Abstract
Background:
The rhizome of Atractylodes lancea (AL) is usually used for the treatment of various diseases such as spleen deficiency syndrome (SDS). Both bran-processed and crude AL is included in Chinese Pharmacopoeia. The different efficacies of bran-processed and crude AL on SDS are largely unknown, and the mechanisms of AL effects have not been fully elucidated.
Objective:
The objective of the study was to compare the effects of bran-processed and crude AL and then assess the mechanisms of treating SDS.
Materials and Methods:
The model of SDS in rats was established using excessive exertion, combined with an irregular diet and intragastric administration of the extract of Sennae Folium, and different doses of bran-processed and crude AL were gavaged. The serum was analyzed by an enzyme-linked immunosorbent assay (ELISA), and small intestinal tissues were analyzed by reverse transcription-polymerase chain reaction (RT-PCR).
Results:
The injury of SDS was alleviated by the treatment of bran-processed and crude AL. Compared to model group, the indexes of trypsin (TRY), amylase (AMS), vasoactive intestinal peptide (VIP), somatostatin (SS), gastrin (GAS), substance P (SP), Na+-K+-ATPase, and succinic dehydrogenase in serum of each administration group were increased by ELISA, and the mRNA expressions of VIP, SS, GAS, and SP in small intestinal tissues were increased by RT-PCR. Furthermore, in a dose-dependent manner, the bran-processed and crude AL increased the levels of TRY, AMS, VIP, and GAS and the mRNA expression levels of VIP. Compared with the crude AL, the bran-processed AL was more effective in treating SDS.
Conclusion:
Through the mechanisms of treating SDS by AL, both bran-processed and crude AL has alleviated the symptoms of SDS.
SUMMARY
Both bran-processed and crude Atractylodes lancea (AL) alleviated symptoms of spleen deficiency syndrome (SDS)
Comparing with crude AL, bran. processed AL was more effective in treating SDS
The efficacy of AL could be partly attributed to digestive enzyme activity, gastrointestinal hormone levels, membrane protein activity, and changes in mitochondrial activity.
Abbreviations used: AL: Atractylodes lancea; TRY: Trypsin; AMS: Amylase; VIP: Vasoactive intestinal peptide; SS: Somatostatin; GAS: Gastrin; SP: Substance P; ELISA: The enzyme-linked immunosorbent assay; mRNA: Messenger ribonucleic acid; SDH: Succinic dehydrogenase; RT-PCR: Reverse transcription-polymerase chain reaction; TCM: Traditional Chinese medicine; SDS: Spleen deficiency syndrome.
Keywords: Atractylodes lancea, bran processing, spleen deficiency syndrome, Traditional Chinese medicine
INTRODUCTION
According to the theory of traditional Chinese medicine (TCM), the spleen includes the spleen described in modern anatomy and the lymphatic and pancreas systems. The comprehensive decreases of these systems are called spleen deficiency syndrome (SDS).[1]
The literature reports that SDS is related to the digestive system diseases, energy metabolism, immune dysfunction of gastrointestinal motility, and stomach function.[2]
Atractylodes lancea (AL) is often used for the treatment of SDS AL belongs to Atractylodes genus, Asteraceae family, which grows widely in China. The rhizome of Atractylodes, including the rhizome of AL, is used for SDS treatment. The rhizome of AL is also frequently used in the treatment of rheumatic diseases, night blindness, influenza, and several other types of digestive problems.[3,4,5]
In the preparation of TCM, processing (pao zhi) is an important procedure based on TCM classics, for example, treatise on cold-induced and Miscellaneous Diseases (Shang Han Za Bing Lun), and Compendium of Materia Medica (Ben Cao Gang Mu). Using different processing methods, the properties of AL can be changed. One common method is stir-frying with wheat bran. In the Chinese Pharmacopoeia, both bran-processed AL and crude AL are listed.
Currently, the literatures on the use of bran-processed and crude AL for treating SDS and evaluation of the mechanisms of action is rare. This paper studied the differences in curative effects of bran-processed and crude AL on SDS. The model of SDS was induced by the irregular diet and intragastric administration of the extract of Sennae Folium. The serum was analyzed by enzyme-linked immunosorbent assay (ELISA), and small intestinal tissues were analyzed by reverse transcription-polymerase chain reaction (RT-PCR). This paper also evaluated the action mechanisms of AL for treating SDS.
MATERIALS AND METHODS
Materials
The crude AL, which was cultivated in Luotian City, Hubei Province in China, was purchased from Beijing Tong Ren Tang Pharmacy of Shenyang Branch, Shenyang City, Liaoning Province, China, in December, 2015, and authenticated by Prof. Rongxiang, Wang, from the School of Pharmacy, Liaoning University of Traditional Chinese Medicine. According to the procedures described in Chinese Pharmacopoeia, the bran-processed AL was stir-fried with wheat bran.[6] Wheat bran was put into a pot that was hot enough for the bran smoke and then the AL was immediately added into the pot. When the surface was dark yellow, stir-frying was quickly stopped. Then, the bran was removed, and the AL was cooled. The bran-processed and crude AL was crushed into fine powder. The powders were suspended in distilled water at three different concentrations for use.
Chemicals
Sennae Folium was obtained from Guangshang County Han Tea Co., Ltd., (Guangshang, China) (Drug approval number: 4115 1402 0035). Shen Ling Bai Zhu Powder (a proprietary Chinese medicine for treating indigestion) was commercially provided from Shanxi Huakang Pharmaceutical Co., Ltd. (Shanxi, China) (Drug approval number: 14020346). Domperidone (for the treatment of indigestion in clinical practice) was purchased from Xian-Janssen Pharmaceutical Co., Ltd. (Xian, China) (Drug approval number: 10910003). Shen Ling Bai Zhu Powder and domperidone were used as positive control drugs. Sennae Folium was extracted with distilled water, domperidone was grounded into powder, and Shen Ling Bai Zhu Powder was suspended in distilled water before use.
TRIzol Reagent was obtained from Invitrogen Corporation (CA, USA). Brilliant III Ultra-Fast SYBR® Green QPCR Master Mix was purchased from Agilent Technologies (USA). Primers for target genes were synthesized by Beijing Genomics Institute (Beijing, China). Mice ELISA Kits were purchased from R and D Techno Co. (MN, USA). Prime Script® RT Reagent Kits with gDNA Eraser for real-time PCR were provided by TaKaRa Biotech Co. (Dalian, China).
Animals
Sprague-Dawley rats (6 weeks old) of both genders (SPF grade) were commercially obtained from Liaoning Chang Sheng Biotechnology Co., Ltd. (SCXK 2015-0001, Liaoning, China), with a mean weight of 190–230 g, housed for 1 week in a controlled temperature laboratory of 23°C ± 1°C and 50% ± 5% humidity, with standard food and distilled water ad libitum. All the rats were deprived of food and distilled water ad libitum for 24 h before the experiments. All procedures were in accordance with the European Community Guidelines for Animal Experimentation. The animal experimentation studies were approved by the Ethics Committee on Animal Research in Liaoning University of TCM.
Spleen deficiency syndrome model and drug administration
All rats were randomly assigned to ten groups (n = 12 in each experimental group, six female rats and six male rats after housing for 1 week): control group (CG), model group (MD), Shen Ling Bai Zhu Powder group (SL, 0.16 mg/ml dose), domperidone group (DO, 0.26 mg/ml dose), bran-processed AL at a high dose group (HP, treated with bran-processed AL 2.5 g/kg dose), bran-processed AL at a medium dose group (MP, treated with bran-processed AL 1.25 g/kg dose), bran-processed AL at a low-dose group (LP, treated with bran-processed AL 0.625 g/kg dose), crude AL at a high-dose group (HC, treated with crude AL 2.5 g/kg dose), crude AL at a medium dose group (MC, treated with crude AL 1.25 g/kg dose), and crude AL at a low-dose group (LC, treated with crude AL 0.625 g/kg dose).
There are a variety of SDS animal models and established methods. In this paper, excessive exertion combined with an irregular diet and intragastric administration with extract of Sennae Folium were used.[7] The first period lasted 9 days. On odd days, the rats were forced to swim and administered lard by gavage. On even days, the rats were fed cabbages only. The second period lasted for 7 days. On these days, the rats were given the extract of Sennae Folium intragastrically.[8]
Clinical manifestations of SDS in humans include fatigue, low appetite, pasty loose stools, and inactivity.[9] The spleen deficiency rats presented pasty loose stools, weight loss, inactiveness, hypotrichotrophy, athrepsy, grouping, bad appetite, and lassitude. The spleen deficiency rats showed all the above signs, these signs are similar to the symptoms of TCM SDS pattern in human.[10]
At the end of the experiment, all the animals were anaesthetized with 10% chloral hydrate (4 ml/kg body wt., i.p.) and the rats' abdomens were opened. Blood was taken from the abdominal aorta of the rats, allowed to settle for 2 h at room temperature, and then centrifuged at 5000 rpm 4°C for 5 min. The supernatant serum was frozen at −70°C until use. A 3 cm portion of the small intestine was removed and washed with ice-cold saline to avoid contamination, kept in TRIzol for mRNA level assays, and stored at −70°C until further analysis.
Determination of trypsin, amylase, vasoactive intestinal peptide, somatostatin, gastrin, substance P, Na+-K+-ATPase, and succinic dehydrogenase in serum by enzyme-linked immunosorbent assay
The serum was first thawed at 4°C before use, and then the supernatants serum was transferred to other tubes. The serum levels of trypsin (TRY), amylase (AMS), vasoactive intestinal peptide (VIP), somatostatin (SS), gastrin (GAS), substance P (SP), Na+-K+-ATPase, and succinic dehydrogenase (SDH) were determined using ELISA kits (Infinite M200, TECAN, Austria) according to the instruction manuals at the wavelength of 450 nm.
The mRNA expressions levels of vasoactive intestinal peptide, somatostatin, gastrin, and substance in small intestine tissues as determined by reverse transcription-polymerase chain reaction
Total RNA isolation
Using the method of quantitative RT-PCR, the mRNA expressions level of VIP, SS, GAS, and SP, which were extracted from the small intestine tissues of rats were determined. The tissue samples weighted about 50–100 mg and were homogenized in 1 ml TRIzol. The total RNA was isolated by precipitation at 4°C for 10 min, at the speed of 12, 000 rpm. Following the instructions, the total RNA was extracted by a TRIzol total kit, which was based on isopropanol precipitation and phenol/chloroform extraction. To measure the concentrations of total RNA, absorbency was set with 260 nm as the reference, and the 260/280 nm absorbance ratio (1.8–2.0 indicates a highly pure sample)[11] was used for the assessment of the isolated RNA quality.
Synthesis of cDNA and polymerase chain reaction
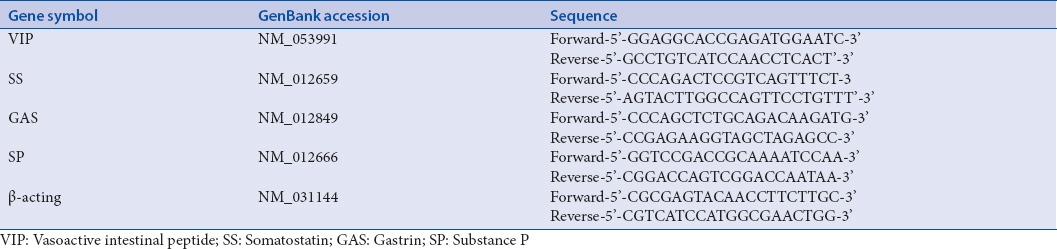
According to the instructions (37°C, 15 min; 85°C, 5 s), 0.5 μg RNA was reversely transcribed to cDNA using Prime-Scripts RT reagent Kit with the gDNA Eraser reagent kit. A brilliant ultra-fast SYBR Green QPCR Master Mix Kit was used for the DNA amplification, and the process was carried out under the following steps: first step was 3 min initial denaturation at 95°C, and then 40 amplification cycles were performed (denaturation for 5 s at 95°C and annealing for 1 min at 60°C) in a Strata-gene MX 3000p PCR System (Agilent, German). In Table 1, the primer for the sequences of the genes is shown. For the verification of the specific PCR products, melting curves were used. For data analysis, the comparative cycle threshold method was used as a means of relative quantization that was normalized to an endogenous reference (β-acting).[12]
Table 1.
Information of polymerase chain reaction primers
Statistical analysis
Statistical calculations were analyzed by Statistical Package for the Social Sciences (SPSS) software (version 17.0, SPSS Inc., Chicago, USA). All the data of results represented with mean ± standard deviation. Statistical differences between the groups were calculated using one-way ANOVA, according to Fisher's protected LSD multiple comparison test or the Dunnette's T3 test. Differences with values of P < 0.05 were considered to be statistically significant.
RESULTS
Effect of Atractylodes lancea on trypsin, amylase, vasoactive intestinal peptide, somatostatin, gastrin, substance P, Na+-K+-ATPase, and succinic dehydrogenase levels in serum as determined by enzyme-linked immunosorbent assay
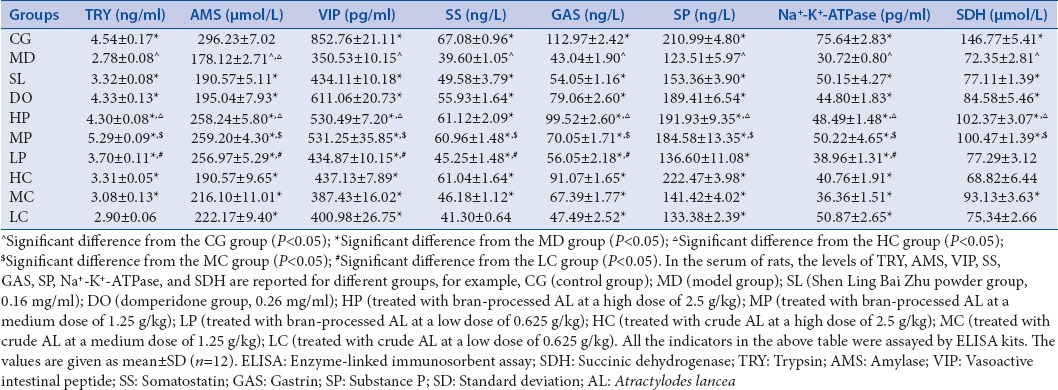
After oral administration of bran-processed and crude AL, TRY, AMS, VIP, SS, GAS, SP, Na+-K+-ATPase, and SDH in serum were detected by ELISA as shown in Table 2.
Table 2.
Levels of trypsin, amylase, vasoactive intestinal peptide, somatostatin, gastrin, substance P, Na+-K+-ATPase, and succinic dehydrogenase in serum as determined by enzyme-linked immunosorbent assay
Determinations of trypsin and amylase in serum
As shown in Table 2, compared with CG rats, lower levels of TRY were found in the serum of MD rats (P < 0.05). In a dose-independent manner, bran-processed and crude AL increased the TRY levels (P < 0.05 in HP, MP, LP, HC, and MC groups). Furthermore, there was a significant difference between HP versus HC, MP versus MC, and LP versus LC (P < 0.05). The result showed that the bran-processed AL had much better effect on the regulation of TRY levels than the crude AL.
Compared with CG rats, lower levels of AMS were found in the serum of MD rats (P < 0.05). In a dose-independent manner, the bran-processed and crude AL increased the AMS levels (P < 0.05 in HP, MP, LP, HC, MC, and LC groups). Furthermore, there was a significant difference between HP versus HC, MP versus MC, and LP versus LC (P < 0.05). The result showed that the bran-processed AL had a much better effect on the regulation of AMS levels than the crude AL.
In conclusion, the effects of bran-processed AL on the regulation of TRY and AMS levels were superior to those of the crude AL.
Effects of Atractylodes lancea on vasoactive intestinal peptide, somatostatin, gastrin, and substance P levels in serum
As shown in Table 2, compared with CG rats, lower levels of VIP were found in the serum of MD rats (P < 0.05). In a dose-independent manner, the bran-processed and crude AL increased the VIP levels (P < 0.05 in HP, MP, LP, HC, MC and LC groups). Furthermore, there was a significant difference between HP versus HC, MP versus MC and LP versus LC (P < 0.05). The result showed that the bran-processed AL had much better effect on the regulation of VIP levels than the crude AL.
Compared with CG rats, lower levels of SS were found in the serum of MD rats (P < 0.05). In a dose-dependent manner, the bran-processed and crude AL increased the SS levels (P < 0.05 in HP, MP, LP, HC and MC groups). Furthermore, there was a significant difference between MP versus MC and LP versus LC (P < 0.05). The result showed that the bran-processed AL had much better effect on the regulation of SS level than the crude AL.
Compared with CG rats, lower levels of GAS were found in the serum of MD rats (P < 0.05). In a dose-dependent manner, the bran-processed and crude AL increased the GAS levels (P < 0.05 in HP, MP, LP, HC, MC and LC groups). Furthermore, there was a significant difference between HP versus HC, MP versus MC and LP versus LC (P < 0.05).The result showed that the bran-processed AL had much better effect on the regulation of GAS level than the crude AL.
Compared with CG rats, lower levels of SP were found in the serum of MD rats (P < 0.05). In a dose-dependent manner, the bran-processed and crude AL increased the SP levels (P < 0.05 in HP, MP, LP, HC, MC, and LC groups). Furthermore, there was a significant difference between HP versus HC and MP versus MC (P < 0.05). The result showed that the bran-processed AL had much better effect on the regulation of SP level than the crude AL.
In conclusion, the regulation effects of the bran-processed AL on VIP, SS, GAS, and SS were superior to the crude AL.
Effects of Atractylodes lancea on Na+-K+-ATPase in serum
As shown in Table 2, compared with CG rats, lower levels of Na+-K+-ATPase were found in the serum of MD rats (P < 0.05). In a dose-independent manner, the bran-processed and crude AL increased the Na+-K+-ATPase levels (P < 0.05 in HP, MP, LP, HC, MC, and LC groups). Furthermore, there was a significant difference between HP versus HC, MP versus MC, and LP versus LC (P < 0.05). The result showed that the bran-processed AL had much better effect on the regulation of Na+-K+-ATPase level than the crude AL.
Effects of Atractylodes lancea on succinic dehydrogenase in serum
As shown in Table 2, compared with CG rats, lower levels of SDH were found in the serum of MD rats (P < 0.05). In a dose-independent manner, the bran-processed and crude AL increased the SDH levels (P < 0.05 in HP, MP, and MC groups). Furthermore, there was a significant difference between HP versus HC and MP versus MC (P < 0.05). The result showed that the bran-processed AL had much better effect on the regulation of the SDH level than the crude AL.
In conclusion, the bran-processed AL had a superior effect on the regulation of Na+-K+-ATPase and SDH levels than the crude AL.
Effects of Atractylodes lancea on the mRNA expression of vasoactive intestinal peptide, somatostatin, gastrin, and substance P in small intestine tissues
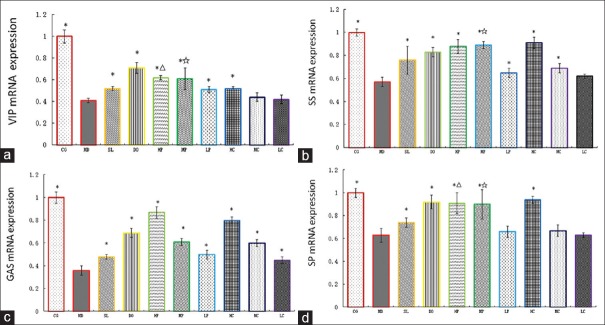
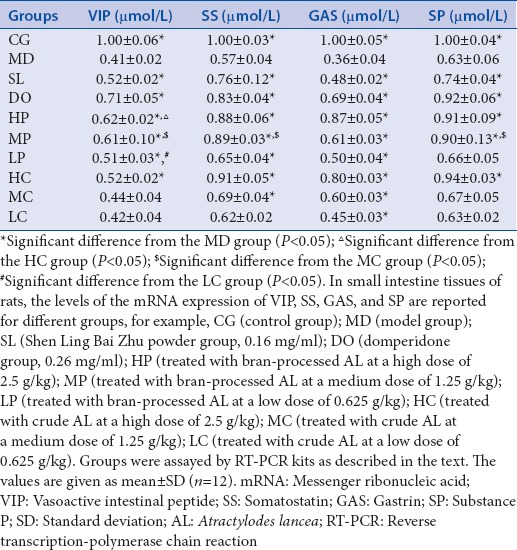
The mRNA expressions levels of VIP, SS, GAS, and SP in the small intestine tissues were detected by RT-PCR. The mRNA expression analysis showed the dissociation curve as a single peak. The absorbance ratios of 260/280 nm ranged from 1.8 to 2.0. After 10-day treatment with AL, except for SS (in the LC group) and SP (in the LP, MC, and LC groups), a significant increase was detected in the expressions levels of other mRNAs as compared to that in the MD group (P < 0.05) [Figure 1]. This measurement was done in a dose-dependent manner. As shown in Table 3, in a dose-independent manner, the bran-processed and crude AL increased the mRNA expressions levels of GAS and SP (P < 0.05 in HP, MP, LP, HC, MC, and LC groups). Furthermore, there was a significant difference between VIP of HP versus that of HC, MP versus MC and LC versus LP, SS of MP versus that of MC, and SP of MP versus that of MC (P < 0.05). The result showed that the bran-processed AL had a superior effect on the regulation of mRNA expression of VIP, SS, GAS, and SP levels than the crude AL.
Figure 1.
(a) VIP mRNA expression); (b) SS mRNA expression; (c) GAS mRNA expression; (d) SP mRNA expression. The mRNA expressions levels of VIP, SS, GAS, and SP in the small intestine tissues of rats. CG (control group); MD (model group); SL (Shen Ling Bai Zhu Powder group, 0.16 mg/ml); DO (domperidone group, 0.26 mg/ml); HP (bran-processed AL at a high dose of 2.5 g/kg); MP (bran-processed AL at a medium dose of 1.25 g/kg); LP (bran-processed AL at a low dose df of 0.625 g/kg); HC (the crude AL at a high dose of 2.5 g/kg); MC (the crude AL at a medium dose of 1.25 g/kg); LC (the crude AL at a low dose of 0.625 g/kg); *Difference from the MD group;Δ Difference from the HC group; ☆Difference from the MC group;# Difference from the LC group. AL: Atractylodes lancea; VIP: Vasoactive intestinal peptide; SS: Somatostatin; GAS: Gastrin; SP: Substance P
Table 3.
Levels of the messenger ribonucleic acid expression of vasoactive intestinal peptide, somatostatin, gastrin, and substance P in small intestine tissues as determined by reverse transcription polymerase chain reaction
DISCUSSION
SDS is a multifactorial etiological disease. There are several factors that play different roles in SDS, for example, digestion, absorption dysfunction membrane structure and function, and energy metabolism.[13] Currently, the incidence of SDS is relatively high in China.
According to the TCM theory, several types of common medicines have been used to treat SDS, and AL is one of them. AL was first recorded in the “Shen Nong Herbal Classic” as a top grade. The taste of AL is spicy and bitter. The meridian is spleen, stomach, and liver. AL removes spleen dampness, expels cold, and improves eyesight, among other functions.[6] Clinically, AL is usually used to treat different diseases, for example, spleen deficiency, diarrhea, cold and dampness, and edema syndrome. Modern pharmacological researches have shown that AL can promote gastrointestinal motility, improve gastrointestinal function, prevent and treat chronic gastrointestinal inflammation, and have other significant effects.
Technology plays an important role in the TCM production process. In ancient times, there were nearly twenty different methods of processing AL, among which wheat bran stir-frying was commonly used. Other processing methods are now rarely known, i.e., there are only two types of AL listed (bran-processed and crude) in Chinese Pharmacopoeia.[6] The bran-processed AL is more widely used in clinical practice. In general, it is believed that after being processed with bran, ALs dry nature decreased, while its role in invigorating the spleen is enhanced. Research has shown that after processing, the content and constitution of volatile oil in AL changes. Studies on the differences in efficacy of bran-processed and crude AL and mechanisms of treating SDS are rarely reported. In the present study, we compared the effects of the bran-processed and crude AL on SDS, as determined by ELIAS and RT-PCR, and further evaluated the mechanisms of action of AL on SDS.
SDS is related to abnormal function of digestive absorption, which is related to digestive enzyme (such as AMS and TRY) activity and gastrointestinal hormone (such as VIP, SS, GAS, and SP) disorders. SDS is also involved in the abnormal changes of cell membrane structure and function with Na+-K+-ATPase as the main indicator, and material and energy metabolism with the SDH as the index.[13] In SDS, cellular level changes induce changes at the molecular and genetic levels in various organs, which in turn can cause changes in the structure and function of digestive enzymes.[14] Thus, the mechanisms of treating SDS could be partly attributed to digestive enzyme activity, gastrointestinal hormone levels, membrane protein activity, and mitochondrial activity changes. The experimental indicators were all related to the mechanisms of treating SDS.
TRY is a food-digesting of mammals, which is now considered to be widely distributed in various organisms.[15] In biochemical language, an important “word” is extensively referenced by the “three system,” which is VIP. The first mention of VIP was in 1970, by Said and Mutt.[16] VIP was originally isolated and purified from the intestine in methanol extract, as a polypeptide with a broad biological activity, it was later described as neuropeptides.[16,17] VIP has the functions of vasodilation, anti-inflammatory immunomodulation,[18] promotion of blood flow, cardiac contraction, lowering of blood pressure, and reduction of peripheral vascular resistance gastrointestinal hormone.[19] SS is a growth hormone, that was first isolated from the extract of pigs and sheep hypothalamus, and identified as an inhibiting hormone.[20] The function of GAS is to regulate gastric acid secretion. It is a kind of peptides secreted by G cells (in the gastric antrum and duodenal mucosa). There is a physiopathological importance in the occurrence of the duodenal diseases.[21] SP was first discovered both in the digestive tract ghrelin and the central nervous system. Related literature suggested that SP can promote bowel movements, strengthen visceral sensitivity, and increase the pressure within the colon. SP can also promote the secretion of water and electrolytes in the digestive system.[22,23] Na+-K+-ATPase is well known for its role in ion transport across the plasma membrane of animal cells. It carries out the transport of Na+ ions out of the cell, and of K+ ions into the cell and thus maintains electrolyte and fluid balance.[24] SDH is a functional component of the citric acid cycle, and aerobic respiration is one part of the hub linking oxidative phosphorylation and electron transport, mitochondria, and a variety of eukaryotic cells and prokaryotic cells, and the aerobic capacity of the electron respiratory chain may provide for mitochondria.[25,26]
According to the results of the ELISA, the average levels of TRY, AMS, VIP, SS, GAS, SP, Na+-K+-ATPase, and SDH decreased significantly in the serum of the MD group. After treatment with bran-processed and crude AL in a dose-dependent manner, the levels of TRY, AMS, VIP, SS, GAS, SP, Na+-K+-ATPase, and SDH increased significantly. According to the statistical difference at two different doses (1.25 and 2.5 g/kg, P < 0.05), the bran-processed AL was more effective than the crude AL. In addition, RT-PCR revealed that the mRNA expression levels of VIP, SS, GAS, and SP increased in the small intestines of the model group. According to the statistical difference at two different doses (1.25 and 2.5 g/kg, P < 0.05), the bran-processed groups showed better curative effects than the crude groups. Although the statistical significance was not reached, there was still a better trend in the effects of the bran-processed AL (at the dose of 0.625 g/kg). Thus, the bran-processed AL showed a better effectiveness.
CONCLUSION
Both the bran-processed and crude ALs are effective in the treatment of SDS. The efficacy of AL on SDS could be partly attributed to digestive enzyme activity through upregulation of the production of TRY and AMS, gastrointestinal hormone levels through upregulation of the production of VIP, SS, GAS, and SP, membrane protein activity through upregulation of the production of Na+-K+-ATPase, and changes in mitochondrial activity through upregulation of the production of SDH. The changes of indicators showed that the mechanisms of AL on the curative effects of SDS are involved with enhancement of digestive enzyme activity, increase in gastrointestinal hormone levels, improvement of membrane protein activity, and changes in mitochondrial activity. According to the previous results, bran-processed AL is more effective than crude AL. As described above, this study can be used for the clinical application of AL in SDS treatment.
Financial support and sponsorship
This work was financially supported by the National Natural Science Foundation of China (No. 81573601).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This work was financially supported by the National Natural Science Foundation of China (No. 81573601). We also thank Prof. Shu-ru Lin, for her technical assistance.
REFERENCES
- 1.Xiong B, Qian H. Effects of Sijunzi decoction and Yupingfeng powder on expression of janus kinase-signal transducer and activator of transcription signal pathway in the brain of spleen-deficiency model rats. J Tradit Chin Med. 2013;33:78–84. doi: 10.1016/s0254-6272(13)60105-3. [DOI] [PubMed] [Google Scholar]
- 2.Peng Y, Peng F, Yi SX, Lin YP, Chang XR, Long YW, et al. Effect of moxibustion on motility, absorption and content of ATP in small intestine of spleen-deficiency rats. Zhongguo Zhen Jiu. 2012;32:246–50. [PubMed] [Google Scholar]
- 3.Duan JA, Wang L, Qian S, Su S, Tang Y. A new cytotoxic prenylated dihydrobenzofuran derivative and other chemical constituents from the rhizomes of Atractylodes lancea DC. Arch Pharm Res. 2008;31:965–9. doi: 10.1007/s12272-001-1252-z. [DOI] [PubMed] [Google Scholar]
- 4.Nakai Y, Kido T, Hashimoto K, Kase Y, Sakakibara I, Higuchi M, et al. Effect of the rhizomes of Atractylodes lancea and its constituents on the delay of gastric emptying. J Ethnopharmacol. 2003;84:51–5. doi: 10.1016/s0378-8741(02)00260-x. [DOI] [PubMed] [Google Scholar]
- 5.Resch M, Heilmann J, Steigel A, Bauer R. Further phenols and polyacetylenes from the rhizomes of Atractylodes lancea and their anti-inflammatory activity. Planta Med. 2001;67:437–42. doi: 10.1055/s-2001-15817. [DOI] [PubMed] [Google Scholar]
- 6.Pharmacopoeia of Peoples Republic of China. Beijing: Chinese Medicine Science and Technology Press Two; 2015. [Google Scholar]
- 7.Xu CX, Liu YC, Liu YZ, Jia R, Cai Q. Therapeutic effect of Atractylodis rhizoma processed with and without stir-frying with bran on rats with spleen disorder due to dampness. Chin Tradit Pat Med. 2016;38:978–83. [Google Scholar]
- 8.Pang X, Liu YQ, Liu XD, Guan MY, Cai Q. Study on the pharmacodynamic comparison of active part in crude Atractylodes lancea and Atractylodes lancea fried with bran. China Pharm. 2016;27:1308–11. [Google Scholar]
- 9.Cheng LX. The Clinical and Experimental Study of Treating Chronic Cholecystitis with Xiangqinglidan Decoction. Shangdong: Shandong University of Traditional Chinese Medicine; 2003. [Google Scholar]
- 10.Gao XL, Guo WF, Li RL, Chen WW. Effects of Sijunzi decoction on urine's xylose excretion rate and ATP in mucosa of spleen deficiency rats. Zhong Yao Cai. 2009;32:1242–5. [PubMed] [Google Scholar]
- 11.Pan J, Xiang Q, Renwick AB, Price RJ, Ball SE, Kao J, et al. Use of a quantitative real-time reverse transcription-polymerase chain reaction method to study the in duct ion of CYP1A, CYP2B and CYP4A forms in precision-cut rat liver slices. Xenobiotica. 2002;32:739–47. doi: 10.1080/00498250210147115. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C (T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Lv L. Experimental Study of Rat Spleen with Spleen Fails Mechanism Liaoning. Shenyang: Liaoning University of Traditional Chinese Medicine; 2012. [Google Scholar]
- 14.Xiu ZC, Yu SY, Shang WP. Study on correlation between spleen deficiency syndrome and enzymology. China J Basic Med Tradit Chin Med. 2003;9:79–81. [Google Scholar]
- 15.Kasai K, Ishii S. Affinity chromatography of trypsin and related enzymes. I. Preparation and characteristics of an affinity adsorbent containing tryptic peptides from protamine as ligands. J Biochem. 1975;78:653–62. doi: 10.1093/oxfordjournals.jbchem.a130952. [DOI] [PubMed] [Google Scholar]
- 16.Said SI, Mutt V. Polypeptide with broad biological activity: Isolation from small intestine. Science. 1970;169:1217–8. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- 17.Said SI, Rosenberg RN. Vasoactive intestinal polypeptide: Abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976;192:907–8. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- 18.Fahrenkrug J. VIP and PACAP[M]. Cellular Peptide Hormone Synthesis and Secretory Pathways. Berlin, Heidelberg: Springer; 2009. pp. 1–20. [Google Scholar]
- 19.Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with giardia spp. Reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology. 2002;123:1179–90. doi: 10.1053/gast.2002.36002. [DOI] [PubMed] [Google Scholar]
- 20.Schally AV, Dupont A, Arimura A, Redding TW, Nishi N, Linthicum GL, et al. Isolation and structure of somatostatin from porcine hypothalami. Biochemistry. 1976;15:509–14. doi: 10.1021/bi00648a009. [DOI] [PubMed] [Google Scholar]
- 21.Xie XZ, Zhao ZG, Qi DS, Wang ZM. Assay of gastrin and somatostatin in gastric antrum tissues of children with chronic gastritis and duodenal ulcer. World J Gastroenterol. 2006;12:2288–90. doi: 10.3748/wjg.v12.i14.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10. [PubMed] [Google Scholar]
- 23.De Wied D, Sigling HO. Neuropeptides involved in the pathophysiology of schizophrenia and major depression. Neurotox Res. 2002;4:453–68. doi: 10.1080/10298420290031432. [DOI] [PubMed] [Google Scholar]
- 24.Reinhard L, Tidow H, Clausen MJ, Nissen P. Na +, K +-ATPase as a docking station: Protein-protein complexes of the Na +, K +-ATPase. Cell Mol Life Sci. 2013;70:205–22. doi: 10.1007/s00018-012-1039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sui B, Jiang P. Effects of treadmill training on rate-limiting enzyme in glycolysis and aerobic oxidation energysupplying system of rats. J Shandong Inst Phys Educ Sports. 2009;25:54–7. [Google Scholar]
- 26.Li J, Wang H. Effects of acute exercise with different intensities on the activities of respiratory chain complexes in mitochondria in rats myocardium. Chin J Sports Med. 2008;26:304–12. [Google Scholar]