Abstract
Background:
Inhabitants of the Eastern Cape Province of South Africa use the roots of Dianthus thunbergii and corms of Hypoxis argentea to treat diabetes mellitus and other ailments.
Objective:
The objective of this study was to analyze the phytochemical composition and antioxidant activities of the aqueous and ethanol extracts of the roots and corms of two plants.
Materials and Methods:
Total phenolics, flavonoids, flavonols, proanthocyanidins, tannins, and alkaloids were determined by standard methods. The scavenging activities of the extracts against 1,1 diphenyl-2-picrylhydrazyl (DPPH), 2'-azino-bis (3-ethylbenthiazoline-6-sulfonic acid (ABTS), nitric oxide (NO), hydrogen peroxide (H2O2), and their ferric-reducing antioxidant potentials (FRAPs) were measured.
Results:
The ethanol extract of H. argentea had the highest content of phenolics (66.71 ± 2.71 mg gallic acid equivalent/g) and tannins (1.18 ± 0.07 mg TAE/g), while the ethanol extract of D. thunbergii gave higher contents of flavonoids and proanthocyanidins (62.21 ± 1.75 mg Qe/g and 432.62 ± 2.43 mg Ca/g, respectively). Flavonols were the most predominant in the aqueous extract of H. argentea (25.51 ± 1.92 mg Qe/g). We observed a concentration-dependent response in the ABTS- and H2O2-scavenging activities and FRAP values of the extracts and standards (Vitamin C, butylated hydroxytoluene, and rutin). The ethanol extracts of both plants generally demonstrated better antioxidant activities against H2O2, NO, and ABTS while also possessing better reducing power than the aqueous extracts. The aqueous extract of D. thunbergii, however, showed the best DPPH scavenging activity.
Conclusion:
The higher content of phytochemicals and antioxidant capacity obtained for the ethanol extracts of D. thunbergii and H. argentea may prove to be valuable information in selecting suitable extraction solvents for the medicinal applications of both plants.
SUMMARY
Ethanol extracts of Hypoxis argentea had the highest levels of phenolics and tannins
Ethanol extracts of Dianthus thunbergii had the highest levels of flavonoids and proanthocyanidins
Ethanol extracts of both plants possess better antioxidant activityagainst hydrogen peroxide, nitric oxide, and ABTS as well as higher reducingpower than the aqueous extracts
Aqueous extract of Dianthus thunbergii had the highest free radical scavenging activity as measured with DPPH.
Abbreviations used: ABTS: 2'-azino-bis (3-ethylbenthiazoline-6-sulfonic acid); BHT: Butylated hydroxytoluene; DPPH: 1,1 diphenyl-2-picrylhydrazyl; DTA: Dianthus thunbergii aqueous extract (16.6%); DTE: Dianthus thunbergii ethanol extract (2.4%); Fe3+-TPTZ: Ferric tripyridyltriazine; FRAP: Ferric-reducing antioxidant potentials; GAE: Gallic acid equivalent; HAA: Hypoxis argentea aqueous extract (3.2%); HAE: Hypoxis argentea ethanol extract (1.8%); Qe: Quercetin equivalence; ROS: Reactive oxygen species; TBA: Thiobarbituric acid;TCA: Trichloroacetic acid.
Keywords: Antioxidants, diabetes, Dianthus thunbergii, Hypoxis argentea, phytochemicals, radical scavenging
INTRODUCTION
Plants have developed secondary biochemical pathways to synthesize a variety of chemicals that enable them to cope with challenges such as herbivores, pathogen attacks, and local nutrient fluctuations in their immediate environment.[1] These phytochemicals, including phenols, flavonoids, tannins, saponins, alkaloids, phenolic glycosides, terpenoids, and other metabolites,[2,3] are sources of compounds that possess medicinal properties against several diseases that affect humans. The pathogenesis of most diseases involves oxidative and inflammatory components, usually arising from excessive production of reactive oxygen species (ROS).[4] ROS include activated oxygen metabolites called free radicals, such as superoxide anion radicals (O2−) and hydroxyl radicals (OH), as well as nonradicals including hydrogen peroxide (H2O2) and singlet oxygen (1O2). Nonradicals have the potential to participate in reactions that eventually give rise to free radicals. Free radicals and nonradicals alike are capable of reacting in vivo with tissue macromolecules, forming products such as guanine adducts with DNA, or lysyl adducts with proteins.[5]
The body of aerobic organisms is equipped with antioxidant defense mechanisms (enzymic and nonenzymic) that protect against tissue damage by ROS.[6] These are, however, not always sufficient to counteract the severity of oxidative challenge that characterizes many disease or toxic states. The imbalance that results between production of oxidants (ROS) and antioxidants, in favor of the oxidants, is described as oxidative stress and is a major underlying factor in the pathogenesis of many chronic diseases such as diabetes, cancer, and atherosclerosis.[3]
In recent times, considerable research efforts have been directed at finding naturally occurring compounds, especially of botanical origin, which offer protection against diseases through their antioxidative and/or anti-inflammatory properties. Dietary supplementation with phytochemicals represents an effective protective strategy to complement the activities of natural antioxidant defense systems, as well as a viable alternative to synthetic drugs which generally have limitations of associated adverse effects.[7,8]
The South African population is one of those plagued with a rising incidence of chronic noncommunicable diseases such as diabetes mellitus.[9] A considerable proportion of the population resorts to the use of herbs for the treatment of various diseases such as diabetes. Ethnobotanical surveys have revealed a large number of these plants reported to be useful in the treatment of diabetes mellitus.[10,11] Dianthus thunbergii, of the family Caryophyllaceae, belongs to a genus of over 300 species, which are traditionally used for medicinal purposes as infusions for the treatment of chest complaints, severe colic, soothing of wounds, urolithiasis, boils and carbuncles, eczema, and itching.[12,13] Extracts from the fresh crushed roots are reportedly used against diabetes and other conditions and are taken as 2 teaspoonfuls orally three times daily.[11] Hypoxis argentea is one of the several species of the genus Hypoxis which are popular in African herbal medicine for the treatment of septic sores, headaches, dizziness, testicular tumors, tuberculosis, asthma, and diabetes.[14,15,16] The corm, the underground part, is usually sought for medicinal use. For diabetes treatment, it is reportedly boiled in water and the infusion is administered orally until a patient is healed.
Despite traditional claims for the effectiveness of D. thunbergii and H. argentea against diabetes mellitus, they have not been investigated scientifically for their phytochemical composition and biological activities. As part of ongoing comprehensive analysis of the medicinal potentials of these plants, the present study aimed to quantify the content of major phytochemicals and also to evaluate the antioxidant activities of the aqueous and ethanol extracts of the roots and corms of D. thunbergii and H. argentea. These properties were compared between the two plants and correlations were made between the phytochemical contents and antioxidant activities of the plants.
MATERIALS AND METHODS
Chemicals
Folin–Ciocalteu reagent, 1,1 diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azino-bis (3-ethylbenthiazoline-6-sulfonic acid) (ABTS), vanillin, ferric chloride, butylated hydroxytoluene (BHT), rutin, Vitamin C potassium ferricyanide, trichloroacetic acid (TCA), thiobarbituric acid, glacial acetic acid, sodium nitroprusside, tannic acid, gallic acid, quercetin, sodium carbonate, aluminum chloride, and potassium acetate were purchased from Merck (Pty) Ltd., Gauteng, South Africa. All the other chemicals and solvents were of analytical grade.
Collection of plant materials and preparation of extracts
The roots of D. thunbergii and corms of H. argentea were collected in May, 2015, at Alice, Eastern Cape, South Africa. They were authenticated at the Giffen Herbarium, University of Fort Hare, South Africa. Both plants were identified with voucher specimen numbers CRY-2502 for D. thunbergii and HYP-1230 for H. argentea. The roots and corms were initially washed free of soil attachments and then oven-dried to constant weight at 30°C. The dried plant materials were then milled into fine powder using an electric blender (Commercial Blender type GB27, Hamilton Beach Brands, Inc., China). A composition of 200 g of each of the powdered plant materials was extracted separately in distilled water and 99.99% ethanol using an orbital shaker (Labcon laboratory service [Pty], South Africa) for 24 h. The suspensions in ethanol were thereafter filtered using Whatman No. 1 filter papers in a Buchner funnel. The aqueous suspensions were initially filtered through a thick layer of sterile cotton wool before their filtration with the filter paper. The ethanol extracts were concentrated to dryness using a rotary evaporator (Heidolph Laborota 4000, Heidolph Instruments, GmbH and Co, Germany) while the aqueous extracts were initially frozen at −40°C and then dried using a freeze dryer. The different extracts were reconstituted in their respective solvents for use in the assays conducted in this study.
Phytochemical screening
Total phenolic content
The content of phenols in the different extracts was determined spectrophotometrically by the Folin–Ciocalteu reagent according to the method of Ozkok et al.[17] A calibration curve was prepared with gallic acid as standard (0.025–0.125 mg/ml in 70% methanol v/v). To 0.5 ml of each of the gallic acid concentrations or extracts (mg/ml), 2.5 ml Folin–Ciocalteu reagent (previously prepared as 10% v/v dilution in distilled water) was added. Thereafter, 2 ml anhydrous sodium carbonate (7.5%) was added, producing a blue-colored solution. The mixtures were vortexed thoroughly and placed in a water bath for 30 min at 45°C. The absorbance was then read at 765 nm. The equation of the calibration curve obtained (Y = 14.885x; R2 = 0.9961) was used to establish the gallic acid equivalence (mg/ml). The total phenolic content was calculated using the formula: T = C × V/m, where T is the total phenolic content, V is the volume of the extract (ml) used in the assay, C is the gallic acid equivalent (GAE) (mg/ml), and m is the weight of the pure plant extract used in the assay. Values were expressed as GAE per gram of dry plant extract (mg GAE/g). All assays were performed in triplicate.
Total flavonoid content
Flavonoid contents in the extracts were determined using the aluminum chloride method as described by Ozkok et al.[17] A calibration curve was prepared with quercetin (0.025–0.125 mg/ml in 80% methanol v/v). Briefly, 0.5 ml of the extracts (prepared at a concentration of 1 mg/ml) or the standard at the different concentrations was mixed with 3 ml of 95% ethanol, 0.2 ml of aluminum chloride (prepared as a 10% aqueous dilution), and 0.2 ml of 1 M potassium acetate, and the whole mixture was made up to 10 ml with distilled water. The resulting solutions, prepared in triplicate, were yellow and were thoroughly vortexed and allowed to stand for 30 min at room temperature, after which the absorbance was read at 420 nm. The equation of the calibration curve obtained (Y = 11.922x; R2 = 0.9955) was used to establish quercetin equivalence (mg/ml) and the total flavonoid content was calculated using the formula: T = C × V/m, where T is the total flavonoid content, V is the volume of the extract (ml) used in the assay, C is the quercetin equivalent (mg/ml), and m is the weight (g) of the pure plant extract used in the assay. Values were expressed as quercetin equivalent per gram of dry plant extract (mg Qe/g).
Total flavonol content
Total flavonols were determined using the method of Wintola and Afolayan,[18] with slight modifications. A calibration curve was prepared with quercetin (0.025–0.125 mg/ml in 80% methanol v/v). Plant extracts were prepared at a final concentration of 1 mg/ml. 2 ml of the extract or the standard was mixed with 3 ml of 95% ethanol, 0.2 ml of aluminum chloride (10% w/v), and 0.2 ml of sodium acetate (50 g/L). The resulting mixture was made up to 10 ml with distilled water and vortexed thoroughly. All assays were done in triplicate and were allowed to stand for 2.5 h at room temperature, after which the absorbance was read at 440 nm. The equation of the calibration curve obtained (Y = 13.128x; R2 = 0.9990, where Y is the absorbance and x is the concentration) was used to establish quercetin equivalence (mg/ml). The total flavonol content was calculated using the formula: T = C × V/m, where T is the total flavonol content, V is the volume of the extract (ml) used in the assay, C is the quercetin equivalent (mg/ml), and m is the weight (g) of the pure plant extract used in the assay. Values were expressed as quercetin equivalent per gram of dry plant extract (mg Qe/g).
Total tannins
The method described by Wintola and Afolayan[18] was used to quantify the tannin content in the plant extracts. A standard curve was prepared using tannic acid (0.002–0.010 mg/ml in distilled water) as standard. Briefly, 0.2 g of each extract in triplicate was dissolved in 20 ml of 50% methanol. This was placed in a water bath at 80°C for 1 h, after which the mixtures were filtered into 100 ml volumetric flasks. To the standard concentrations of tannic acid as well as the filtrates from the extracts, 20 ml of distilled water, 2.5 ml of Folin–Ciocalteu reagent, and 10 ml of 17% sodium carbonate were added in that order. All mixtures in triplicate were then made up to 100 ml with distilled water and were allowed to stand for 20 min. A bluish green color was developed at the end of the reaction and the absorbance of the mixtures was read at 760 nm. Tannic acid equivalence (mg/ml) was established from the equation of the standard curve (Y = 154.45x; R2 = 0.9585), where y is the absorbance and x is the tannic acid equivalent in mg/ml. Total tannin content (T) in milligram tannic acid equivalent per gram of dry extract was calculated using the formula: T = C × V/m, where V is the volume of the extract (ml) used in the assay, C is the tannic acid equivalent (mg/ml), and m is the weight (g) of the pure plant extract used in the assay.
Total proanthocyanidins
Total proanthocyanidins were measured using the method described by Oyedemi et al.[19] The extracts were prepared at a final concentration of 1 mg/ml. To 0.5 ml of this in triplicate, 3 ml of 4% vanillin-methanol solution and 1.5 ml of hydrochloric acid were added. These were thoroughly mixed and allowed to stand for 15 min at room temperature. The absorbance was read at 500 nm. Total proanthocyanidin content as catechin equivalent was evaluated from the equation Y = 0.5825x; R2 = 0.9277, where y is the absorbance and x is the catechin equivalent (mg/ml). The amount of proanthocyanidin in the extracts in mg/g was calculated with the following formula: T = C × V/m, where V is the volume of the extract (ml) used in the assay, C is the catechin equivalent (mg/ml), and m is the weight (g) of the pure plant extract used in the assay.
Alkaloids
Alkaloid content was determined using the method of Harbone[20] with slight modifications. Briefly, 0.5 g of the dried extract was dissolved in 20 ml of 20% acetic acid in ethanol v/v. The mixture was allowed to stand for 4 h after which it was filtered. The filtrate was then placed in a water bath for about 30 min at boiling temperature. Thereafter, concentrated ammonium hydroxide was added dropwise which produced some effervescence and precipitation. The collected precipitate was washed with dilute ammonium hydroxide and then filtered with already weighed filter papers. The residue left in the filter papers was then dried in an oven and the resulting dried papers with residue were also weighed. The alkaloid content was determined using the formula: Alkaloid (%) = final weight of the residue/initial weight of the extract used × 100. All the determinations were done in triplicate.
Antioxidant activity assays
The antioxidant capacities of the different extracts were measured using DPPH radical scavenging activity, ferric-reducing power, ABTS radical scavenging activity, H2O2, and nitric oxide (NO) scavenging activities. These measurements were made against standard antioxidants including Vitamin C, BHT, and Rutin.
1,1 diphenyl-2-picrylhydrazyl radical scavenging activity
The method described by Liyana-Pathiranan and Shahidi[21] was used in this assay. 1 ml of the extracts or the standards at different concentrations (0.025–0.50 mg/ml), prepared in triplicates, was mixed with 1 ml of DPPH (0.135 mM) prepared in methanol. The mixtures were vortexed thoroughly and left in the dark for 30 min at room temperature. The absorbance was then measured spectrophotometrically at 517 nm. The percentage of DPPH scavenging activity of the extract or standard was calculated with the following formula: % DPPH radical scavenging activity = [(AC–AS)/AC] × 100, where AC is the absorbance of the control and AS is the absorbance of the test samples (extract or standard).
Ferric-reducing antioxidant power
The method described by Aiyegoro and Okoh[22] was used for the determination of the ferric-reducing activities of the plant extracts. The assay is based on the reduction of ferric-tripyridyltriazine (Fe3+-TPTZ) complex by the action of electron-donating antioxidants at low pH to the ferrous form. Each extract or standard was initially prepared in distilled water in increasing concentrations from 0.025 to 0.5 mg/ml. 1 ml of each of the extract or the standard at the different concentrations was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of potassium ferricyanide. The mixture was incubated for 20 min at 50°C. This was followed by the addition of 2.5 ml of TCA (10% w/v) and centrifugation at 3000 rpm for 10 min. Thereafter, 2.5 ml of the supernatant was withdrawn and mixed with 2.5 ml of distilled water and 0.5 ml FeCl3 (0.1% w/v). The absorbance was read at 700 nm with distilled water as blank. An increase in absorbance with increasing concentration of extract or standard corresponds to the formation of the bluish-green color of the reduced form of TPTZ. The average absorbance of the reactions performed in triplicate was obtained and plotted against the different concentrations of each extract and standard.
2'-azino-bis (3-ethylbenthiazoline-6-sulfonic acid) radical scavenging activity
This assay was performed according to the method of Adedapo et al.[23] It is based on the reaction between ABTS and potassium persulfate, to produce the ABTS radical cation (ABTS+), a bluish-green chromogen, which is converted to a colorless solution in the presence of an antioxidant reductant.[24] A working solution was prepared by mixing equal amounts of 7 mM ABTS and 2.4 mM potassium persulfate. These were allowed to react for about 12 h in the dark at room temperature. 1 ml of the resulting solution was mixed with 60 ml methanol and the absorbance was adjusted to 0.706 ± 0.001 units at 734 nm by addition of drops of the original ABTS/potassium persulfate solution. Thereafter, 1 ml of each extract and the standards prepared at different concentrations (0.025–0.50 mg/ml) in methanol was mixed with 1 ml of the ABTS/methanol solution. The absorbance of the resulting solutions was read at 734 nm after about 7 min. ABTS radical scavenging activity was indicated by different degrees of decolorization of the dark-green color of the ABTS solution. All assays were done in triplicate. The percentage of ABTS radical scavenging activity was calculated from the following equation: % ABTS scavenging activity = [(AC–AS)/AC] × 100, where AC is the absorbance of the control (ABTS + methanol) and AS is the absorbance of the test samples (extract or standard).
Hydrogen peroxide scavenging activity
H2O2 scavenging activity was determined according to the method described by Wintola and Afolayan.[18] H2O2 in the presence of an antioxidant is converted to water and oxygen, thus decreasing the concentration of H2O2 as the concentration of antioxidant increases. 1 ml of the extract or the standard prepared at different concentrations (0.025–0.5 mg/ml) in distilled water was mixed with 0.6 ml of 40 mM H2O2 (prepared in 0.1 mM phosphate buffer, pH 7.4). This was left to react for 10 min after which the absorbance was read at 230 nm using a UV–VIS 3000 PC spectrophotometer, against a blank containing phosphate buffer without H2O2. The inhibition of H2O2 was calculated as the percentage of H2O2 remaining in the solutions[25] using the following formula: % H2O2 remaining = [(AC–AS)/AC] × 100, where AC is the absorbance of the control (phosphate buffer + H2O2) and AS is the absorbance of the test samples (extract or standard).
Nitric oxide scavenging activity
The method described by Wintola and Afolayan[18] was used for the assay of the NO radical scavenging activity of the extracts. The procedure is based on the principle that sodium nitroprusside in aqueous solution at physiological pH spontaneously generates NO, which interacts with oxygen to produce nitrite ions. The nitrite ions are detected in solution by the Griess reagent which contains sulfanilamide and naphtylethylene diaminedihydrochloride. Compounds that scavenge NO compete with oxygen, leading to a reduced production of nitrite ions (Ebrahimzadeh et al.,[26]), For the essay, (0.5 ml of the extracts or standard was mixed with 2 ml of 10 mM sodium nitroprusside [prepared in 0.5 mM phosphate-buffered saline, pH 7.4]). The mixture was incubated for 2.5 h at 25°C. 0.5 ml of the mixture was mixed with 0.5 ml of Griess reagent (prepared by mixing 1 ml sulfanilic acid [0.33% in 20% glacial acetic acid] with 1 ml of naphthalene diaminedihydrochloride [0.1% w/v]). The mixture was incubated for 30 min at room temperature and the absorbance was measured at 540 nm. The percentage of NO scavenging ability of the plant extracts and standard compounds was calculated using the following formula: % NO scavenged = [(AC–AS)/AC] × 100, where AC is the absorbance of the control reaction and AS is the absorbance of the test samples (extract or standard).
Statistical analysis
All values have been expressed as mean ± standard deviation of three replicates. Microsoft Excel® Professional for Windows (Version 2013) was used for statistical analyses and determination of IC50 for the antioxidant assays. Significant differences (P < 0.05) were examined by ANOVA using GraphPad Prism Software (GraphPad Software, Inc; www.graphpad.com).
RESULTS
The yields of the extracts obtained are D. thunbergii aqueous extract (DTA; 16.6%), D. thunbergii ethanol extract (DTE; 2.4%), H. argentea aqueous extract (HAA; 3.2%), and H. argentea ethanol extract (HAE; 1.8%).
Phytochemical constituents
The phytochemical composition of the different extracts of D. thunbergii roots and H. argentea corms is presented in Table 1. The results indicate significant differences (P < 0.05) in the contents of the different phytochemicals between the two plants and the extraction solvents used in this study, water and ethanol.
Table 1.
Phytochemical composition of aqueous and ethanol extracts of Dianthus thunbergii and Hypoxis argentea
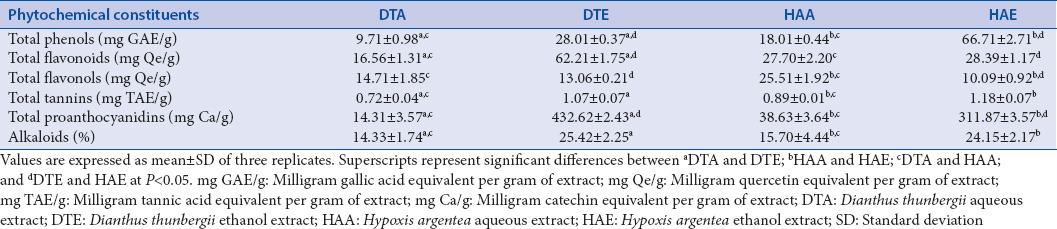
The ethanol extracts of each plant had significantly higher content (P < 0.05) of phenols than the aqueous extracts. HAE had the highest total phenolic content while DTA had the least. The results, however, indicate a significantly higher content (P < 0.05) of total phenols in H. argentea than in D. thunbergii. Total flavonoid contents in the extracts were in the following order DTE > HAE > HAA > DTA, implying that the aqueous extracts of each plant had higher contents of flavonoids than the ethanol extracts. The contents of flavonols, on the other hand, were significantly higher (P < 0.05) in the aqueous extracts than the ethanol extracts. HAA had the highest content of total flavonols while HAE had the least amount. High concentrations of proanthocyanidins were detected in the two plants, the majority of which were concentrated in the ethanol extracts in the following order: DTE > HAE > HAA > DTA.
Tannin content was generally low in all the extracts compared to the other phytochemicals evaluated. As with most phytochemicals, the ethanol extracts contained higher contents of tannins than the aqueous extracts, the amount in H. argentea being slightly higher than in D. thunbergii. The percentage of alkaloids detected in the plant extracts also showed that the ethanol extracts had significantly higher alkaloid contents (P < 0.05) than the aqueous extracts. There were no significant differences in the alkaloid contents of both plants when extracted with the same solvent. This indicates a trend which suggests that ethanol may extract greater amounts of phytochemicals, despite the relatively lower yields of ethanol extracts produced from the original plant material, compared to the aqueous extracts.
Antioxidant activities
The antioxidant activities of the plant extracts compared favorably with those of the standard antioxidants (Vitamin C, BHT, and Rutin) employed in the antioxidant assays.
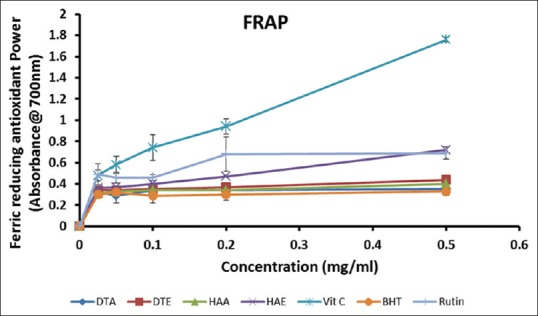
Ferric-reducing power
As shown in Figure 1, the extracts and standards exhibited a concentration-dependent increase in their ferric-reducing abilities, indicated by an increase in absorbance with increasing concentration of extract or standard. Vitamin C exhibited the most potent ferric-reducing ability of all other compounds and extracts tested. The overall order of the ferric-reducing abilities at the highest concentration (0.50 mg/ml) was as follows: Vitamin C > HAE > Rutin > DTE > HAA > DTA > BHT. This result suggests that the ethanol extracts of both D. thunbergii and H. argentea possessed higher ferric-reducing capacities than the aqueous extracts. This observation appears to bear significant correlation with the total phenolic and flavonoid contents of the extracts.
Figure 1.
1,1 diphenyl-2-picrylhydrazyl radical scavenging activities of the aqueous and ethanol extracts of Dianthus thunbergii and Hypoxis argentea
1,1 diphenyl-2-picrylhydrazyl scavenging activity
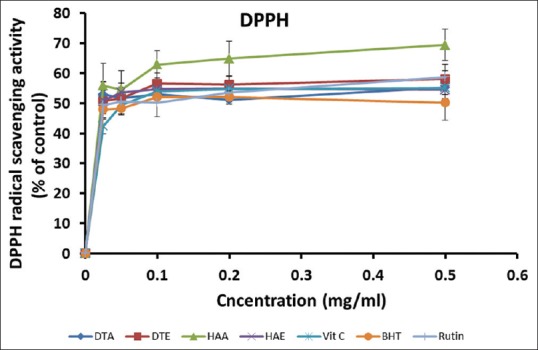
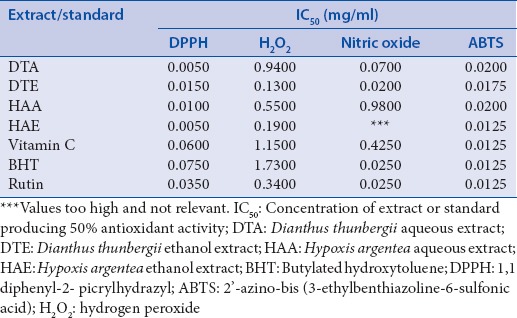
The DPPH radical scavenging activities of the extracts and standards are presented in Figure 2.At the maximum concentration tested (0.50 mg/ml), HAA exhibited the highest DPPH scavenging activity (69.42 ± 5.26%), followed by Rutin (58.69 ± 4.25%), DTE (58.06 ± 2.83%), DTA (55.18 ± 2.23%), Vitamin C (55.06 ± 2.25%), HAE (54.19 ± 2.83%), and BHT (50.19 ± 5.77%), in decreasing order. It appears that this effect correlated with the contents of flavonols in the extracts. IC50 values [Table 2], however, indicate that DTA and HAE required the lowest concentration (0.005 mg/ml) to produce 50% inhibition, followed by Vitamin C (0.092 mg/ml).
Figure 2.
Ferric-reducing activities of the aqueous and ethanol extracts of Dianthus thunbergii and Hypoxis argentea
Table 2.
IC50 values for antioxidant activities of the extracts
2'-azino-bis (3-ethylbenthiazoline-6-sulfonic acid) radical scavenging activity
The results of this assay [Figure 3] show that all the extracts and standards possessed considerable ABTS radical scavenging activities, even at the low concentrations, and this activity compared favorably with the standards. At the highest concentration tested (0.50 mg/ml), all the standards produced up to 99% inhibition, while values for DTA, DTE, HAA, and HAE were (84.14 ± 4.56%), (98.16 ± 0.14%), (95.52 ± 2.02%), and (99.15 ± 0.14%), respectively. Among the extracts, HAE had the lowest IC50 value (0.0125 mg/ml), similar to the standards, followed by DTE (0.175 mg/ml), DTA (0.020 mg/ml), and HAA (0.020 mg/ml) [Table 2].
Figure 3.
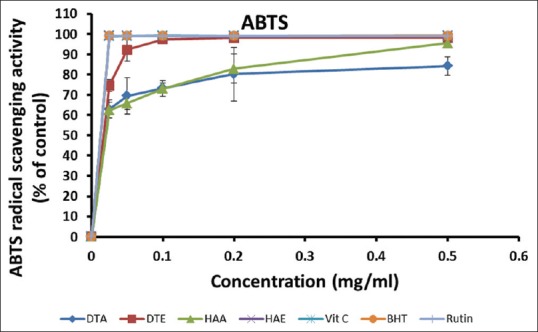
2'-azino-bis (3-ethylbenthiazoline-6-sulfonic acid) radical scavenging activities of the aqueous and ethanol extracts of Dianthus thunbergii and Hypoxis argentea
Nitric oxide inhibition
The percentage NO inhibitory activities of the extracts and standards [Figure 4] were in the following order: DTE > BHT > DTA > Rutin > Vitamin C > HAA > HAE, at the highest concentration (0.50 mg/ml). This seems to suggest that extracts of D. thunbergii exhibited a more potent NO inhibitory activity than those of H. argentea at higher concentrations. DTE also possessed the lowest IC50 [Table 2], indicating its strong NO scavenging activity. In general, increasing the concentration of the extracts or standards did not seem to significantly affect their NO inhibitory potentials.
Figure 4.
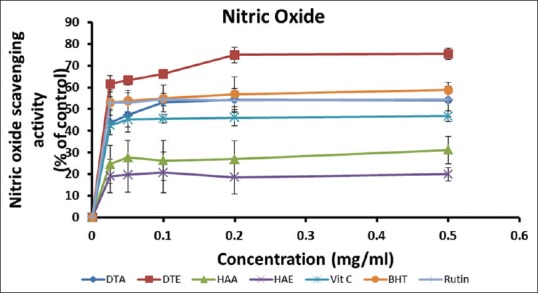
Nitric oxide inhibitory activities of the aqueous and ethanol extracts of Dianthus thunbergii and Hypoxis argentea
Hydrogen peroxide scavenging activity
As shown in Figure 5, the extracts and standards exhibited a concentration-dependent inhibition of H2O2, as the contents of H2O2 remaining in the reaction mixtures reduced with increasing concentration of extracts or standards. At the highest concentration (0.50 mg/ml), the order of inhibition was as follows: DTE > HAE > Rutin > HAA > DTA > Vitamin C > BHT, suggesting that the ethanol extracts possessed higher H2O2 scavenging abilities than that of the aqueous extracts. The result also indicates that the plant extracts had better antioxidant activity than most of the standards with respect to removal of H2O2.
Figure 5.
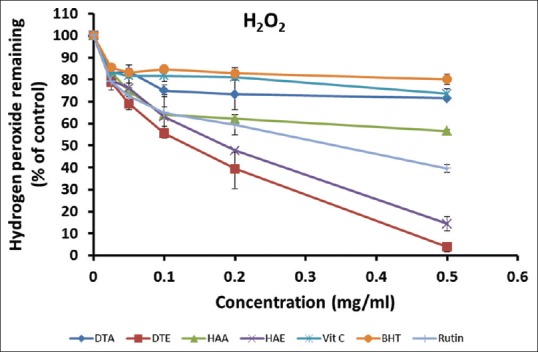
Hydrogen peroxide scavenging activities of the aqueous and ethanol extracts of Dianthus thunbergii and Hypoxis argentea
DISCUSSION
The pathogenesis of many chronic ailments such as diabetes mellitus usually involves oxidative stress mechanisms.[27] In oxidative stress, the production of ROS such as hydroxyl radicals, superoxide radicals, and H2O2 exceeds the capacity of exogenous antioxidants to detoxify them, leading to cellular or tissue damage due to lipid peroxidation and other mechanisms.[5] The use of herbal preparations as alternative or complementary therapeutic strategies against several diseases is a common practice in many African communities. Various groups of phytochemicals in plant extracts are believed to possess potent antioxidant and anti-inflammatory activities, which makes them useful in disease prevention and treatment.[28] For example, some flavonoids, tannins, alkaloids, saponins, and polycyclic glycosides have been reported to have hypoglycemic and antidiabetic activities.[29]
In this study, we evaluated the phytochemical composition and antioxidant activities of extracts from the roots of D. thunbergii and corms of H. argentea, two commonly used plants in the Eastern Cape province of South Africa for the management of diabetes mellitus. We aimed to compare the parameters between the two plants and the solvents (water and ethanol) used for the preparation of these plants for medicinal use. We also attempted to find any correlations between the antioxidant activities and the content of the major phytochemicals in these extracts. With respect to the extracting ability of the solvents, we observed that the ethanol extracts of the two plants contained significantly higher contents of phenols, flavonoids, proanthocyanidins, tannins, and alkaloids, compared to the aqueous extracts. The differences in the extracting abilities may be related to the differences in polarity of the components extracted and that of the solvents.[30] Total flavonol contents of the extracts, however, were higher in the aqueous extracts than in the ethanol extracts. This latter observation is similar to findings in another research.[31] The aqueous extract of H. argentea contained the highest amount of flavonols followed by the aqueous extract of D. thunbergii.
Flavonols, a class of flavonoids, are a group of poly-hydroxylated phenolics which are highly water soluble as a result of their hydroxyl groups. They are, therefore, more soluble in aqueous solvents than in organic solvents.[32] Comparison of total flavonol content with DPPH radical scavenging activities of the extracts at 0.5 mg/ml revealed a positive correlation. The DPPH radical is relatively stable. Its unpaired electron can become paired upon reaction with hydrogen-donating antioxidants.[33] Flavonoids have the ability to easily donate the hydrogen atoms in their hydroxyl groups[34] and therefore, it appears that this group of phytochemicals must have played a major role in the DPPH radical scavenging activities of the extracts in this study.
The order of increasing total phenolic content in the extracts was DTA (9.71 mg/g) < HAA (18.10 mg/g) < DTE (28.01 mg/g) < HAE (66.71 mg/g). In line with this result, extractions with ethanol or ethanol in water (50% v/v) have been reported to yield higher total phenolic contents than extraction with water alone.[35] At 0.5 mg/ml, we observed a highly positive correlation between the total phenolic contents of the extracts and their ferric-reducing abilities. The ability of compounds to convert ferric ion into ferrous form is a good indicator of antioxidant potential.[36] This ferric-reducing ability has been attributed to the presence of phenols in plant extracts, as well as the number and position of the hydroxyl groups in these compounds.[37] This finding is consistent with reports from several other researchers.[36,38,39,40]
The concentration of proanthocyanidins was higher in the ethanol extracts of both plants compared to that of the aqueous extracts, being highest in the ethanol extract of D. thunbergii. The tannin contents of the extracts were considerably low compared to the other groups of phytochemicals. The ethanol and aqueous extracts of each plant had similar contents of alkaloids. Although almost all plant species possess these different phytochemicals, their quantitative distribution varies, and this is believed to be responsible for the different medicinal potentials they exhibit.[41] Tannins have been reported to also possess the ability to reduce Fe3+ to the Fe2+ form and can inhibit the 5-lipoxygenase in arachidonic acid pathways, which highlights their importance in inflammatory processes. Interestingly, we found a similar trend of positive correlation between tannin content and the ferric-reducing abilities of the extracts, as obtained for total phenolic contents. This, therefore, suggests that the antioxidant activity of the extracts is a combination of the roles played by the different groups of phytochemicals, rather than any one group.
The NO radical is produced in vivo by phagocytes and endothelial cells and it is very important in inflammatory processes. However, its overproduction can result in tissue damage seen in many inflammatory conditions including diabetes and arthritis.[42] This radical can combine with the superoxide radical to form the highly reactive peroxynitrite anion (ONOO−).[43] In our study, the ethanol extract of D. thunbergii exhibited the most potent NO inhibitory activity, which was even higher than those of Rutin, Vitamin C, and BHT. The NO scavenging activity was lowest in the extracts of H. argentea. The significantly higher contents of flavonoids and proanthocyanidins in the ethanol extracts of D. thunbergii might have been responsible for this observation. This finding suggests that D. thunbergii than H. argentea may be more beneficial in counteracting inflammatory signaling processes involving NO.
H2O2 is a nonradical which is not very reactive, but becomes toxic when it forms the highly reactive hydroxyl radical in the presence of transition metals such as Fe2+.[44] We observed a concentration-dependent pattern of H2O2 scavenging activity in the extracts and standards used in this study. This activity was highest in the ethanol extract of D. thunbergii which gave the least amount of H2O2 remaining in the reaction mixtures at the highest concentration tested and the lowest IC50, while the ethanol extract of H. argentea also demonstrated high activity. It is noteworthy that the activity of these extracts in scavenging H2O2 was higher than those exhibited by the standards, again highlighting the significant antioxidant capacities of the plants. Further confirmation of the potent antioxidant activities of these extracts was indicated by their pronounced ABTS radical scavenging activities, even at the lowest concentrations. This effect was comparable to those of the standard antioxidants, Vitamin C, BHT, and Rutin.
CONCLUSION
Our study shows that extracts from D. thunbergii and H. argentea are easily accessible sources of phytochemicals possessing significant antioxidant activities, attributes which may prove useful for their effectiveness in the treatment of diseases such as diabetes mellitus as claimed by traditional healers in the Eastern Cape. With respect to the solvents used for extraction, the ethanol extracts had higher contents of phytochemicals than the aqueous extracts, an observation which correlated positively with the higher antioxidant activities exhibited by the ethanol extracts. The two solvents used in this study have been chosen to represent those commonly used by the local traditional healers in the preparation of these plants for medicinal use.
Further studies are, however, required to determine the best extraction solvents for these plants. Overall, both plants exhibited superior antioxidant activities than the other in different respects, depending on the nature of radical being scavenged. This may be related to the variations in their respective contents of the different phytochemicals. This latter observation may explain, in part, the traditional approach of mixing different plants in concoctions, rather than the use of individual plants for the treatment of different diseases. Further studies are currently ongoing to investigate the acclaimed roles of the plants used in this study in the treatment of diabetes mellitus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2:32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal E, Salim KA, Lim LB. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ Sci. 2015;27:224–32. [Google Scholar]
- 4.Sylvie DD, Anatole PC, Cabral BP, Veronique PB. Comparison of in-vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: Acalypha racemosa, Garcinia lucida and Hymenocardia lyrata. Asian Pac J Trop Biomed. 2014;4:625–32. [Google Scholar]
- 5.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Zhu H, Yang Z, Liu Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol. 2013;45:395–8. doi: 10.4103/0253-7613.115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahidi F, Naczk M. Phenolics in Food and Nutraceuticals. Boca Raton: CRC Press; 2003. [Google Scholar]
- 8.Borneo R, Leon AE, Aguirre A, Ribotta P, Cantero JJ. Antioxidant capacity of medicinal plants from the province of Cordoba (Argentina) and their in-vitro testing in a model food system. Food Chem. 2009;112:664–70. [Google Scholar]
- 9.Erasmus RT, Soita DJ, Hassan MS, Blanco-Blanco E, Vergotine Z, Kegne AP, et al. High prevalence of diabetes mellitus and metabolic syndrome in a South African coloured population: Baseline data of a study in Bellville, Cape Town. S Afr Med J. 2012;102:841–4. doi: 10.7196/samj.5670. [DOI] [PubMed] [Google Scholar]
- 10.Erasto P, Adebola PO, Grierson DS, Afolayan AJ. An ethnobotanical study of plants used for the treatment of diabetes in the Eastern Cape Province, South Africa. Afr J Biotechnol. 2005;4:1458–60. [Google Scholar]
- 11.Oyedemi SO, Bradley G, Afolayan AJ. Ethnobotanical survey of medicinal plants used for the management of diabetes mellitus in the Nkonkobe municipality of South Africa. J Med Plants Res. 2009;3:1040–4. [Google Scholar]
- 12.Dold AP, Cocks ML. The trade in medicinal plants in the Eastern Cape Province, South Africa. S Afr J Sci. 2002;98:589–97. [Google Scholar]
- 13.Dold T, Cocks M. Voices from the Forest: Celebrating Nature and Culture in Xhosaland. Gauteng, South Africa: Jacan Media; 2012. p. 204. [Google Scholar]
- 14.Ncube B, Ndhlala AR, Okem A, Van Staden J. Hypoxis (Hypoxidaceae) in African traditional medicine. J Ethnopharmacol. 2013;150:818–27. doi: 10.1016/j.jep.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Ojewole JA. Antinociceptive, anti-inflammatory and antidiabetic properties of Hypoxis hemerocallidea Fisch. & C.A. Mey (Hypoxidaceae) corm ['African Potato'] aqueous extract in mice and rats. J Ethnopharmacol. 2006;103:126–34. doi: 10.1016/j.jep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Koduru S, Grierson DS, Afolayan AJ. Ethno-botanical information of medicinal plants used for the treatment of cancer in the Eastern Cape Province, South Africa. Curr Sci. 2007;92:906–8. [Google Scholar]
- 17.Ozkok A, D'arcy B, Sorkun K. Total phenolic acid and total flavonoid content of Turkish pine honeydew honey. J ApiProd ApiMed Sci. 2010;2:65–71. [Google Scholar]
- 18.Wintola OA, Afolayan AJ. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox Mill. Pharmacogn Mag. 2011;7:325–33. doi: 10.4103/0973-1296.90414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyedemi SO, Bradley G, Afolayan AJ. In vitro and in vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr J Pharm Pharmacol. 2010;4:70–8. [Google Scholar]
- 20.Harbone JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed. New Delhi: Springer Pvt; 2005. [Google Scholar]
- 21.Liyana-Pathiranan CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–40. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 22.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement Altern Med. 2008;8:54. doi: 10.1186/1472-6882-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahoo S, Ghosh G, Das D, Nayak S. Phytochemical investigation and in vitro antioxidant activity of an indigenous medicinal plant Alpina nigra BL Burtt. Asian Pac J Trop Biomed. 2013;3:871–6. [Google Scholar]
- 25.Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–8. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- 26.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR. Antioxidant and free radical scavenging activity of H. officinalis L. var. Angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci. 2010;23:29–34. [PubMed] [Google Scholar]
- 27.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rupasinghe HP, Jackson CJ, Poysa V, Di Berardo C, Bewley JD, Jenkinson J, et al. Soyasapogenol A and B distribution in soybean (Glycine max L. Merr.) in relation to seed physiology, genetic variability, and growing location. J Agric Food Chem. 2003;51:5888–94. doi: 10.1021/jf0343736. [DOI] [PubMed] [Google Scholar]
- 30.Yusha'u M, Gabari DA, Dabo NT, Hassan A, Dahiru M. Biological activity and phytochemical constituents of Tamarindus indica stem bark extracts. Sky J Microbiol Res. 2014;2:67–71. [Google Scholar]
- 31.Shah MD, Hossain MA. Total flavonoids content and biochemical screening of the leaves of tropical endemic medicinal plant Merremia borneensis. Arab J Chem. 2014;7:1034–8. [Google Scholar]
- 32.Qian H, Nihorimbere V. Antioxidant power of phytochemicals from Psidium guajava leaf. J Zhejiang Univ Sci. 2004;5:676–83. doi: 10.1007/BF02840979. [DOI] [PubMed] [Google Scholar]
- 33.Bhagat M, Pandita RM, Saxena AK. In vitro and in vivo biological activities of Nardostachys jatamansi roots. Med Aromat Plants. 2013;2:142. [Google Scholar]
- 34.Lugasi A, Hóvári J, Sági KV, Bíró L. The role of antioxidant phytonutrients in the prevention of diseases. Acta Biol Szeged. 2003;47:119–25. [Google Scholar]
- 35.Agbor GA, Oben JE, Ngogang JY, Xinxing C, Vinson JA. Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods. J Agric Food Chem. 2005;53:6819–24. doi: 10.1021/jf050445c. [DOI] [PubMed] [Google Scholar]
- 36.Rao AS, Reddy SG, Babu PP, Reddy AR. The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran. BMC Complement Altern Med. 2010;10:4. doi: 10.1186/1472-6882-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birasuren B, Kim NY, Jeon HL, Kim MR. Evaluation of the antioxidant capacity and phenolic content of Agryophyllum pungens seed extracts from Mongolia. Prev Nutr Food Sci. 2013;18:188–95. doi: 10.3746/pnf.2013.18.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alia M, Horcajo C, Bravo L, Goya L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res. 2003;23:1251–67. [Google Scholar]
- 39.Anwar F, Latif S, Przybylski R, Sultana B, Ashraf M. Chemical composition and antioxidant activity of seeds of different cultivars of mungbean. J Food Sci. 2007;72:S503–10. doi: 10.1111/j.1750-3841.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 40.Adedapo AA, Jimoh FO, Koduru S, Afolayan AJ, Masika PJ. Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement Altern Med. 2008;8:53. doi: 10.1186/1472-6882-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razali N, Razab R, Junit SM, Aziz AA. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale) Food Chem. 2008;111:38–44. [Google Scholar]
- 42.Taylor BS, Kim YM, Wang Q, Shapiro RA, Billiar TR, Geller DA, et al. Nitric oxide down-regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997;132:1177–83. doi: 10.1001/archsurg.1997.01430350027005. [DOI] [PubMed] [Google Scholar]
- 43.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 44.Chang LW, Yen WJ, Huang SC, Duh PD. Antioxidant activity of sesame coat. Food Chem. 2002;78:347–54. [Google Scholar]


