Abstract
Background:
Cabbage, Brassica oleracea var. capitata L., is one of the most common vegetables in the world. Because of its high levels of flavonoids and anthocyanins, cabbage has long been used as a herbal medicine. The antioxidant and anti-inflammatory properties of cabbage were also recently been reported.
Objective:
This study was designed to investigate the anti-inflammatory effects of cabbage in mice with contact dermatitis (CD).
Materials and Methods:
We investigated the effects of methanol extract of B. oleracea var. capitata L. (MEBO) on ear swelling, erythema, and histopathological changes in CD mice. Moreover, the effects on cytokine production and the spleen/body weight ratio were investigated.
Results:
Topical treatment with MEBO inhibited ear swelling and erythema significantly. MEBO also significantly inhibited epidermal hyperplasia and infiltration of immune cells. Furthermore, the levels of tumor necrosis factor-alpha, interferon-gamma, interleukin-6, and monocyte chemotactic protein-1 in inflamed tissues were effectively lowered by MEBO. Finally, MEBO did not affect body weight gain or spleen body weight ratio.
Conclusions:
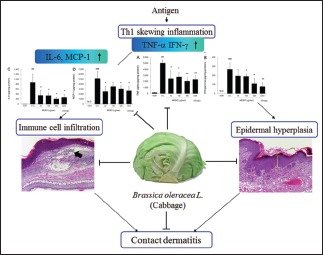
These results indicate that cabbage can be used for the treatment of skin inflammation and that its anti-inflammatory activity is closely related to the inhibition of Th1 skewing reactions.
SUMMARY
MEBO inhibited ear thickness, weight, and erythema in inflamed skin
MEBO also prevented epidermal hyperplasia and infiltration of immune cells
The levels of tumor necrosis factor-α, interferon-γ, interleukin-6, and monocyte chemotactic protein-1 in inflamed tissues were lowered by MEBO.
Abbreviations used: AOO: Acetone and olive oil (4:1), CBA: Cytometric bead array, CD: Contact dermatitis, DEX: Dexamethasone, DNFB: 1-fluoro-2,4-dinitrofluorobenzene, GM-CSF: Granulocyte-macrophage colony-stimulating factor, ICAM-1: Intercellular Adhesion Molecule-1, LPS: Lipopolysaccharide, MEBO: Methanol extract of Brassica oleracea, MCP-1: Monocyte chemotactic protein-1, NO: Nitric oxide.
Keywords: Brassica oleracea, cabbage, contact dermatitis, herbal medicine, inflammation
INTRODUCTION
Cabbage (Brassica oleracea var. capitata L.), which belongs to the family Brassicaceae, is one of the most common vegetables grown worldwide. Cabbage is often referred to as green cabbage to distinguish it from red cabbage, which has the same scientific name. Due to its antioxidant and anti-inflammatory properties,[1,2] cabbage has been widely used as an herbal medicine to treat gastrointestinal disorders such as gastritis, peptic and duodenal ulcers, and irritable bowel syndrome, as well as wounds and mastitis.[1,3,4]
Many people have believed cabbage can ameliorate various skin ailments such as xeroderma, skin troubles, and acne. In the theory of traditional medicine, malfunction of gastrointestinal tract is closely related in skin problems.[5] In addition, cabbage is recognized as good supplement for both gastrointestinal disorders and skin problems in Korea. For this reason, Korean people have used cabbage to improve skin ailments through amelioration of gastrointestinal malfunctions. Furthermore, grated cabbage is used as ingredient of face mask and its usages are easily found on websites.[6]
Occupational contact dermatitis (CD) is characterized by dry and flaking skin, as well as by pruritus, macular erythema, hyperkeratosis, and vesiculation. Although this condition is not life-threatening, it leads to severe impairment of workplace efficiency and the quality of life of workers. In addition, it has a very high-financial burden. For example, almost 13 million workers in the United States have the potential to CD each year, resulting in costs exceeding US$ 1 billion annually.[7]
CD is an inflammatory response to dermal exposure to a harmful agent, which is known as a sensitizer. This condition tends to be chronic, repetitive, and consistent because workers have no choice but to be exposed to the sensitizer. For this reason, patients with occupational CD tend to use corticosteroids frequently and continuously, even if they have had adverse reactions to such treatments.[8]
We have recently been investigating the anti-inflammatory effects of plant materials, which have relatively low toxicity, to identify candidates for complementary or alternative medicine to corticosteroids. As part of these efforts, we investigated the anti-inflammatory effects of B. oleracea var. capitata L., in mice with CD induced by 1-fluoro-2,4-dinitrofluorobenzene (DNFB).
MATERIALS AND METHODS
Preparation of samples
Cabbage, B. oleracea var. capitata L., cultivated in Jeju Island, Korea, in 2014, was purchased from a public market (Top Mart, Yansan, Korea). The extracting processes were conducted according to our standard procedure.[9] A total of 50 g minced cabbage was immersed in 500 ml methanol, then sonicated for 15 min, after which the sample was extracted for 24 h. Next, the supernatant was transferred and the sample was extracted in 500 ml of methanol for an additional 24 h. The extract was subsequently filtered through Whatman filter paper No. 20 and evaporated under reduced pressure using a vacuum evaporator (Eyela, Tokyo, Japan). The condensed extract was then lyophilized using a freeze dryer (Labconco, Kansas City, MO, USA). Finally, 4.1 g of lyophilized powder (methanol extract of B. oleracea var. capitata L., [MEBO]) was obtained (yield, 8.2%). An aliquot of the extract was deposited at the Division of Pharmacology, School of Korean Medicine, Pusan National University (MEBO, Voucher No. MH 2014-033).
Animals
Male 6-week-old Balb/c mice were purchased from Samtaco (Incheon, Korea). Mice were housed under specific pathogen-free conditions with a 12 h light/dark cycle and free access to standard rodent food and water. All animal experiments were approved by our Animal Care and Use Committee and conducted according to institutional guidelines (PNU-2012-0140).
Induction of contact dermatitis and experimental design
Experimental CD was induced using DNFB and all procedures were described in our previous reports.[9] Mice were sensitized by painting 50 μL of DNFB (0.2%, v/v) in acetone: olive oil (AOO, 4:1) onto the shaved back of each animal for 3 consecutive days. Four days after sensitization, each mouse was challenged by painting 20 μL of DNFB (0.2%, v/v) in AOO onto the dorsum of both ears every 2 days (4 times). MEBO solution (30, 100, or 300 μg/ear) was then applied onto the dorsum of both ears for 6 consecutive days. Naïve animals were treated with vehicle (AOO) and then painted with vehicle (n = 4). Control animals (CTL) were sensitized and challenged with DNFB, after which they were painted with vehicle (n = 8). MEBO-treated animals were sensitized and challenged with DNFB, then painted with 30 μg/ear (0.1%, w/v), 100 μg/ear (0.33%, w/v), and 300 μg/ear (1%, w/v) of MEBO (n = 8). DEX-treated animals were sensitized and challenged with DNFB, then painted with 75 μg/ear dexamethasone (DEX) as a positive control (n = 6). The experimental schedule is summarized in Figure 1.
Figure 1.
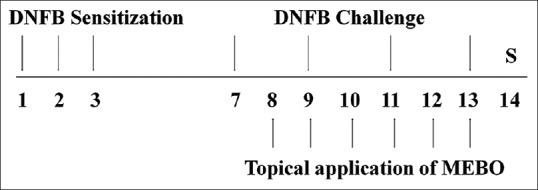
Experimental schedule – the experimental groups, except for the naïve group, were sensitized by painting with 1-fluoro-2,4-dinitrofluorobenzene on days 1, 2, and 3. Mice were challenged by 1-fluoro-2,4-dinitrofluorobenzene on days 7, 9, 11, and 13. The naïve group was treated with vehicle (acetone: olive oil) in the same way. Methanol extract of Brassica oleracea var. capitata L. and DEX groups were topically treated with methanol extract of Brassica oleracea var. capitata L. (30, 100, or 300 μg/ear in ethanol and acetone: olive oil) or DEX (75 μg/ear in ethanol and acetone: olive oil) for 6 days from day 8–13. All animals were sacrificed on day 14
Measurement of ear thicknesses and weights
Mice were sacrificed with CO2, after which the thicknesses of both ears were measured using vernier calipers (Mitutoyo, Kanagawa, Japan). The weights of earpieces obtained through dermal punch (5 mm in diameter) were also determined using a microbalance (Sartorius AG, Göttingen, Germany).
Evaluation of erythema index
At the end of the experiment, resected ears were photographed using a digital camera (Olympus, Tokyo, Japan). To measure the effects on erythema, the levels of flushing and vasodilatation in the ear skin were evaluated using arbitrary units as follows: Grade 0, no flushing and normal range of blood vessels in the center; Grade 1, no flushing and slight vasodilatation in the center; Grade 2, slight flushing and vasodilatation in the center; Grade 3, flushing and vasodilatation in the edge and in the center; and Grade 4, severe flushing, petechia, and vasodilatation in the edge and in the center. Erythema index values are presented as the mean ± standard deviation (SD).
Histopathological examination
Following measurement of ear thicknesses and weights, ear tissues were resected and embedded in paraffin. Sections were then stained with hematoxylin and eosin for histopathological changes such as epidermal hyperplasia, hyperkeratosis, and immune cell infiltration. Stained tissues were observed at ×50 and ×100 using a light microscope.
Evaluation of epidermal hyperplasia and immune cell infiltration
For evaluation of epidermal hyperplasia and immune cell infiltration, five nonoverlapping fields per slide were randomly selected and images were captured with a light microscope. To measure the thickness of the epithelium, the vertical length between the basal lamina and the top of the outermost stratum granulosum was quantified. For each slide, five lengths were measured at random, after which the average values of epithelial thickness in all experimental groups were used for analysis. To evaluate immune cell infiltration, infiltrated immune cells were counted using a cell counting grid.
Measurement of cytokine production
Cytokine levels in ear tissues were measured using the cytometric bead array (CBA) method. Briefly, resected ear tissues were lysed and homogenized with protein extraction solution (Intron Bio, Daejeon, Korea) using a bullet blender (Next Advance, New York, NY, USA) to obtain tissue lysates. Next, 50 μg of each lysate was used to measure the levels of tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ interleukin-6 (IL-6), and monocyte chemotactic protein-1 (MCP-1). Cytokine levels were measured using a CBA kit (BD Bioscience, CA, USA). All experimental procedures were conducted according to the manufacturer's guidelines.
Statistical analysis
Mann–Whitney test was used for all statistical comparisons, and Prism 5 for Windows Version 5.01 (GraphPad Software, CA, USA) was used for all analyses. All data are presented as the mean ± SD. The P < 0.05 was considered statistically significant.
RESULTS
Methanol extract of Brassica oleracea var. capitata L. effectively inhibited ear swelling in inflamed tissues.
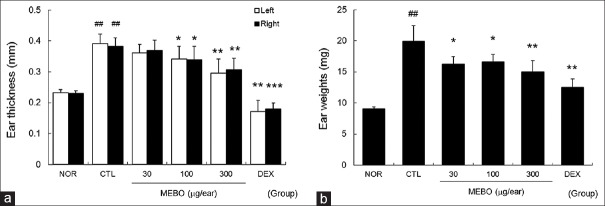
Repeated painting of DNFB enlarged ear thickness and weight significantly compared to nontreated normal mice. Treatment with more than 100 μg/ear of MEBO effectively prevented enlargement of ear thickness. In addition, MEBO lowered ear weights significantly [Figure 2].
Figure 2.
Effects of methanol extract of Brassica oleracea var. capitata L. on ear swelling in inflamed tissues. The ear thickness and weight were measured on day 14. NOR, nontreated normal mice; CTL, nontreated contact dermatitis mice; 30, 100 or 300, 30, 100, or 300 μg/ear of methanol extract of Brassica oleracea var. capitata L-treated contact dermatitis mice; DEX, 75 μg/ear of dexamethasone-treated contact dermatitis mice. (a) Ear thickness; (b) ear weight. All values are presented as the mean ± standard deviation.##P < 0.01 and###P < 0.001 vs. nontreated normal mice (NOR), *P < 0.05, **P < 0.01 and ***P < 0.001 versus nontreated contact dermatitis mice (CTL)
Methanol extract of Brassica oleracea var. capitata L. effectively lowered the erythema index in the skin
Severe flushing and vasodilatation were observed in the edge and center of the ear in the CTL group. However, treatment with more than 100 μg/ear of MEBO effectively prevented the flushing and vasodilatation induced by DNFB in a dose-dependent manner [Figure 3].
Figure 3.
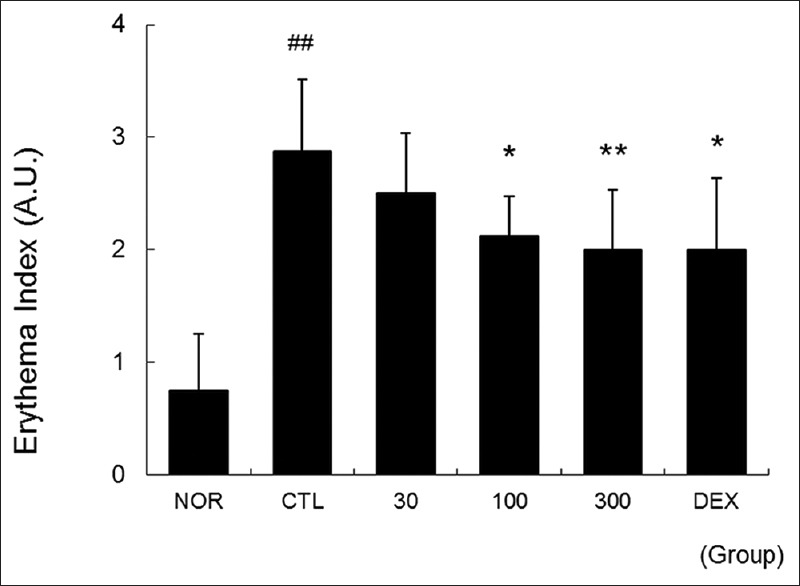
Effects of methanol extract of Brassica oleracea var. capitata L. on erythema index of the skin. Ear skins were observed using a digital camera on day 14. The effects of methanol extract of Brassica oleracea var. capitata L. on the erythema index were evaluated using arbitrary units as shown in the materials and methods. Abbreviations are the same as in Figure 2; A.U., arbitrary unit. All values are presented as the mean ± standard deviation.##P < 0.01 versus nontreated NOR mice, *P < 0.05 and **P < 0.01 versus nontreated contact dermatitis mice
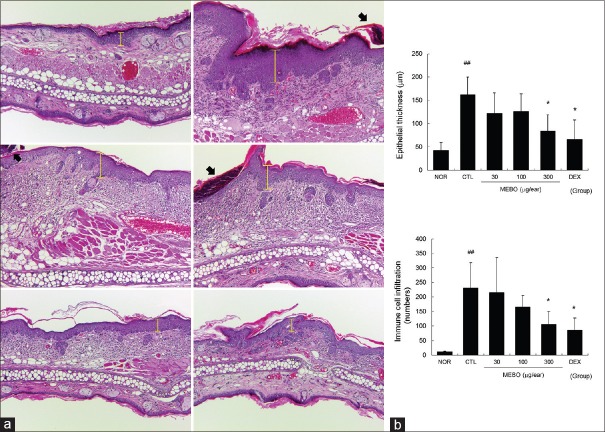
Methanol extract of Brassica oleracea var. capitata L. effectively inhibited epidermal hyperplasia, hyperkeratosis, and immune cell infiltration in inflamed tissues
Multiple applications of DNFB induced epidermal hyperplasia (yellow bars) and hyperkeratosis, as well as immune cell infiltration [Figure 4a]. Topical application of MEBO inhibited epidermal hyperplasia, hyperkeratosis, and immune cell infiltration [Figure 4]. As shown in Figure 4b, 300 μg/ear of MEBO significantly prevented epithelial hyperplasia. In addition, MEBO prevented immune cell infiltration induced by DNFB in a dose-dependent manner [Figure 4b].
Figure 4.
Effects of methanol extract of Brassica oleracea var. capitata L. on histopathological changes in contact dermatitis mice. Ear tissues were stained with hematoxylin and eosin and observed using a light microscope. Abbreviations are the same as in Upper left, NOR; upper right, CTL; middle left, 30 μg/ear of MEBO-treated; middle right, 100 μg/ear of MEBO-treated; bottom left, 300 μg/ear of MEBO-treated; bottom right, DEX (X50). Yellow bars indicate epidermal thicknesses, filled arrows mean hyperkeratotic areas (a). The epithelial thickness and immune cell infiltration were evaluated in a quantitative manner. Abbreviations are the same as in Figure 2. All values are presented as the mean ± standard deviation.##P < 0.01 versus nontreated NOR mice, *P < 0.05 versus nontreated contact dermatitis mice (b)
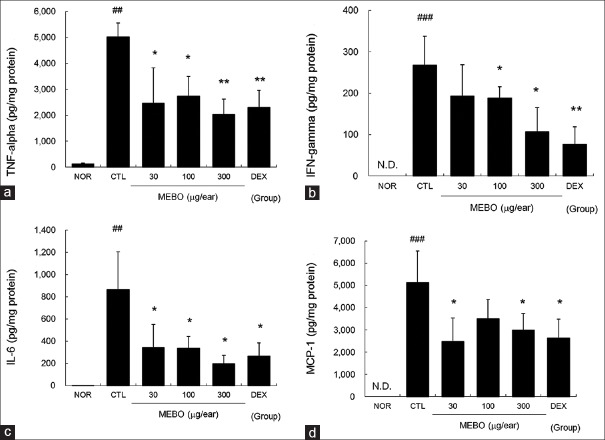
Methanol extract of Brassica oleracea var. capitata L. reduced the levels of tumor necrosis factor-alpha, interferon-gamma, interleukin-6, and monocyte chemotactic protein-1 in inflamed tissues of contact dermatitis mice
Marked increases in TNF-α, IFN-γ, IL-6, and MCP-1 production were observed in the CTL group. MEBO effectively inhibited the TNF-α, IFN-γ, IL-6, and MCP-1 production induced by repeated application of DNFB [Figure 5].
Figure 5.
Effects of methanol extract of Brassica oleracea var. capitata L. on levels of tumor necrosis factor-alpha, interferon-gamma, interleukin-6, and monocyte chemotactic protein-1 in contact dermatitis mice. The production levels of tumor necrosis factor-alpha, interferon-gamma, interleukin-6, and monocyte chemotactic protein-1 in inflamed tissues were measured using the cytometric bead array method. A total of 50 μg of tissue lysates were used to measure the cytokine levels. Abbreviations are the same as in Figure 2. (a) Tumor necrosis factor-alpha; (b) interferon-gamma; (c) interleukin-6; (d) monocyte chemotactic protein-1. All values are presented as the mean ± standard deviation.##P < 0.01 and###P < 0.001 versus nontreated NOR mice, *P < 0.05 and **P < 0.01 versus nontreated contact dermatitis mice (CTL)
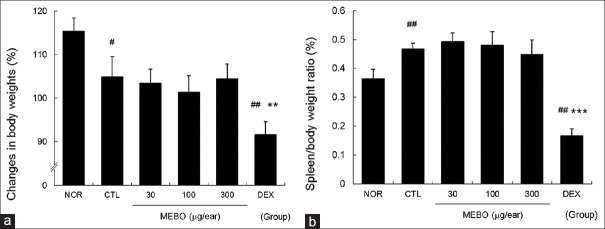
Methanol extract of Brassica oleracea var. capitata L. did not affect spleen body weight ratio of contact dermatitis mice
Slight inhibition of body weight gain was observed in the CTL group, while MEBO had no effect on body weight gain. Conversely, topical application of dexamethasone inhibited body weight gain significantly [Figure 6a]. In addition, multiple applications of DNFB elevated the spleen body weight ratio compared to nontreated normal mice, while MEBO had no effect on spleen body weight ratio in CD mice. On the other hand, dexamethasone significantly reduced the spleen body weight ratio compared to nontreated normal and control mice [Figure 6b].
Figure 6.
Effects of methanol extract of Brassica oleracea var. capitata L. on spleen body weight ratio in contact dermatitis mice. The spleen body weight ratio was calculated using body and spleen weights measured on day 14. Abbreviations are the same as in Figure 2. (a) Changes in body weights; (b) spleen body weight ratio. All values are presented as the mean ± standard deviation.#P < 0.05 and##P < 0.01 versus nontreated NOR mice, **P < 0.01 and ***P < 0.001 versus nontreated contact dermatitis mice (CTL)
DISCUSSION
CD induced by superantigens such as 2,4-Dinitrochlorobenzene (DNCB) and DNFB is characterized by severe skin lesions such as marked scaling, crust, and erythematous eruptions in the skin surface, Th1 skewing reactions such as elevated levels of TNF-α and IFN-γ, and immune cell infiltration into the inflamed tissues.[10] In addition, it is well known that topical application of DNFB can enlarge skin thickness and weight in experimental animals.[9] In our model, repeated application of DNFB induced ear swelling and erythema in ear skin, as well as epithelial hyperplasia, hyperkeratosis and immune cell infiltration in ear tissues. Marked increases in inflammatory cytokines such as TNF-α, IFN-γ, IL-6, and chemokine (MCP-1) were also observed. These results were in accordance with those of previous studies conducted using an animal model of CD and indicate that our model mimics human CD well.
Cabbage has high levels of flavonoids[11] and anthocyanins,[12] which possess antioxidant properties and can increase the levels of antioxidant enzymes, leading to protection of the free-radical action and high nutritional value.[13] The antioxidant and anti-inflammatory mechanisms of cabbage are reportedly linked to stimulation of the Nrf2 pathway.[14,15] In addition, Sarandy et al. recently reported that cabbage ointment matured the extracellular matrix in skin wounds of Wistar rats.[14] Taken together, these findings suggest that cabbage can ameliorate skin lesions and pathophysiological changes in CD.
Th 1 skewing inflammatory reactions such as elevated production levels of TNF-α and IFN-γ and immune cell infiltration play an important role in the pathophysiology of CD and are considered hallmarks and therapeutic targets of CD. In this study, treatment with MEBO effectively reduced the production levels of TNF-α and IFN-γ in ear tissues [Figure 5], indicating that MEBO is an anti-inflammatory agent against the Th 1 skewing reaction of T cells.
IL-1β, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) contribute to the activation and migration of epidermal Langerhans cells during induction of CD, and strongly upregulate chemokines, resulting in recruitment of leukocytes.[16] In addition, IFN-γ accelerates the release of mediators such as chemokine (C-X-C motif) receptor (CXCR) 3 agonist and MCP-1.[17] TNF-α released by activated immune cells and keratinocytes can induce surface expression of intercellular adhesion molecule-1 and various chemokines such as IL-8, MCP-1, and chemokine (C-C motif) ligand 27 (CCL27) in both an autocrine and paracrine manner.[18] MCP-1, which is also known as chemokine (C-C motif) ligand 2 (CCL2), can recruit T cells, monocytes, and dendritic cells to inflammatory sites.[19,20] In our study, MEBO effectively lowered production levels of two representative Th1 cytokines, TNF-α and IFN-γ, and the chemokine, MCP-1 [Figure 5a and d]. In addition, MEBO prevented immune cell infiltration induced by DNFB in inflamed tissue [Figure 4b and c]. These results imply that MEBO can prevent immune cell infiltration through inhibition of MCP-1 production and that inhibition of MCP-1 by MEBO may be closely related to regulation of Th1 skewing cytokine production.
Keratinocytes play a pivotal role in the initiation and progression of CD as an effector cell with other immune cells. During initiation of CD, sensitizers, especially haptens, induce activation of keratinocytes, resulting in the release of inflammatory mediators such as TNF-α, IL-1, IL-6, and GM-CSF.[21] Among them, IL-6, which is mainly secreted from skin tissues after injury, can accelerate the proliferation and migration of keratinocytes.[22] Moreover, inflammatory cytokines such as TNF-α and IFN-γ can activate keratinocytes in both an autocrine and paracrine manner, leading to epidermal hyperplasia in the case of inflammatory skin diseases such as psoriasis and eczema.[23] In our experiment, MEBO significantly lowered production levels of TNF-α, IFN-γ, and IL-6 [Figure 5] and prevented hyperplasia in the epidermis [Figure 4a and b]. These results will help elucidate the action mechanism by which MEBO inhibits enlargement of ear thickness and weight [Figure 2].
Both erythema and swelling are major hallmarks of inflammation that are closely related to vasodilatation.[24] In the present study, MEBO effectively lowered the erythema index [Figure 3] and prevented ear swelling [Figure 2]. These results imply that the anti-inflammatory effects of MEBO in the skin tissues of CD and the mechanisms that lead to these effects are closely related to inhibition against vasodilatation through regulation of Th1 skewing immune responses.
Rokayya et al. reported that cabbage can effectively scavenge free radicals and slightly inhibit nitric oxide (NO) production in Raw 264.7 cells.[1] We also investigated the effects of MEBO on cytotoxicity and NO production. We found that treatment with up to 100 μg/ml MEBO did not affect cell viability or NO production induced by 1 μg/ml of lipopolysaccharide (LPS) for 20 h (data not shown). These discrepancies may be due to use of different experimental systems and extraction methods. We treated MEBO for 4 h and discarded MEBO containing media, after which cells were stimulated using LPS for 20 h. Conversely, Rokayya et al. treated samples for 24 h and did not describe the stimulation method. We also extracted minced cabbage using absolute methanol, while Rokayya et al. used ground cabbage and 80% methanol. Considering these differences, the yield of extraction by Rokayya et al. may be higher than that obtained by us; however, they did not report their yield.
We also investigated the inhibitory effects of MEBO on degranulation of mast cells. We found that MEBO did not inhibit degranulation of mast cells induced by phorbol 12-myristate 13-acetate plus A23187 (data not shown). At present, we are considering whether MEBO can inhibit activation of keratinocytes or not. We already found that treatment with up to 200 μg/ml MEBO did not affect cell viability of HaCaT keratinocytes (data not shown).
Both dexamethasone and MEBO showed similar anti-inflammatory efficacies in CD mice. However, dexamethasone significantly lowered the spleen/body weight ratio, which can be used as a hallmark of immune reaction,[25,26,27] compared to the normal group and the CTL group [Figure 6b]. In addition, average body weight gain was significantly lowered by treatment with dexamethasone [Figure 6a]. These results mean that MEBO did not act as dexamethasone which suppresses general immune reaction.
Taken together, these findings demonstrate that MEBO can be used for the treatment of inflammatory skin diseases. These findings indicate that the therapeutic mechanisms of MEBO are involved in the inhibition of Th1 immune reactions.
CONCLUSIONS
In the present study, we showed that MEBO reduced Th1 skewing reactions such as production of TNF-α, IFN-γ, and MCP-1, resulting in reduced epidermal hyperplasia and immune cell infiltrations. These consecutive anti-inflammatory reactions of MEBO ultimately led to the inhibition of ear swelling and erythema. The effects of MEBO were similar or slightly weaker than those of dexamethasone, and there was no general immune suppression observed in the dexamethasone-treated group. Taken together, these findings indicate that MEBO can be used as a relatively safe alternative to corticosteroids.
Financial support and sponsorship
The National Research Foundation of Korea grant funded by the Korean Government (MSIP; grant no. 2015R1A2A2A04005619 and 2014R1A5A2009936).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rokayya S, Li CJ, Zhao Y, Li Y, Sun CH. Cabbage (Brassica oleracea L. Var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac J Cancer Prev. 2014;14:6657–62. doi: 10.7314/apjcp.2013.14.11.6657. [DOI] [PubMed] [Google Scholar]
- 2.Lin JY, Li CY, Hwang IF. Characterisation of the pigment components in red cabbage (Brassica oleracea L. Var.) juice and their anti-inflammatory effects on LPS-stimulated murine splenocytes. Food Chem. 2008;109:771–81. doi: 10.1016/j.foodchem.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Noess K. Ulcer-fiber-cabbage and Vitamin U. Tidsskr Nor Laegeforen. 1986;106:693–4. [PubMed] [Google Scholar]
- 4.Doll R, Pygott F. Clinical trial of robaden and of cabbage juice in the treatment of gastric ulcer. Lancet. 1954;267:1200–4. doi: 10.1016/s0140-6736(54)92262-x. [DOI] [PubMed] [Google Scholar]
- 5.Gabrielle H. Encyclopedia of Folk Medicine: Old World and New World Traditions. Santa Barbara, CA: ABC-CLIO; 2004. pp. 59–60. [Google Scholar]
- 6.Wordpress.org. Word Press Foundation. c2000-01. [Last updated on 2016 May 28]. Available from: http://www.cabbage-vi17.tk/some-tips-cures-folk-cabbage/
- 7.Lurati AR. Occupational risk assessment and irritant contact dermatitis. Workplace Health Saf. 2015;63:81–7. doi: 10.1177/2165079914565351. [DOI] [PubMed] [Google Scholar]
- 8.Peate WE. Occupational skin disease. Am Fam Physician. 2002;66:1025–32. [PubMed] [Google Scholar]
- 9.Han HY, Ryu MH, Lee G, Cheon WJ, Lee C, An WG, et al. Effects of Dictamnus dasycarpus turcz. root bark on ICAM-1 expression and chemokine productions in vivo and vitro study. J Ethnopharmacol. 2015;159:245–52. doi: 10.1016/j.jep.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 11.Hassimotto NM, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem. 2005;53:2928–35. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Zheng Y, Yang Z, Cao S, Shao X, Wang H, et al. Domestic cooking methods affect the nutritional quality of red cabbage. Food Chem. 2014;161:162–7. doi: 10.1016/j.foodchem.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Kim JM, Shim SH, Chang HI. Anthocyanins accelerate the healing of naproxen-induced gastric ulcer in rats by activating antioxidant enzymes via modulation of Nrf2. J Funct Foods. 2014;7:569–79. [Google Scholar]
- 14.Sarandy MM, Novaes RD, da Matta SL, Mezencio JM, da Silva MB, Zanuncio JC, et al. Ointment of Brassica oleracea var. capitata matures the extracellular matrix in skin wounds of wistar rats. Evid Based Complement Alternat Med. 2015;2015:919342. doi: 10.1155/2015/919342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirota A, Kawachi Y, Yamamoto M, Koga T, Hamada K, Otsuka F, et al. Acceleration of UVB-induced photoageing in Nrf2 gene-deficient mice. Exp Dermatol. 2011;20:664–8. doi: 10.1111/j.1600-0625.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 16.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 17.Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999;162:494–502. [PubMed] [Google Scholar]
- 18.Goebeler M, Trautmann A, Voss A, Br, Vos EV, Toksoy A, Gillitzer R, et al. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Pathol. 2001;158:431–40. doi: 10.1016/s0002-9440(10)63986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60:365–71. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 21.Bonneville M, Chavagnac C, Vocanson M, Rozieres A, Benetiere J, Pernet I, et al. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J Invest Dermatol. 2007;127:1430–5. doi: 10.1038/sj.jid.5700726. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami M, Kaneko N, Anada H, Terai C, Okada Y. Measurement of interleukin-6, interleukin-10, and tumor necrosis factor-alpha levels in tissues and plasma after thermal injury in mice. Surgery. 1997;121:440–8. doi: 10.1016/s0039-6060(97)90315-9. [DOI] [PubMed] [Google Scholar]
- 23.Corsini E, Galli CL. Epidermal cytokines in experimental contact dermatitis. Toxicology. 2000;142:203–11. doi: 10.1016/s0300-483x(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 24.Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327–41. doi: 10.1016/j.jaad.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Jo S, Ryu J, Jeong HS, Lee G, Ryu MH, et al. Effects of Schisandra chinensis turcz. fruit on contact dermatitis induced by dinitrofluorobenzene in mice. Mol Med Rep. 2015;12:2135–9. doi: 10.3892/mmr.2015.3618. [DOI] [PubMed] [Google Scholar]
- 26.Jeklova E, Leva L, Jaglic Z, Faldyna M. Dexamethasone-induced immunosuppression: A rabbit model. Vet Immunol Immunopathol. 2008;122:231–40. doi: 10.1016/j.vetimm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed SA, Sriranganathan N. Differential effects of dexamethasone on the thymus and spleen: Alterations in programmed cell death, lymphocyte subsets and activation of T cells. Immunopharmacology. 1994;28:55–66. doi: 10.1016/0162-3109(94)90039-6. [DOI] [PubMed] [Google Scholar]