Abstract
Background:
The apigenin has important medicinal value. However, the low solubility of apigenin in water significantly reduced its application.
Objective:
In this study, the apigenin was extracted, and the complex of apigenin and lecithin was obtained by the solvent method and its physical and chemical properties were investigated.
Materials and Methods:
The apigenin was extracted from the leaves of Adinandra nitida. Afterward, its apigenin was obtained by hydrolysis and recrystallization. The solvent method was used to synthesis the complex of apigenin and lecithin. Tetrahydrofuran was used as the solvent. The physicochemical properties of the complex were investigated by the various methods such as ultraviolet (UV)-visible spectrometry, infrared spectrometry (IR), scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and X-ray diffractometry.
Results:
No distinct difference was examined between the complex and physical mixture according to the UV analysis. While the result of Fourier transform-IR analysis indicated the characteristic absorption peaks of apigenin was subdued by the absorption peaks of lecithin. SEM showed the irregular form of the complex. In the DSC thermogram of the complex, the characteristic endothermic peak belonging to apigenin disappears, and the apparent amorphous properties are shown in the X-ray X-diffractograms of the complex.
Conclusion:
The synthetic process does not break the conjugated structure of apigenin. The complex is held together by Hydrogen bonding and van der Waals force and processes new physical and chemical characteristics. The industrial application of apigenin might be enhanced by the increase of the bioavailability.
SUMMARY
The apigenin was extracted from the leaves of Adinandra nitida
The complex of apigenin and lecithin was obtained by solvent method
The physical and chemical properties of the complex were investigated
The complex is held together by Hydrogen bonding and van der Waals force and processes new physical and chemical characteristics.
Abbreviation used: SLA: The synthesis of the complex of soy lecithin and apigenin, PMSLA: The manufacture of a physical mixture of soybean lecithin and apigenin, UV: Ultraviolet, IR; Infrared Radiation, FT-IR: Fourier transform infrared, NMR: Nuclear magnetic resonance, SEM: Scanning electron microscopy, DSC: Differential scanning calorimetry, XRD: X-ray diffractometry.
Keywords: Apigenin, complex, differential scanning calorimetry, infrared, lecithin, scanning electron microscopy, ultraviolet, X-ray diffractometry
INTRODUCTION
Adinandra nitida is a wild plant growing in the ethnic minority areas of southern China and is often used as a substitute for tea. Its leaves not only possess the flavor of tea but also process various activities such as anti-inflammation, detoxification, anti-hypertensive, antifebrile, and antibleeding.[1,2] It has been used as a Chinese herbal medicine for treating diseases such as inflammation, innominate swelling, and injury[3,4] for hundreds of years. Recent studies have shown that it has a positive effect on a variety of diseases, such as lowering blood pressure, antibacterial, antioxidant, and analgesia.[5] In previous studies,[6,7] the most impressive is its more than 20% flavonoid content. These flavonoids can be hydrolized into apigenin which possesses some beneficial activities such as anti-tumor activity, spasmolysis, reducing blood pressure, diastolic blood vessels, anti-inflammatory effects by inhibiting the expression of macrophage nitric oxide synthase, sedating, and anti-depression effect by increasing the neurotransmitter of gamma-aminobutyric acid and the inhibition effect against Gram-negative bacteria.[8,9,10,11,12] These beneficial activities indicated the promising industrial value of apigenin. However the low solubility of apigenin in water significantly reduced the bioavailability of its oral administration and thus limited its application in the food and medical industry. Lecithin, an important substance consisted of cytomembrane, contains both the hydrophobic fatty acid ester and the hydrophilic phosphate group. Hence, it is a substance process dual solubility in both water and oil. Studies showed that it can be reacted with compounds with certain structures to form complexes under certain conditions. These complexes formed with lecithin have better bioavailability in comparing to the original compounds due to improved physical and chemical properties. With the advantage of simple preparation method and low cost, this method can be used to synthesis a wide variety of complexes with higher bioavailability. In this study, flavonoid was extracted from the leaves of A. nitida. The apigenin was prepared from the extract by acid hydrolysis and recrystallization. Afterward, the complex of apigenin and lecithin was synthezed, and its physical and chemical properties were studied.
MATERIALS AND METHODS
Materials and chemicals
A. nitida was acquired in JinXiu, GuangXi Province, China in the October of 2016. The material was confirmed by Prof. Korbanjhon Brad in Yili Normal University (College of Chemistry and Environmental Sciences) and was preserved with the identification number of YLNU2016111207 in the specimen preservation site of the university. The Soy lecithin was provided by Sangon Biotech. All the chemicals and solvents used in the experiment were of analytically pure agent.
The preparation and purification of apigenin
The apigenin was extracted from the leaves of A. nitida. Afterward, its apigenin was obtained by hydrolysis and recrystallization.[13] The structural characteristics of apigenin were identified by various methods such as ultraviolet (UV), Fourier transform-infrared (FT-IR), nuclear magnetic resonance (NMR), mass spectrum, and differential scanning calorimetry (DSC) analysis to certify the obtained crystal is apigenin of high purity.
The synthesis of the complex of soy lecithin and apigenin
A total of 100 mg of apigenin and 200 mg of soy lecithin were dissolved in 50 mL tetrahydrofuran and the mixture was magnetically stirred at 25°C for 3 h. Then, the solvent was removed by vacuum concentration to obtain the yellow substance of soy lecithin and apigenin (SLA).
The manufacture of a physical mixture of soybean lecithin and apigenin
Hundred milligram of apigenin were fully mixed with 200 mg of soy lecithin to obtain the physical mixture.
The ultraviolet and Fourier transform infrared analyses
One milligram of soy lecithin, apigenin, SLA, physical mixture of SLA (PMSLA) was dissolved in 10 mL of methanol separately for the UV spectra analysis. The UV spectra analysis was conducted under the wavelength of 220–500 nm on a scanning UV spectrophotometer (UV-2500PC, Shimadzu, Japan).
The sample for FT-IR analysis was prepared by 1 mg of sample and 150 mg of dried KBr. In brief, after being fully mixed and crushed to powder, the powder were pressed into a 1 mm thick disk for the analysis. Fourier transformed IR spectrophotometer (VECTOR22, Bruker, Germany) was used to carry out the experiment. 4000–400 cm−1 was set as the measuring range. All data processing is done by OPUS software (Bruker, Germany).
Scanning electron microscopy
The testing samples of soy lecithin, apigenin, SLA, PMSLA were adhered on a copper testing stub before a layer of gold was evenly sputtered on them. The experimental condition was set as 10 kV and low vacuum. A SU1510 (Hitachi, Japan) scanning electron microscope was used to observe the microstructure of samples.
Differential scanning calorimetry
The temperature was increased from 30°C to 300°0 at the speed of 10°0eeda protected by nitrogen. The type of DSC equipment (DSC60, Shimadzu, Japan). The recorded data was processed by software (Shimadzu, Japan).
X-ray diffractometry
Moderate amounts of soy lecithin, apigenin, SLA, and PMSLA were used to conduct the XRD experiment.
A D8 Advance X-ray diffractometer (XRD-6100, Shimadzu, Japan) was used to generate monochromatic Cu Ka radiation (wavelength = 1.54056 A°). 40 kV was set as the tube voltage while 40 mA was set as the tube current. The scanning regions of the diffraction angle (2 θ) were set between 5°C and 70°C with the scanning speed of 4°C/min.
RESULTS AND DISCUSSION
The identification of compounds
UV, FT-IR, NMR, mass spectrum, and DSC analyses were conducted to identify the structural characteristics of the apigenin.
These data were inconsistency with the references, and thus, it can be identified that the compound was apigenin.[14,15,16]
Spectra analyses
The UV spectra of lecithin, apigenin, SLA, and PMSLA are shown in Figure 1. No difference was observed between the spectrum of SLA and PMSLA. The same as that of apigenin, characteristic absorption peaks were observed at 268 and 334 nm in these two samples. This suggested that no change happened in the absorption generated and conjugated structures existed in the structure of apigenin that causes these absorption peaks. It could be observed from infrared spectra of these four [Figure 2]. Judging from the fact that the similarity of characteristic absorption peaks of SLA and PMSLA, the figures of these two were resulted from the pilling up of lecithin and formononetin. The characteristic absorption peak of apigenin is inhibited by the peaks of lecithin, and the fact between 500/cm and 1750/cm can be demonstrated. In the meantime, the broad peaks of -OH in the wavelength of 3500–2800 cm−1 have changed into narrow peaks with high intensity, a conclusion could be reached that no hydrogen bond was formed during the synthesis of SLA and that the conjugated structure of both lecithin and apigenin remained intact in the complex.
Figure 1.
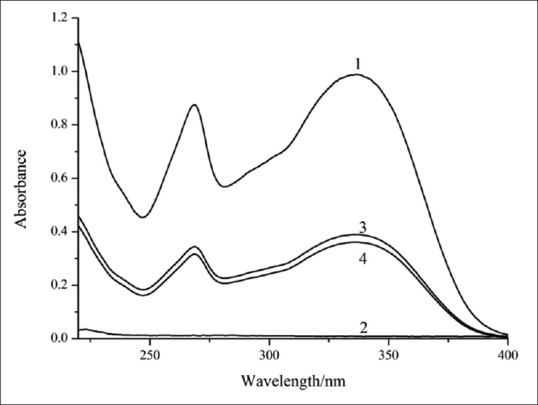
Ultraviolet spectra of lecithin (2), apigenin (1), their physical mixture (3) and complex (4)
The UV data indicated that the conjugated groups of apigenin which caused the absorption peaks remained unchanged. Judging from the similarity of characteristic absorption peaks of SLA and PMSLA, the figures of these two were resulted from the pilling up of lecithin and apigenin. Meanwhile, some characteristic absorption peaks of apigenin were subdued by that of lecithin and thus not occurred in the absorption peak of SLA. This indicated that the basic chemical of both apigenin and lecithin remained unchanged in SLA and PMSLA.
Scanning electron microscopy analysis
The SEM images of lecithin, apigenin, SLA, and PMSLA are shown in Figure 3, the surface of lecithin showed irregular structure while that of apigenin appeared as crystals; Both the irregular structure of lecithin and the crystals of apigenin were observed in the sample of PMSLA. This indicated that lecithin and apigenin in PMSLA were simply mixed. The SEM image of SLA showed the irregular structure of lecithin. This indicated that apigenin was successfully incubated in the structure of lecithin.
Figure 2.
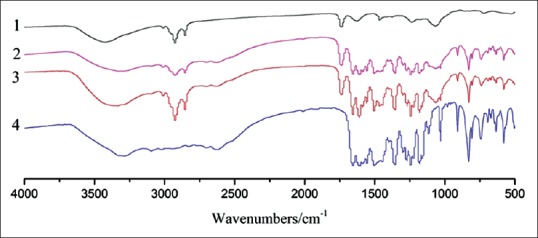
Infrared spectra of lecithin (1), apigenin (4), their physical mixture (2) and complex (3)
Thermal characteristics
The DSC thermograms of lecithin, apigenin, SLA and PMSLA are shown in Figure 4. The thermogram of the apigenin showed an endothermal peak at around 355°C. It was at this temperature that apigenin started to melt. The characteristic absorption peaks of apigenin were completely disappeared in the thermogram of PMSLA. This might be resulted from the fact that the characteristics of apigenin were covered by that of lecithin in the melting process. The endothermal peak of apigenin was disappeared in the thermograms of the complex of the two. This showed that crystal structures were absent in the complex and that the complex existed in the form of irregularity. Overall, the results suggested that apigenin was evenly dispersed in the structure of lecithin and that the two may be connected by hydrogen bonding and van der Waals force. No obvious endothermal peak was observed in that of lecithin and SLA, the possible reason is that the polarity end of the apigenin and the lecithin were interacted with each other and that the regularity decreased between the fatty hydrocarbon chain of phospholipid molecules in lecithin with the interaction of apigenin.
Figure 3.
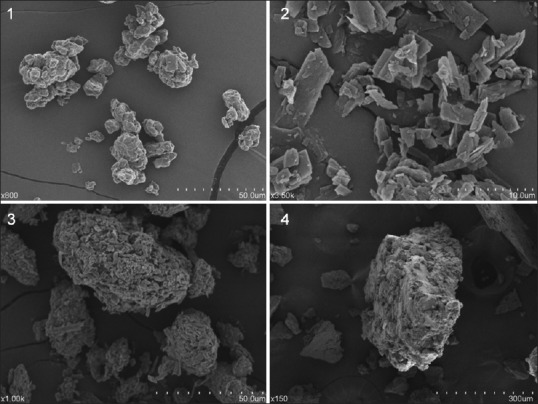
Scanning electron micrographs of lecithin (1), apigenin (2), their physical mixture (3) and complex (4)
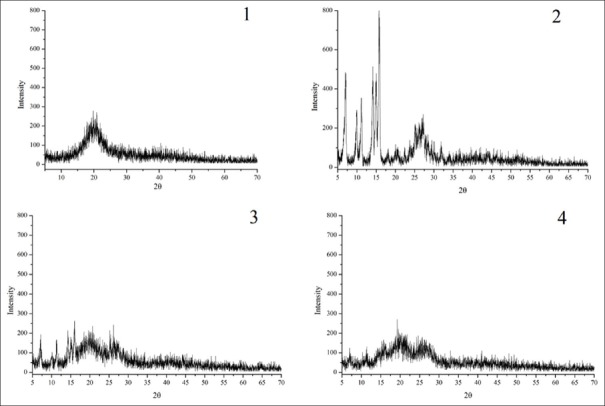
X-ray diffractometry analysis
As it is shown in Figure 5, sharp crystalline peaks in the figure of apigenin indicated the presence of the crystalline organic molecule. By contrast, the amorphous property without the crystalline peaks was observed in the diffraction pattern of lecithin. Effected by the presence of lecithin, the crystalline peaks of the apigenin was hardly visible, in addition, several peaks have been covered by that of lecithin and the intensity is reduced. The polarity bond of apigenin and lecithin might be connected with each other in the complex of the two, which fact was evident in the characteristic peaks of the apigenin were completely disappeared in the SLA. The result suggested that the apigenin was evenly dispersed in the structure of lecithin and that it existed in the form of irregularity.
Figure 4.
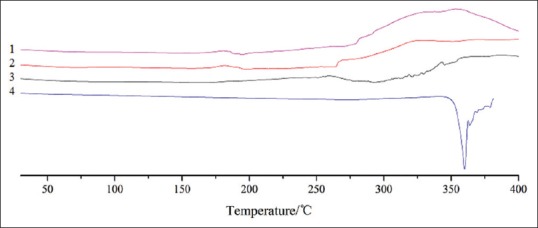
Differential scanning calorimetry curves of lecithin (1), apigenin (4), their physical mixture (3) and complex (2)
Figure 5.
X-ray diffraction patterns of lecithin (1), apigenin (2), their physical mixture (3) and complex (4)
CONCLUSION
The apigenin of high purity in the form of the transparent crystal was prepared by extraction, acid hydrolysis, and recrystallization. Various methods such as NMR, Mass spectrometry, UV, and DSC analyses were used to identify the transparent crystal to be apigenin.
Different ratios of the apigenin to the lecithin had been tried to synthesis the SLA. The optimum ratio was found out to be 1:2 (m/m). The ratio of the mole is close to 1:1 when the Mw of apigenin is considered to be 270 and the Mw of lecithin is considered to be 750. SLA was synthesized in tetrahydrofuran. It showed better solubility in comparing to apigenin which is in consistency with the reports of several other complexes.
The polarity end of lecithin and apigenin were connected with each other in tetrahydrofuran by hydrogen bonding and van der Waals force. The synthesized SLA processing with new characteristics showed a significant change in comparing with the original chemicals as well as the physical mixture.
The data of UV, XRD, IR, and DSC were inconsistent with each other. These data suggested that the characteristics of SLA were significantly different from that of PMSLA, lecithin, and apigenin. The results also showed that neither the conjugated groups in apigenin changed, nor formed during the synthesis process. The apigenin was only evenly dispersed in the structure of lecithin and that the complex existed in the form of irregularity. The two were connected by hydrogen bonding and van der Waals force. The complex may enhance the potential value in the industrial application of apigenin.
Financial support and sponsorship
The project supported by the National Science Foundation of China (31401663).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The project supported by the National Science Foundation of China (31401663).
REFERENCES
- 1.Chen Y, Chen G, Fu X, Liu RH. Phytochemical profiles and antioxidant activity of different varieties of Adinandra tea (Adinandra jack) J Agric Food Chem. 2015;63:169–76. doi: 10.1021/jf503700v. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Yang J, Duan J, Liang Z, Zhang L, Huo Y, et al. Quantitative and qualitative analysis of flavonoids in leaves of Adinandranitida by high performance liquid chromatography with UV and electrospray ionization tandem mass spectrometry detection. Anal Chim Acta. 2005;532:97–104. [Google Scholar]
- 3.Wang Y, Chen SB, Ni J, Yao X, Ye WC, Zhao SX. Chemical studies on Adinandranitida. J Chin Pharm Univ. 2003;34:407–2. [Google Scholar]
- 4.Wang Y, Ye WC, Yin ZQ, Zhao SX. Triterpene saponins from Adinandra nitida. Yao Xue Xue Bao. 2008;43:504–8. [PubMed] [Google Scholar]
- 5.Yuan ED, Liu BG, Ning ZX. Preparation and antioxidant activity of camellianin a from Adinandra nitida leaves. J Food Process Pres. 2008;32:785–12. [Google Scholar]
- 6.Liu BG, Ning ZX, Zhan Y, Xu KY, Gao JH. Characterization and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of methanol and supercritical carbon dioxide extracts from leaves of Adinandra nitida. J Food Biochem. 2008;32:431–11. [Google Scholar]
- 7.Liu BG, Zhan Y, Ning ZX, Gao JH, Xu KY. Characterization and antioxidant activity of flavonoid extract from leaves of Adinandra nitida Merr. ex Li. Chem Ind Forest Prod. 2008;28:6–10. [Google Scholar]
- 8.Mariappan G, Sundaraganesan N, Manoharan S. The spectroscopic properties of anticancer drug apigenin investigated by using DFT calculations, FT-IR, FT-raman and NMR analysis. Spectrochim Acta A Mol Biomol Spectrosc. 2012;95:86–99. doi: 10.1016/j.saa.2012.04.089. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Y, Krisanapun C, Lee SH, Nualsanit T, Sams C, Peungvicha P, et al. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur J Cancer. 2010;46:3365–74. doi: 10.1016/j.ejca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R, Dajas F, et al. Apigenin, a component of matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–6. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- 11.Martini ND, Katerere DR, Eloff JN. Biological activity of five antibacterial flavonoids from combretum erythrophyllum (Combretaceae) J Ethnopharmacol. 2004;93:207–12. doi: 10.1016/j.jep.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Eumkeb G, Chukrathok S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant enterobacter cloacae. Phytomedicine. 2013;20:262–9. doi: 10.1016/j.phymed.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Liu BG, Ning ZX, Gao JH, Xu KY. Preparing apigenin from leaves of Adinandra nitida. Food Technol Biotech. 2008;46:32–5. [Google Scholar]
- 14.Yuan ED, Liu BG, Ning ZX, Chen CG. Preparative separation of flavonoids in Adinandra nitida leaves by high-speed counter-current chromatography and their effects on human epidermal carcinoma cancer cells. Food Chem. 2009;115:1158–5. [Google Scholar]
- 15.Feng WS, Chen X, Zheng XK, Li DD. Study on chemical constituents of Lonicerae japonicae Flos. Chin Pharm J. 2011;46:338–40. [Google Scholar]
- 16.Yin XJ, Qin MJ. Advances in chemical and pharmacological studies on medicinal plants of Medicago. Chin Wild Plant Res. 2008;27:4–7. [Google Scholar]