesting that quercetin can reduce oxidative stress through its antioxidant properties. HP, a member of the biflavonoid group, is a potent antioxidant with effects similar to Vitamin E. It exhibits various biological effects in many mammalian cell systems. Its antimicrobial, antiviral, antihypertensive, hypolipidemic, antiulcerogenic, antineoplastic, anti-inflammatory, antioxidant, and antihepatotoxic effects were shown in in vitro and in vivo studies.[40,41] In their study, Akiyama et al.[42] reported that HP decreased glucose levels and increased insulin levels in the STZ-induced diabetic model. In another study, Mahmoud et al.[43] established a diabetes model with STZ and reported that HP administration significantly reduced the elevated levels of TG and LDL-C in the diabetic group. It was also observed in our study that blood TG and LDL-C levels decreased significantly and insulin levels increased in the quercetin and HP -treated groups in comparison to the DM group, in accordance with the literature.
In the studies investigating the correlation of oxidative stress with diabetes and diabetes-related complications, it is suggested that the damage occurred in the β-cells, which are known to be very susceptible to oxidative stress, may be due to the toxic effects of hyperglycemia.[35,38] It is also underlined in these studies that, tissue damage, which is the result of energy metabolism, increased the production of free radicals and lipid peroxidation and suppresses antioxidant system. Studies on oxidant-antioxidant status in rats before and after diabetes and determined that the level of MDA, the end product of lipid peroxides, were significantly increased in the diabetic group, indicating that, an increase in lipid peroxidation may be important criteria for the development of DM and related complications.[9,44,45]
In recent years, studies on experimental diabetes-induced rats and diabetic patients revealed that free oxygen radicals and lipid peroxidation increase considerably and take an active role in the etiology and development of diabetes.[44] The most important problem in DM is the accumulation of glucose in blood when it is not metabolized and the increase of harmful free radicals due to the use of lipids in energy production. In the case of diabetes, autoxidation of glucose increases and oxidized glucose is transformed into glucose acids, and in the meantime, it leads to the formation of free radicals.[43] We found a statistically significant difference in MDA levels of the STZ-induced DM group in comparison to the control group, which was compatible with the literature. We determined a statistically significant difference in MDA levels of our DM + Q and DM + HP groups in comparison to DM group. We can suggest that elevated MDA levels in diabetic rats might reflect increased rate of superoxide radical production while reduced MDA levels may be related to the capture of the resulting radicals by quercetin and HP. We have the conviction that the administration of quercetin and HP has a positive effect on lipid peroxidation, which may be related to the enhancement of the endogenous antioxidant defense system by HP and quercetin to scavenge the radicals from the environment, hence, prevent the formation of MDA.
GSH is an important antioxidant molecule capable of scavenging free radicals and repairing biological damage from free radicals. GSH, which exists in reduced form in the cell, reduces endogenously produced peroxides as it gets oxidized. Most of the GSH must be kept in a reduced state so that it can effectively protect the cell. GSH inhibits free radicals by participating in detoxification reactions and supports sustaining the normal cell structure and function by protecting redox homeostasis. In the studies, GSH levels are reported to be significantly lower in diabetic patients than in healthy controls.[9,46] In our study, a statistically significant decrease in GSH levels was determined in the DM group in comparison to the control group. The decrease determined in GSH levels in diabetes indicates that its consumption gets increased by the body against oxidative stress. In our study, administration of quercetin and HP caused an increase in GSH levels more than those subjects in DM group, but this increase was not statistically significant. Soliman[47] pointed out that hyperglycemia increased oxidative stress and remarked that the resultant antioxidant depletion should be considered as a risk factor for the development of diabetic complications. It was also concluded that the increase in MDA levels and the decrease in GSH levels can lead to imbalance and instability between plasma oxidant and antioxidant systems.[47]
Most of the interactions between immune system cells are controlled by soluble mediators called cytokines. Cytokines are not only the kind of proteins regulating the local or systemic inflammatory responses through cell-to-cell interactions but they also play a role in wound healing, hematopoiesis and many other biological events. TNF-α, one of the major proinflammatory cytokines released from mononuclear cells, was reported to induce the changes in endothelial cell morphology and behavior, also stimulate cytokine synthesis and extracellular matrix proteins.[48,49] There are findings that IL-6 contributes to the development processes of both types of diabetes and has influence on glucose balance and metabolism through pancreatic beta cells.[14] These two cytokines were also suggested to be associated with diabetic microvascular complications, and TNF-α was involved in the pathogenesis of diabetes as a consequence of increased serum TNF-α levels in DM.[50] Fidan et al.[51] determined that serum TNF-α and IL-6 levels in diabetic female rats induced with STZ for 6–8 weeks were similar to control subjects. Altinova et al.[52] showed no statistically significant difference for serum TNF-α and IL-6 levels between patient groups with and without microvascular complications of DM. In the studies examining TNF-α levels in diabetes, there are reports showing similar as well as increased TNF-α levels, in comparison to the control subjects.[53,54,55] In our study results, TNF-α and IL-6 levels statistically significantly decreased in STZ-induced DM group with respect to the control group. There are many diabetes studies on cytokines in the literature with conflicting results and it is not clear yet whether cytokine parameters can be used to assess the pathogenesis of diabetes. At the end of our study, weights of the rats of STZ-induced DM group decreased statistically significantly compared to the control group. IL-6 and TNF-α are considered as adipokines secreted from adipose tissue macrophages. Inflammatory markers in plasma such as IL-6 and TNF-α were reported to increase constantly in obesity and correlate with adiposity.[56,57] Studies reported the secretion of TNF-α and IL-6 by subcutaneous fat tissue.[58,59] In our study, decreased levels of TNF-α and IL-6 in the DM group can be possibly attributed to the ability of the adipose tissue to produce[60,61] and secrete TNF-α and IL-6.[62] We speculate that weight loss determined in the rats of the DM group can result in decreased production of TNF-α and IL-6 levels secreted by adipose cells due to decreased fat tissue.
DM is characterized by permanent destruction of pancreatic beta cells. This destruction leads to degranulation and decreased insulin secretion.[63] In our study, we observed some morphological changes in the pancreas of STZ-induced diabetic rats, that regressed by the administration of HP and quercetin, which is likely to be the result of their antioxidant effect. There are many studies in the literature reporting different results. This difference may be due to measurement of TNF-α or TNF-α receptor level. The reason is, there is evidence that the level of TNF-α receptor may better reflect TNF-α activity, yet there is no consensus on this conception. Some studies measure TNF-α levels while others TNF-α receptor levels. In our study, HP and quercetin administration increased TNF-α levels statistically significantly as compared to DM group while there was no significant difference in IL-6 levels.
As a result, in this study we determined that HP and quercetin, which are natural flavonoids and favorable as active antioxidants, showed positive effects on insulin, TG, LDL-C, and MDA levels of the experimental diabetic rats induced with STZ and we also hypothesize that they may play an effective role in regulating insulin metabolism in diabetes. However, considering the incompatibility of various results in the literature as well as our own results, we think that the actual role of cytokines in the pathogenesis of diabetes is one of the issues that need to be clarified in further studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by Coordinator of Scientific Research Projects (2015.M80.02.05) at University of Artvin Coruh.
REFERENCES
- 1.Ahmed I, Adeghate E, Cummings E, Sharma AK, Singh J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol Cell Biochem. 2004;261:63–70. doi: 10.1023/b:mcbi.0000028738.95518.90. [DOI] [PubMed] [Google Scholar]
- 2.Buyukleblebici O, Karagul H. The biochemical effects of dietary chromium in experimental diabetic rats with streptozotocin. Kafkas Univ J Vet Med. 2012;18:21–6. [Google Scholar]
- 3.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–73. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 5.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–73. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–4. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 7.Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–28. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 8.Ozgun GS, Eskiocak S, Sut N. The effects of l-carnitine on protein oxidation of streptozotocin-ınduced diabetic rats. Turk J Biochem. 2010;35:183–9. [Google Scholar]
- 9.Abou-Seif MA, Youssef AA. Evaluation of some biochemical changes in diabetic patients. Clin Chim Acta. 2004;346:161–70. doi: 10.1016/j.cccn.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–42. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 11.Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005;20:2601–4. doi: 10.1093/ndt/gfi155. [DOI] [PubMed] [Google Scholar]
- 12.Douglas D. Inflammatory cytokines tied to risk of type 2. Diabetes. 2003;52:812–7. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 13.Swaroop JJ, Rajarajeswari D, Naidu JN. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012;135:127–30. doi: 10.4103/0971-5916.93435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: The good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–24. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 15.Qu D, Liu J, Lau CW, Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol. 2014;171:3595–603. doi: 10.1111/bph.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HX, Ng TB. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65:2663–77. doi: 10.1016/s0024-3205(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 17.Stephen Irudayaraj S, Sunil C, Duraipandiyan V, Ignacimuthu S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) lam. Leaves in streptozotocin induced diabetic rats. J Ethnopharmacol. 2012;143:515–23. doi: 10.1016/j.jep.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Babujanarthanam R, Kavitha P, Mahadeva Rao US, Pandian MR. Quercitrin a bioflavonoid improves the antioxidant status in streptozotocin. 2011;358:121–9. doi: 10.1007/s11010-011-0927-x. [DOI] [PubMed] [Google Scholar]
- 19.Coldiron AD, Jr, Sanders RA, Watkins JB., 3rd Effects of combined quercetin and coenzyme Q (10) treatment on oxidative stress in normal and diabetic rats. J Biochem Mol Toxicol. 2002;16:197–202. doi: 10.1002/jbt.10035. [DOI] [PubMed] [Google Scholar]
- 20.Casagrande R, Georgetti SR, Verri WA, Jr, Jabor JR, Santos AC, Fonseca MJ, et al. Evaluation of functional stability of quercetin as a raw material and in different topical formulations by its antilipoperoxidative activity. AAPS PharmSciTech. 2006;7:E10. doi: 10.1208/pt070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama S, Katsumata S, Suzuki K, Nakaya Y, Ishimi Y, Uehara M, et al. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in goto-kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem. 2009;73:2779–82. doi: 10.1271/bbb.90576. [DOI] [PubMed] [Google Scholar]
- 22.Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res. 2004;24:851–74. [Google Scholar]
- 23.Kakadiya J, Mulani H, Shah N. Protective effect of hesperidin on cardiovascular complication in experimentally induced myocardial infarction in diabetes in rats. J Basic Clin Pharm. 2010;1:85–91. [PMC free article] [PubMed] [Google Scholar]
- 24.Demir EA, Gergerlioglu HS, Oz M. Antidepressant-like effects of quercetin in diabetic rats are independent of hypothalamic-pituitary-adrenal axis. Acta Neuropsychiatr. 2016;28:23–30. doi: 10.1017/neu.2015.45. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Lobo AM, Buonfiglio DC, Cipolla-Neto J. Streptozotocin-induced diabetes disrupts the body temperature daily rhythm in rats. Diabetol Metab Syndr. 2015;7:39. doi: 10.1186/s13098-015-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewanjee S, Das AK, Sahu R, Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: Effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 2009;47:2679–85. doi: 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 28.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed I, Goldstein B. Diabetes mellitus. Clin Dermatol. 2006;24:237–46. doi: 10.1016/j.clindermatol.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Erukainure OL, Ebuehi OA, Adeboyejo FO, Aliyu M, Elemo GN. Hematological and biochemical changes in diabetic rats fed with fiber-enriched cake. J Acute Med. 2013;3:39–44. [Google Scholar]
- 31.Demir E, Yılmaz O. Effect of pine oil on antihyperglycemic and some biochemical parameters in serum of streptozotocin ınduced tip-2 diabetic rats. Mufbed. 2013;25:140–56. [Google Scholar]
- 32.Maciel RM, Costa MM, Martins DB, França RT, Schmatz R, Graça DL, et al. Antioxidant and anti-inflammatory effects of quercetin in functional and morphological alterations in streptozotocin-induced diabetic rats. Res Vet Sci. 2013;95:389–97. doi: 10.1016/j.rvsc.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Pawelczyk T, Sakowicz M, Szczepanska-Konkel M, Angielski S. Decreased expression of adenosine kinase in streptozotocin-induced diabetes mellitus rats. Arch Biochem Biophys. 2000;375:1–6. doi: 10.1006/abbi.1999.1548. [DOI] [PubMed] [Google Scholar]
- 34.Sanders RA, Rauscher FM, Watkins JB., 3rd Effects of quercetin on antioxidant defense in streptozotocin-ınduced diabetic rats. J Biochem Mol Toxicol. 2011;15:143–9. doi: 10.1002/jbt.11. [DOI] [PubMed] [Google Scholar]
- 35.Abdelmoaty MA, Ibrahim MA, Ahmed NS, Abdelaziz MA. Confırmatory studıes on the antıoxıdant and antıdıabetıc effect of quercetın ın rats. Indian J Clin Biochem. 2010;25:188–92. doi: 10.1007/s12291-010-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo M, Spagnuolo C, Tedesco I, Bilotto S, Russo GL. The flavonoid quercetin in disease prevention and theraphy: Facts and fancies. Biochem Pharmacol. 2012;83:6–15. doi: 10.1016/j.bcp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Leiherer A, Mündlein A, Drexel H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul Pharmacol. 2013;58:3–20. doi: 10.1016/j.vph.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and ß cell damage in rat pancreas. Pharmacol Res. 2005;51:117–23. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 40.Guardia T, Rotelli AE, Juardez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effect of rutin, quarcetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 41.Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 42.Akiyama S, Katsumata S, Suzuki K, Ishimi Y, Wu J, Uehara M, et al. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 2010;46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoud AM, Ashour MB, Abdel-Moneim A, Ahmed OM. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26:483–90. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Altan N, Dincel AS, Koca C. Diabetes mellitus and oxidative stres. Turk J Biochem. 2006;31:51–6. [Google Scholar]
- 45.Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109:8–14. doi: 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Pari L, Saravanan R. Beneficial effect of succinic acid monoethyl ester on erythrocyte membrane bound enzymes and antioxidant status in streptozotocin-nicotinamide induced type 2 diabetes. Chem Biol Interact. 2007;169:15–24. doi: 10.1016/j.cbi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Soliman GZ. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008;49:129–36. [PubMed] [Google Scholar]
- 48.Hsu DH, Moore KW, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992;4:563–9. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 49.Camussi G, Albano E, Tetta C, Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991;202:3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- 50.Saraheimo M, Teppo AM, Forsblom C, Fagerudd J, Groop PH. Diabetic nephropathy is associated with low-grade inflammation in Type 1 diabetic patients. Diabetologia. 2003;46:1402–7. doi: 10.1007/s00125-003-1194-5. [DOI] [PubMed] [Google Scholar]
- 51.Fidan I, Yüksel S, Kalkanci A, Imir T, Kustimur S. Evaluation of the natural killer cytotoxicity and the levels of cytokines in rats with type I diabetes mellitus. Mem Inst Oswaldo Cruz. 2005;100:883–7. doi: 10.1590/s0074-02762005000800010. [DOI] [PubMed] [Google Scholar]
- 52.Altınova EA, Toruner FB, Karakoç A. Serum levels of TNF-α and IL-6 in patients with Type I diabetes mellitus with and without microvascular complications. JDE. 2006;2:88–92. [Google Scholar]
- 53.Lechleitner M, Koch T, Herold M, Dzien A, Hoppichler F. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk factors. J Intern Med. 2000;248:67–76. doi: 10.1046/j.1365-2796.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 54.Erbaǧci AB, Tarakçioǧlu M, Coşkun Y, Sivasli E, Sibel Namiduru E. Mediators of inflammation in children with type I diabetes mellitus: Cytokines in type I diabetic children. Clin Biochem. 2001;34:645–50. doi: 10.1016/s0009-9120(01)00275-2. [DOI] [PubMed] [Google Scholar]
- 55.Mohamed-Ali V, Armstrong L, Clarke D, Bolton CH, Pinkney JH. Evidence for the regulation of levels of plasma adhesion molecules by proinflammatory cytokines and their soluble receptors in type 1 diabetes. J Intern Med. 2001;250:415–21. doi: 10.1046/j.1365-2796.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 56.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–45. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 57.Mehta S, Farmer JA. Obesity and inflamation: A new look at an old problem. Curr Atheroscler Rep. 2007;9:134–8. doi: 10.1007/s11883-007-0009-4. [DOI] [PubMed] [Google Scholar]
- 58.Warne JP. Tumour necrosis factor alpha: A key regulator of adipose tissue mass. J Endocrinol. 2003;177:351–5. doi: 10.1677/joe.0.1770351. [DOI] [PubMed] [Google Scholar]
- 59.Zulet MA, Puchau B, Navarro C, Martí A, Martínez JA. Inflamotory biomarkers: The link between obesity and associated pathologies. Nutr Hosp. 2007;22:511–27. [PubMed] [Google Scholar]
- 60.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 61.Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F, et al. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221–2. [PubMed] [Google Scholar]
- 62.Orban Z, Remaley AT, Sampson M, Trajanoski Z, Chrousos GP. The differential effect of food intake and adrenergic stimulation on adipose derived hormones and cytokines in man. J Clin Endocrinol Metab. 1999;84:2126–33. doi: 10.1210/jcem.84.6.5747. [DOI] [PubMed] [Google Scholar]
- 63.Mooradian AD, Reed RL, Meredith KE, Scuderi P. Serum levels of tumor necrosis factor and IL-1 alpha and IL-1 beta in diabetic patients. Diabetes Care. 1991;14:63–5. doi: 10.2337/diacare.14.1.63. [DOI] [PubMed] [Google Scholar]


