Abstract
Background:
Medicinal herbs are significantly effective against a variety of liver disorders and Trapa natans was traditionally used for the treatment of anti-inflammatory, pain disorder, and various types of hepatic ailment.
Objective:
The purpose of this study was to evaluate the hepatoprotective activity of T. natans fruit peel extract against antitubercular drugs (isoniazid + rifampicin [INH + RIF])-induced hepatotoxicity in rats.
Materials and Methods:
Liver toxicity was induced by INH + RIF at a dose level of 50 mg/kg each, intraperitoneally. for 15 days. Fifty percent ethanolic extract of T. natans (TNE) at a dose of 200 and 400 mg/kg was administered orally once daily for 15 days. The hepatoprotective activity was assessed using various biochemical parameters such as aspartate transaminase, alanine transaminase, alkaline phosphate, lactate dehydrogenase, albumin, cholesterol, and bilirubin. Furthermore, in vivo antioxidant activities and histopathological investigation were performed to assess hepatoprotective activity.
Results:
Obtained results demonstrated that the level of liver marker enzymes and antioxidant parameters were significantly altered by INH + RIF treatment. Treatment with T. natans peel extract causes significant (P < 0.01 to P < 0.001) reduction in liver injury & normalized all altered liver marker enzymes. In addition, TNE significantly normalized the activity of antioxidant enzymes, namely, lipid peroxidation (P < 0.01 to P < 0.001), reduced glutathione (P < 0.05 to P < 0.001), superoxide dismutase (P < 0.05 to P < 0.001), and catalase (P < 0.01 to P < 0.001) in the liver tissue of INH + RIF-treated groups. Histological observations of the liver tissues correlated with the biochemical observations.
Conclusion:
These findings powerfully support that the protective effect of T. natans fruit peel extract against liver injury which may be attributed to its hepatoprotective activity due to normalizes the altered liver marker enzymes and antioxidant defense status and thereby contributed to its antihepatotoxic potential.
SUMMARY
Hepatotoxicity was induced in rats by intraperitoneal injection of antitubercular drugs (Isoniazid + Rifampicin at dose level of 50 mg/kg each) for 15 days
The liver was screened for various marker enzymes and antioxidant parameters, which showed significant increase in the level of marker enzymes confirming induced hepatotoxicity
Hepatotoxic rats on treatment with T. natans peel extract (200 and 400 mg/kg) resulted in significant (P < 0.01 to P< 0.001) reduction in liver marker enzymes and antioxidant parameters
Thus, it can be concluded that Trapa natans fruit peel extract showed hepatoprotective potential against antitubercular drugs induced hepatotoxicity.
Abbreviations used: INH: Isoniazid; RIF: Rifampicin; DIH: Drug-induced hepatotoxicity; CMC: Carboxy methyl cellulose; ALT: Alanine transaminase; ALP: Alkaline phosphate; CHL: Total cholesterol; ALB: Albumin; LDH: Lactate dehydrogenase; LPO: Lipid peroxidation; CAT: Catalase; GSH: Reduced glutathione; SOD: Superoxide dismutase; TNE: Trapa natans extract.
Keywords: Antioxidant, antitubercular drugs, hepatoprotective, Trapa natans
INTRODUCTION
The dysfunction or damage of liver occurred due to overload of drugs or xenobiotics is known as hepatotoxicity. Not only some chemicals but also some of the drugs which are in clinical practice are categorized as hepatotoxic agents. Usually, many drugs are implicated with hepatic injury, including routinely used paracetamol, analgesics, first-line antitubercular agents, and frequently abused alcoholic beverages. Such hepatotoxicity induced by a drug not only limits their further use but also might interfere with essential metabolic functions.[1]
The most common reason leading to interruption of TB chemotherapy is due to the development of drug-induced hepatotoxicity (DIH) during the chemotherapy period. Broad variations have been observed in the hepatotoxic reactions' incidences during short course chemotherapy from different countries with the reported frequency being 4% in the UK, 3% in the USA, 9.9% in Argentina, 11% in Germany, 36% in Japan, 13% in Hong Kong, 8%–36% in India, and 26% in Taiwan.[2]
At present, the antitubercular chemotherapeutic regimens contain isoniazid (INH) and rifampicin (RIF) and cause potentially severe adverse effects, leading to liver toxicity caused due to DIH. Separately, both the drugs are potentially hepatotoxic, and when they are given in combination, their hepatotoxic effects are synergistically enhanced. Synthetic drugs browbeaten in the liver disease treatment are ineffectual, and at times, it may lead to serious side effects. Hence, an adept approach of herbal therapy has emerged along with good values in hepatic disease treatment.
Trapa natans (Family: Trapaceae), commonly known as water chestnut, is an annual aquatic floating herb occurring in ponds and lakes throughout the Indian subcontinent. T. natans comprises carbohydrates and also the vitamins, which includes Vitamin A, Vitamin B-complex (riboflavin, pyridoxine, thiamine, pantothenic acids, and nicotinic acid), Vitamin C and D – amylase, and also a significant amount of phosphorylase. Photochemically, it also contains gallic acid, caffeic acid, quercetin, and kaempferol (flavonols) in seeds and pericarp of green-, red-, and black-colored singhara fruits.[3] Inorganic constituents such as acids, minerals, calcium, phosphorus, iron, copper, manganese, magnesium, sodium, and potassium are also available in T. natans.[4]
From the literature review, it was concluded that T. natans has got different activities such as neuroprotective, immunomodulatory, anti-inflammatory, anticancer, analgesic, antiulcer, antioxidant, antidiabetic, antifungal, and antibacterial. T. natans fruit peel (shell) extract is reported to reduce hepatotoxicity induced by CCl4 and paracetamol.[5,6] There is no information available about their hepatoprotective properties in favor of intoxicated liver injury induced by antitubercular drugs. Accordingly, our objective is to perform the study of the hepatoprotective properties of hydroalcoholic extract of T. natans fruit peel against antitubercular drug-intoxicated rats as an experimental model.
MATERIALS AND METHODS
Plant extraction and standardization
Analytical grade chemicals were used for this study. The fruit peel of of T. natans (Trapaceae) were collected from local market of Lucknow. The plant material was authenticated by botanist Mr. Muhammad Arif (Assistant Professor). A voucher specimen of T. natans (IU/PHAR/HRB/14/01) was deposited in the institute for further reference. To remove dirt and soil, the freshly collected fruit peel of T. natans were washed with distilled water and dried in shade in a ventilated place at room temperature.
Dried plant materials were sliced into diminutive pieces and reduced to power in coarse form by the aid of mechanical grinder; further extraction was carried out with 50% hydroalcoholic by cold percolation method to avoid heat damage. The extract, so prepared, was filtered and concentrated under reduced pressure below 40°C ± 1°C using Roteva Vacuum rotary evaporator to dryness to get a constant weight. The percentage yield was calculated and found to be 14.14% w/w. Temperature of −20°C was set of the freezer to store the extract and used for pharmacological investigation.
Animal
Adult Wistar rats (male) weighing 160 ± 20 g were procured from the National Laboratory Animal Center, Central Drug Research Institute, Lucknow. For the housing of animals, separate polypropylene cages were acclimatized at a temperature of 23°C ± 2°C and 50%–60% relative humidity, with a 12 h light/dark cycle 1 week before and during the experimental instigation. Animals were kept on a standard pellet diet (Dayal animal feed, Unnao, India) and drinking water ad libitum throughout the housing period. Administration of extract was done orally, and that for toxicant was done by intraperitoneal (i.p.) route in the morning session throughout the study period. All investigational proceedings concerned with animals were conducted as per the guidelines of Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA). The study protocol was approved by the Ethical Institutional Animal Ethical Committee.
Experimental design
Male Wistar rats, five groups were divided each group having six rats in it (n = 6), were used in this study. Group I, control group, received vehicle 0.3% carboxy methyl cellulose (CMC). Group II, toxic group, received vehicle 0.3% CMC by the oral route (p.o) followed by the administration of anti-TB drugs (INH + RIF, 50 mg/kg/day b.w., each), by i.p. route for 15 days.[7] Group III and IV were administered with T. natans fruit peel extract (200 mg/kg and 400 mg/kg, b.w.), respectively, followed by anti-TB drugs administration, by i.p. route (INH + RIF, 50 mg/kg/day b.w., each) for 15 days.[8] Group V, standard group, received silymarin, which is a standard drug (100 mg/kg b.w.) followed by the administration of anti-TB drugs by i.p. route (INH + RIF, 50 mg/kg/day b.w., each).[9]
At the end of the treatment, i.e., after 24 h of the last dose administration of anti-TB drugs, the animals were anaesthetized and by retro-orbital plexus, blood was collected which was followed by heart puncture and permitted to clot. After the blood withdrawal, rats were sacrificed. The blood was centrifuged at 3000 rpm at 4°C for 20 min, and serum was separated and collected for the examination of assorted biochemical parameters. The liver tissue was isolated and washed twice with ice cold saline, then blotted, and dried and finally weighed to calculate the relative liver weight, as the percentage ratio of liver weight to the body weight. A small tissue portion was fixed in the formalin for histopathological examination.[10,11]
Assessments of liver function test
The action of biochemical parameters such as serum alanine transaminase (ALT), alkaline phosphate (ALP), bilirubin, total cholesterol (CHL), albumin (ALB), and serum lactate dehydrogenase (LDH) was determined using standard kit methods. All estimation was done using ultraviolet spectrophotometer (Shimadzu) as per standard kit methods.
Assessment of antioxidant parameters
Tissues of rats' liver were homogenized (10%) in the basic phosphate buffer (pH 7.4) with the help of Potter-Elvehjem glass homogenizer. The homogenate prepared was centrifuged at 12,000 rpm for 20 min at 4°C to acquire and collect postmitochondrial supernatant (PMS), and further, it was used for the lipid peroxidation (LPO) estimation.[12] The catalase (CAT), reduced glutathione (GSH), and superoxide dismutase (SOD) activities in the liver PMS were estimated by the respective methods described by Aebi, Kakkar et al., and Upadhyay.[13,14,15]
Histopathological assessment
For histological studies, the tissues of liver were fixed with 10% neutral formalin buffered with phosphate buffer; further, it is dehydrated in graded (50%–100%) alcohol and then firmly fitted in the paraffin. Thin sections of about 5 μm were cut and stained with regular hematoxylin and eosin stain for the assessment of microscopic photograph. This qualitative examination was done and the intension of estimating the histopathological lesions in the isolated liver tissues.
Statistical analyses
The data estimated and calculated were represented as mean standard error of the mean of six rats. Analysis of variance test was done, which is further followed by individual comparison test by Newman–Keuls test method using GraphPad Prism software (Version 6.05, GraphPad Software, Inc. USA) for the level of significance determination. Statistically, P < 0.05 was contemplating to be significant.
RESULTS
Effect of 50% ethanolic extract of Trapa natans on body weight, liver weight, and relative liver weight in control and antitubercular drugs (isoniazid + rifampicin)-induced hepatotoxicity in rats
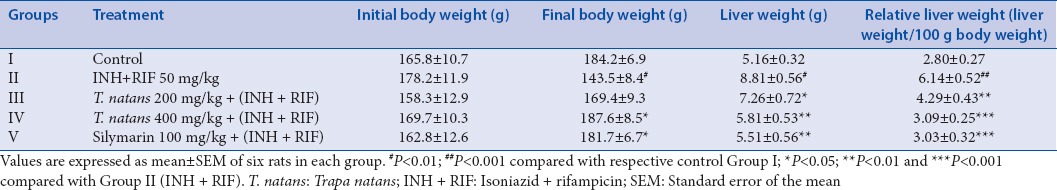
The final body weight of normal Group I rats showed 184.2 ± 6.9 g compared to Group II rats following INH + RIF treatment, which was significantly decreased to 143.5 ± 8.4 g (P < 0.01). In ethanolic extract of T. natans (TNE)-treated Group III (200 mg/kg) and IV (400 mg/kg) rats, the final body weights significantly increased 169.4 ± 9.3 and 187.6 ± 8.5 g (P < 0.05) when compared to the Group II treated with INH + RIF, respectively.
INH + RIF treatment significantly increased the relative liver weight to (P < 0.01) 8.81 ± 0.56/100 g body weight when compared to the control (Group I, 5.16 ± 0.32/100 g body weight). Administration of 200 and 400 mg/kg TNE significantly reduced (P < 0.05 and P < 0.01) the relative liver weight to 7.26 ± 0.72 and 5.81 ± 0.53/100 g body weight, respectively, compared to 5.71 ± 0.52/100 g in INH + RIF treatment. TNE-treated Group IV activity was less to standard silymarin-treated Group V rats at the concentration used [Table 1].
Table 1.
Effect of Trapa natans extract on body weight, liver weight, and relative liver weight of control and isoniazid + rifampicin
Effect of 50% ethanolic extract of Trapa natans on liver injury in control and antitubercular drug-induced hepatotoxicity in rats
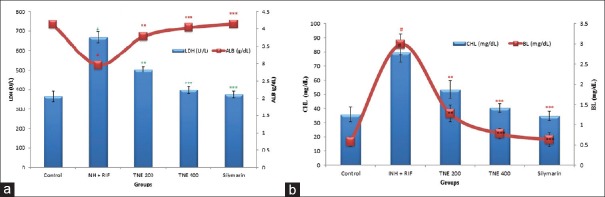
The effect of TNE on liver injury is shown in Figures 1 and 2a, b. INH + RIF-treated Group II rats showed increased serum aspartate transaminase (AST) (291.21 ± 25.94 U/L, P < 0.001), ALT (157.15 ± 12.71 U/L, P < 0.001), ALP (176.97 ± 12.83 U/L, P < 0.001), LDH (668.24 ± 28.52 U/L, P < 0.001), CHL (79.68 ± 6.89 mg/dl, P < 0.001), bilirubin (2.98 ± 0.26 mg/dl, P < 0.001), and decreased serum ALB (2.96 ± 0.08 g/dl, P < 0.001) compared to control Group I rats (90.74 ± 11.54 U/l, 39.23 ± 6.23 U/L, 64.32 ± 8.45 U/L, 364.11 ± 26.21 U/L, 35.87 ± 5.21 mg/dl, 0.58 ± 0.12 mg/dl, and 4.12 ± 0.11 g/dl), respectively. The TNE-treated Groups III and IV rats at 200 and 400 mg/kg significantly decreased AST (223.84 ± 21.33 and 118.12 ± 13.11 U/L, P < 0.01 and P < 0.001), ALT (87.58 ± 09.11 and 47.47 ± 6.27 U/L, P < 0.01 and P < 0.001), ALP (97.45 ± 10.65 and 73.87 ± 7.52 U/L, P < 0.01 and P < 0.001), LDH (502.21 ± 13.29 and 397.21 ± 18.57 U/L, P < 0.01 and P < 0.001), CHL (53.41 ± 6.11 and 40.47 ± 2.89 mg/dl, P < 0.01 and P < 0.001), bilirubin (1.28 ± 0.21 and 0.78 ± 0.13 mg/dl, P < 0.01 and P < 0.001), and significantly increase the ALB (3.78 ± 0.10 and 4.03 ± 0.07 g/dl, P < 0.01 and P < 0.001), respectively, compared to Group II animals.
Figure 1.
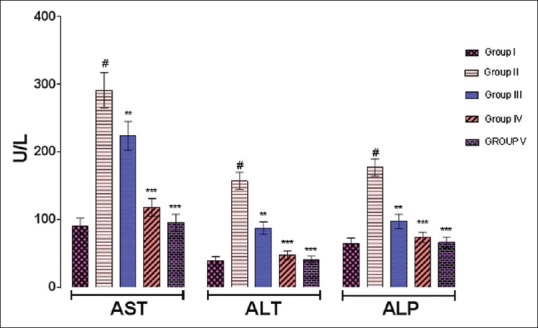
Effect of Trapa natans on serum aspartate transaminase (U/L), alanine transaminase (U/L), and alkaline phosphate (U/L) against antitubercular drugs (isoniazid + rifampicin)-induced hepatotoxicity in rats. P values:#<0.001 compared with respective control Group I. P values: **<0.01, ***<0.001 compared with Group II (rifampicin + isoniazid)
Figure 2.
(a and b) Effect of Trapa natans on serum lactate dehydrogenase (U/L), albumin (g/dl), cholesterol (mg/dl), and bilirubin level (mg/dl) against antitubercular drugs (isoniazid + rifampicin) induced hepatotoxicity in rats. Values are expressed as a mean ± standard error of the mean of six rats in each group. P values:#<0.001 compared with respective control Group I. P values: **<0.01, ***<0.001 compared with Group II (rifampicin and isoniazid)
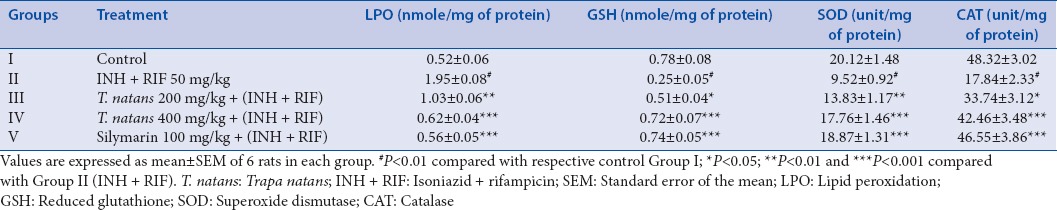
Effect of 50% ethanolic extract of Trapa natans on lipid peroxidation and levels of antioxidant enzymes in liver of control and paracetamol-induced hepatotoxicity in rats
The LPO levels in liver homogenate were found to be significantly increased in the INH + RIF-treated Group II rats. The LPO value of control Group I was 0.52 ± 0.06 U/mg protein which increased to 1.95 ± 0.08 U/mg protein (P < 0.001) [Table 2]. Administration of TNE showed a significant reduction in LPO as 1.03 ± 0.06 and 0.62 ± 0.04 U/mg protein, P < 0.01 and P < 0.001 at doses 200 and 400 mg/kg, respectively. INH + RIF-treatment (Group II) decreased the levels of hepatic GSH, CAT, and SOD from 0.78 ± 0.08 to 0.25 ± 0.05 (P < 0.001), 48.32 ± 3.02 to 17.84 ± 2.33 (P < 0.001), and 20.12 ± 1.48 to 9.52 ± 0.92 U/mg protein (P < 0.001), respectively, when compared to Group I animals [Table 2]. However, TNE at 200 and 400 mg/kg significantly increased the levels of GSH, CAT, and SOD from 0.51 ± 0.04 to 0.72 ± 0.07 (P < 0.05 to P < 0.001), 33.74 ± 3.12 to 42.46 ± 3.48 (P < 0.05 to P < 0.001), and 13.83 ± 1.17 to 17.76 ± 1.46 U/mg protein (P < 0.01 to P < 0.001), respectively, when compared to Group II rats. Silymarin at 100 mg/kg significantly reduced the elevated LPO level (0.56 ± 0.05 U/mg protein, P < 0.001) but increased the levels of GSH, CAT, and SOD (0.74 ± 0.05 [P < 0.001], 46.55 ± 3.86 [P < 0.001], and 18.87 ± 1.31 [P < 0.001], U/mg protein), respectively, compared to INH + RIF-treated Group II rats.
Table 2.
Effect of Trapa natans extract on liver lipid peroxidation, reduced glutathione, superoxide dismutase, and catalase against antitubercular dugs (isoniazid + rifampicin)-induced hepatotoxicity in rats
Histopathological observation
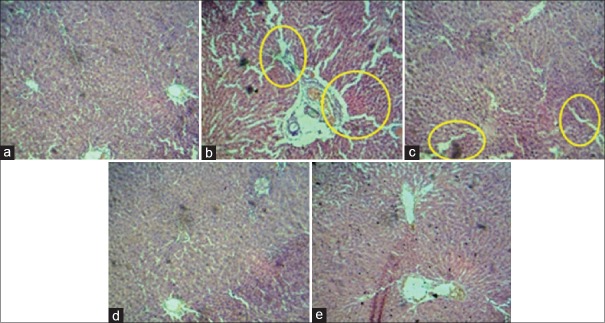
The histopathological examination of the liver tissues of experimental groups of rats (hematoxylin and eosin) was observed as follows. (a) Liver section of normal control rats shows near to normal architecture with small vesicular pyknotic nuclei. (b) Liver section of antitubercular drug-treated rats showing necrosis and disturbed architecture (indicated by circle); section shows smaller vesicular nuclei and eosinophilic cytoplasm with indistinct cell boundaries showing proliferation. (c) Liver section of antitubercular drug and TNE (200 mg/kg) pretreated rats showing approximately normal architecture with less infiltration. Section shows proliferated normal vesicular nuclei and abundant eosinophilic distinct cell boundaries. (d) Liver section of antitubercular drug and TNE (400 mg/kg) pretreated rats shows nontoxic effects, hepatocytes showing normal morphology and normal architecture with normal vascularity. (e) Liver section of rats treated with antitubercular drug and 100 mg/kg of silymarin showing well-maintained architecture along with normal vesicular nuclei and abundant eosinophilic cytoplasm [Figure 3].
Figure 3.
Histopathology of liver from control and experimental groups of animals, circle mark indicated that damage of liver cell. (a) Liver section of normal control Group I, (b) liver section of antitubercular drug-treated Group II, (c) liver section of antitubercular drug and ethanolic extract of Trapa natans (200 mg/kg)-treated Group III, (d) liver section of antitubercular drug and ethanolic extract of Trapa natans (400 mg/kg)treated Group IV, and (e) liver section of rats treated antitubercular drug and 100 mg/kg of silymarin Group V
DISCUSSION
Here, in this study, T. natans was assessed for the hepatoprotective activity using antitubercular drug (INH + RIF)-induced toxicity of liver in rat. Antitubercular chemotherapeutic regimens containing INH and RIF result in DIH which is a major cause for adverse reaction. Earlier, it has been delineated that this combination results in liver toxicity by causing hepatocellular steatosis and centrilobular necrosis, possibly associated with cholestasis; also the toxic metabolites of INH covalently get binds with the cell macromolecules in the case studies of both animal and human.[16]
Cytochrome P450-generated INH metabolite monoacetyl hydrazine is also found to cause liver toxicity. RIF stimulates cytochrome P450 enzyme activity resulting in increased production of toxic metabolites from acetyl hydrazine (AcHz). Furthermore, RIF can hasten metabolism of INH into the isonicotinic acid and hydrazine, both of which are hepatotoxic. The plasma half-life of AcHz (metabolite of I) is reduced by RIF and AcHz is rapidly changed to its active metabolites by increasing the oxidative elimination rate of AcHz, which is associated with the greater incidence of liver necrosis caused by RIF and INH in combination.[9] Apart from the mechanisms stated above, hepatotoxicity may possibly also be produced through hepatic injury induced due to oxidative stress which is an important cause for antitubercular drugs' hepatotoxicity.[17] The INH and RIF combination was reported to cause inhibition of secretion of biliary at a higher rate and also cause to hasten the lipid peroxidation (LPO) in liver cell, and it is also observed that a synergistic effect of cytochrome P450 was considered to be involved in the synergistic effects of RIF over INH. The levels of number serum hepatic enzymes behave as diagnostic indicators for hepatic injury.
This study indicted a significant (P < 0.001) level increase of AST, ALT, ALP, LDH, CHL, and bilirubin and a reduced level of ALB in serum of the animals of INH- and RIF-induced toxicity (Group II) certainly implicating hepatocellular impairment. An augment in the serum levels of these marker enzymes occurred due to the enzymes leakage from liver into the circulation as a consequence of liver damage.[9] The AST and ALT actions are responsive indicators of acute hepatic necrosis, and the ALP level is indicator of hepatobiliary disease. Serum marker enzyme levels were considerably decreased almost near to normal when T. natans 50% ethanolic extract was administered to animals at the dose of 200 and 400 mg/kg, respectively, indicating protection against liver damage. It is proposed that 50% TNE aided liver parenchymal cell regeneration and thereby protecting membrane integrity by decreasing enzyme leakage against the hepatotoxicity induced by antitubercular drug. It is a general reflection that the serum bilirubin levels (SBL) are considerably increased in hepatic injury. A marked raise was experiential in SBL in INH + RIF-induced toxic group of rats, whereas albumin level in the serum was significantly decreased. It was observed that after the liver intoxication with the hepatotoxicants leads to lessening in synthesizing proteins. As observed in the silymarin-treated group and 50% TNE, all studied parameters were significantly restored to normal condition from the abnormal ones. The underlying cause for liver damage during antitubercular drug therapy has been the initiation of oxidative stress, an outcome of hepatic antioxidant dysfunction in defence system which has been observed in earlier experiments carried over rats.[17]
It is been observed that the body has a defense mechanism working effectively to avert and neutralize the damage produced by free radical. The activities of SOD and CAT were significantly reduced observed in the rats with hepatic damage administered with anti-TB drugs, whereas on treatment with 200 and 400 mg/kg of TNE group showed a significant increase in the enzyme levels due to the ability of scavenge reactive oxygen species. Lipid peroxides (LPO) level elevation reflects the liver damage.
If the free radical production increases and/or a reduction of antioxidant defense mechanism leads to deterioration, the balance of the pro-oxidants-antioxidant, which results in cell death induced by oxidative stress.[18] Depletion of GSH is acknowledged to result in increased LPO and lipid peroxidation in excessive amount can cause amplified GSH consumption, as observed in this study which indicates the antioxidant activity of the TNE.[19] Furthermore, on treatment with different doses of TNE (200 and 400 mg/kg) significantly reduced the level of lipid peroxidation (LPO), which is a significant destruction causing damage to hepatocellular membranes, and rise in the GSH level in liver. The increase in hepatic GSH level in animals treated with TNE possibly because of the de novo GSH synthesis or GSH regeneration. Histopathological examinations further substantiated the overall hepatoprotective effect of the T. natans extract. On phytochemical screening, T. natans indicated the occurrence of flavonoids, steroidal alkaloids, triterpenes, and glycosides are the major chemical constituents. These phytochemical moieties are acknowledged for their inherent antioxidant property; hence, it is possible that the T. natans extract may reduce the induced oxidative stress by INH + RIF in addition to the other properties such as analgesic and thereby preventing liver damage. Hence, it is possible that the mechanism of hepatoprotection of T. natans might be because of its inherent antioxidant property present in these phytochemicals by reducing the induced oxidative stress occurred due to the administration of antitubercular drugs in addition to others akin to analgesic and curative property which possibly will prevent hepatic damage.[8,20,21,22] Additional investigations are required for the identification of specific active constituents accountable for the hepatoprotection.
CONCLUSION
Commencing the above results, it is clear from that the 50% TNE has shown a dose-dependent decrease in liver marker enzymes, lipid peroxidation (LPO), and the level of antioxidant enzymes get increased against hepatotoxicity induced by INH + RIF in experimental rats. Liver histopathology examinations evidenced that T. natans attenuated the hepatocellular necrosis and led to a reduction in inflammatory cells infiltration, which perhaps ascribed to its hepatoprotective effects. Clinically, application of T. natans extract for liver protection against liver injury induced by antitubercular drug in patient demands further investigation, yet proving to be the promising herbal drug to be included in the list of herbal drugs against antitubercular drug-induced hepatotoxicity.
Financial support and sponsorship
This research was supported by Deanship of Scientific Research at the University of Hail (0150391), KSA.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The author(s) would like to thank the Dean, College of Pharmacy, University of Hail, Hail, and Honorable Vice Chancellor, Integral University for providing necessary encouragement and facilities for this work.
REFERENCES
- 1.Shoba S, Patil PA, Vivek V. Hepatoprotective activity of Daucus carota L. aqueous extract against paracetamol, isoniazid and alcohol induced hepatotoxicity in male wistar rats. Pharmacologyonline. 2008;3:776–87. [Google Scholar]
- 2.Singla R, Sharma SK, Mohan A, Makharia G, Sreenivas V, Jha B, et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2010;132:81–6. [PubMed] [Google Scholar]
- 3.Niranjan A, Verma S, Lehri A, Amla D. High-performance thin-layer chromatographic analysis for the simultaneous quantification of four phenolic compounds in green, red, and black fruits of Trapa natans var. bispinosa Roxb. (Singhara) J Planar Chromatogr Mod TLC. 2013;26:316–21. [Google Scholar]
- 4.Imtiyaz S, Anwar M, Ali SJ, Tariq M, Chaudhury SS. Trapa bispinosa Roxb.: An Ethnopharmacological review. Int Res J Pharm Plant Sci. 2013;1:13–20. [Google Scholar]
- 5.Kang WY, Li YY, Gu XZ, Huang X. Hepatoprotective activity of Trapa acornis shell extracts against CCl4-induced liver injury in rats. Afr J Pharm Pharmacol. 2012;6:2856–61. [Google Scholar]
- 6.Mondal M, Bhattacharya S, Biswas M. Hepatoprotective activity of Trapa natans fruit peel extracts against paracetamol-induced liver damage in rats. Elixir Pharm. 2013;60:16461–3. [Google Scholar]
- 7.Desai SK, Gavitre BB, Patil MD, Mathapati SS, Gaikwad DT, Kulkarni VS, et al. Evaluation of hepatoprotective activity of a polyherbal formulation (PHF-A) by using isoniazid and rifampmicin-induced hepatotoxicity in rats. Int J Pharmacol Ther. 201;:32–41. [Google Scholar]
- 8.Agrahari AK, Khaliquzzama M, Panda SK. Evaluation of analgesic activity of methanolic extract of Trapa natans L. var. Bispinosa roxb. Roots. J Curr Pharm Res. 2010;1:8–11. [Google Scholar]
- 9.Hussain T, Gupta RK, K S, Khan MS, Hussain MD, Arif MD, et al. Evaluation of antihepatotoxic potential of Solanum xanthocarpum fruit extract against antitubercular drugs induced hepatopathy in experimental rodents. Asian Pac J Trop Biomed. 2012;2:454–60. doi: 10.1016/S2221-1691(12)60075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain T, Siddiqui HH, Fareed S, Vijayakumar M, Rao CV. Chemopreventive evaluation of Tephrosia purpurea against N-nitrosodiethylamine-induced hepatocarcinogenesis in wistar rats. J Pharm Pharmacol. 2012;64:1195–205. doi: 10.1111/j.2042-7158.2012.01503.x. [DOI] [PubMed] [Google Scholar]
- 11.Mujahid M, Hussain T, Siddiqui HH, Hussain A. Evaluation of hepatoprotective potential of Erythrina indica leaves against antitubercular drugs induced hepatotoxicity in experimental rats. J Ayurveda Integr Med. 2017;8:7–12. doi: 10.1016/j.jaim.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem. 1993;125:115–25. doi: 10.1007/BF00936440. [DOI] [PubMed] [Google Scholar]
- 13.Aebi H. In: Methods in Enzymology. Vol. 105. New York: Academic Press; 1984. Catalase in vitro; pp. 121–6. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 15.Upadhyay SN. Therapeutic potential of immunomodulatory agents from plant products. In: Upadhyay SN, editor. Immunomodulation. New Delhi: Narosa Publishing House; 1997. pp. 149–54. [Google Scholar]
- 16.Saraswathy SD, Suja V, Gurumurthy P, Devi S. Effect of Liv-100 against anti-tubercular drugs (isoniazid, rifampicin and pyrazinamide) induced hepatotoxicity in rats. Indian J Pharmacol. 1998;30:233–8. [Google Scholar]
- 17.Kale BP, Kothekar MA, Tayade HP, Jaju JB, Mateenuddin M. Effect of aqueous extract of Azadirachta indica leaves on hepatotoxicity induced by antitubercular drugs in rats. Indian J Pharmacol. 2003;35:177–80. [PubMed] [Google Scholar]
- 18.Sodhi CP, Rana SV, Mehta SK, Vaiphei K, Attari S, Mehta S, et al. Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug Chem Toxicol. 1997;20:255–69. doi: 10.3109/01480549709003881. [DOI] [PubMed] [Google Scholar]
- 19.Onyema OO, Farombi EO, Emerole GO, Ukoha AI, Onyeze GO. Effect of Vitamin E on monosodium glutamate induced hepatotoxicity and oxidative stress in rats. Indian J Biochem Biophys. 2006;43:20–4. [PubMed] [Google Scholar]
- 20.Kim BJ, Kim JH, Kim HP, Heo MY. Biological screening of 100 plant extracts for cosmetic use (II): Anti-oxidative activity and free radical scavenging activity. Int J Cosmet Sci. 1997;19:299–307. doi: 10.1046/j.1467-2494.1997.171726.x. [DOI] [PubMed] [Google Scholar]
- 21.Malviya N, Jain S, Jain A, Jain S, Gurjar R. Evaluation of in vitro antioxidant potential of aqueous extract of Trapa natans L. Fruits. Acta Pol Pharm. 2010;67:391–6. [PubMed] [Google Scholar]
- 22.Kar DM, Maharana L, Si SC, Kar MK, Sasmal D. Antiulcer activity of ethanolic extract of fruit of Trapa bispinosa Roxb. in animals. Pharm Lett. 2010;2:190–7. [Google Scholar]