Abstract
Background:
Inhibition of adipogenesis has been a therapeutic target for reducing obesity and obesity-related disorders such as diabetes, hypertension, atherosclerosis, and cancer. For decades, anti-adipogenic potential of many herbal extracts has been investigated. One example is Garcinia cambogia extract (GE) containing (-)-hydroxycitric acid as an active ingredient. GE is currently marketed as a weight loss supplement, used alone or with other ingredients. Pear pomace extract (PE), another natural product, has been also shown to have anti-adipogenic activity in a recent report.
Objective:
It was tested if the mixture of PE and GE (MIX) would produce more effective anti-adipogenic activity than PE or GE alone.
Materials and Methods:
Differentiation of 3T3-L1 preadipocyte was induced by adding insulin, dexamethasone, and isobutylmethylxanthine and lipid accumulation was measured by Oil Red O staining. Cellular markers for adipogenesis and lipolysis such as CCAAT/enhancer binding protein (C/EBP-α), peroxisome proliferator-activated receptor gamma (PPAR-γ), fatty acid synthase (FAS), and hormone-sensitive lipase (HSL) was measured using immunocytochemistry.
Results:
MIX, compared to PE or GE alone, showed greater inhibition of lipid accumulation. Furthermore, MIX reduced the expression of adipogenesis-related factors C/EBP-α, PPAR-γ, and FAS more than PE or GE alone did. In contrast, the expression of HSL the enzyme required for lipolysis was further enhanced in MIX-treated adipocytes compared to the PE or GE alone treated groups.
Conclusions:
Anti-adipogenic effect of PE and GE appears synergistic, and the MIX may be a useful therapeutic combination for the treatment of obesity and obesity-related diseases.
SUMMARY
PE and GE efficiently inhibited adipocyte differentiation by suppressing the expression of adipogenic transcription factor CEBP-α and PPAR-γ.
PE and GE significantly decreased the expression of adipogenic enzyme FAS.
PE and GE increased the expression of lipid degrading enzyme HSL.
Mixture of PE and GE exhibited additive or moderately synergistic effect on adipocyte differentiation and lipid accumulation.
Abbreviations used: CEBP-a: CCAT/enhancer binding protein alpha, CI: Combination Index, FAS: Fatty acid synthase, GE: Garcinia cambogia extract, HSL: Hormone sensitive lipase, PE: Pear pomace extract, PPAR-γ: Peroxisome proliferator-activated receptor gamma.
Keywords: Adipogenesis, anti-obesity, garcinia cambogia extract, pear pomace extract
INTRODUCTION
Obesity is one of the most common metabolic diseases. Obesity develops if energy intake exceeds energy expenditure, leading to excessive fat accumulation.[1] Excessive accumulation of body lipid may have an adverse effect on health such as hypertension, type II diabetes mellitus, cardiovascular disease, cancer, and osteoarthritis.[2] The regulation of adipogenesis is now a new key target for ameliorating obesity.[3] Adipogenesis is defined as the process of differentiation of preadipocytes into lipid accumulating mature adipocytes.[4] This process is regulated by a number of adipogenic transcription factors such as peroxisome proliferator-activated receptor gamma (PPAR-γ) and CCAAT/enhancer binding protein (C/EBP).[5,6] For decades, studies have been conducted on many natural products to control the process of adipogenesis.[3] Among those extracts, Garcinia cambogia is one of the most popular supplements inhibiting adipogenesis.[7] Hydroxycitric acid (HCA) is an active ingredient extracted from G. cambogia. It inhibits adenosine triphosphate citrate lyase which is required for fatty acid and cholesterol biosynthesis.[8] However, there has been a precaution against prolonged use of G. cambogia extract (GE) due to a possible hepatotoxicity.[9] Consequently, an alternative strategy to utilize anti-adipogenic effect of GE while minimizing hepatotoxicity has been seeked. Mixtures of natural products are often adopted for the treatment and prevention of a disease because the mixture containing diverse ingredients could evoke additive or even synergistic effect through multiple mode of action.[10] Pear is popular fruit which is commercialized in form of juices and soft drinks. Pear contains phenolic compounds such as arbutin, catechin, epicatechin, caffeic acid, and coumaric acid and is reported to prevent hyperglycemia and dyslipidemia.[11] Recent studies showed pear pomace, a manufacturing byproduct of pear juice could be utilized to suppress adipocyte differentiation[11] and to improve some obesity-related disorders.[12] Here, our aim is to test if the combination of pear pomace extract (PE) and GE would produce more effective anti-adipogenic activity than PE or GE alone.
MATERIALS AND METHODS
Materials and reagents
3T3-L1 differentiation
To induce preadipocyte differentiation, 3T3-L1 preadipocytes were cultured until confluent in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat activated fetal calf serum (FCS). Two days after confluence (D0), the cells were differentiated with DMEM containing 10% fetal bovine serum (FBS), 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone, and 10 μg/ml insulin for 3 days (D3). Cells were then maintained in 10% FBS DMEM containing 10 μg/ml insulin for another 4 days (D7). Cells at this condition were converted to mature adipocytes with accumulated fat droplet.
Cell viability (MTS-PMS assay)
Cells were seeded at 5000 cells/well in 96-well plate. After 24 h, cells were incubated in DMEM containing 10% FCS and various concentrations of PE, GE, and mixture of PE and GE (MIX) for 48 h. The treatment media were removed and cells were washed twice with sterile phosphate buffered saline (PBS). Cells were treated with 100 μL MTS working solution (0.5 mg/mL) and incubated for 4 h. The obtained colored solution was measured at test wavelength of 490 nm. The optical density (OD) value of the control group was represented as 100% while the OD of other treatment groups was expressed as percentage of the control group.
Oil Red O staining
It was performed on D7 of cell differentiation. Cells were washed twice with PBS and fixed with 10% formalin for 60 min. The cells were then washed with 60% propanol and dried. Oil red O working solution was added and incubated for 10 min at room temperature. Then, the solution was removed and cells were washed with PBS. Oil Red O-stained lipid droplets were photographed using a phase-contrast microscope (Olympus CKX53). Then, Oil Red O was extracted with isopropanol and OD at 520 nm was measured.
Immunocytochemistry
Cells were cultured on 4 well Lab-Tek™ (Thermo Fisher scientific, cat#154526) chambers and differentiated as described above. At D7, cells were washed with cold PBS followed by fixation with 4% paraformaldehyde for 15 min. The cells were incubated in blocking/permeabilizing buffer (PBS with 5% normal donkey serum and 0.25% Triton X-100) for 1 h at room temperature. Then, the preparations were incubated overnight at 4°C with the primary antibodies diluted in the blocking/permeabilizing buffer. After 3 washes fluorescence-labeled secondary antibodies diluted in the blocking/permeabilizing buffer was reacted in samples for 1 h at room temperature. Samples were then rinsed once with blocking/permeabilizing buffer and twice with PBS. Using Fluorsave® mounting medium, the samples were mounted on slides. Specific immunolabeling was initially examined under epifluorescence microscope, and confocal images were obtained using a confocal laser scanning microscope (LSM710, Zeiss). Antibody against C/EBP-α (Santa cruz biotechnology, cat#sc-61), PPAR-γ (Santa cruz biotechnology, cat#sc-7273), fatty acid synthase (FAS) (Santa cruz biotechnology, cat#sc-20140), and hormone-sensitive lipase (HSL) (Abcam, cat#sc-ab45422) were used at 1:500. Alexa Fluor 488 (Invitrogen, cat#A21206) and Alexa Fluor 555 (Invitrogen, cat#A31570) tagged secondary antibodies generated in donkey were used at 1:1000. Control preparations, in which the primary antibody was omitted, were imaged under comparable exposure conditions to test for specificity. No specific labeling was observed in this condition.
Statistical analysis
Data were presented as mean ± standard deviation. Group differences were assessed by One-way analysis of variance and Tukey's multiple comparison post hoc tests. The differences between groups at P < 0.05 were considered statistically significant. Synergism in the combination of GE and PE was determined by Chou-Talalay method.[13] The combination index (CI) for MIX was calculated using CompuSyn software (Combosyn Inc.).
RESULTS
Effect on cell viability and adipocyte differentiation of 3T3-L1 preadipocytes
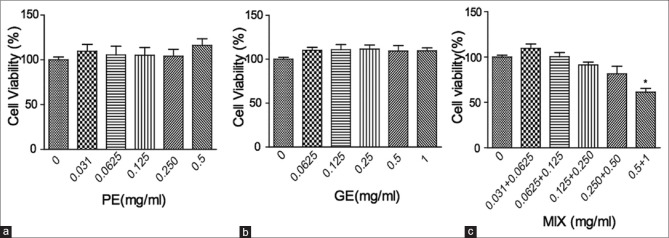
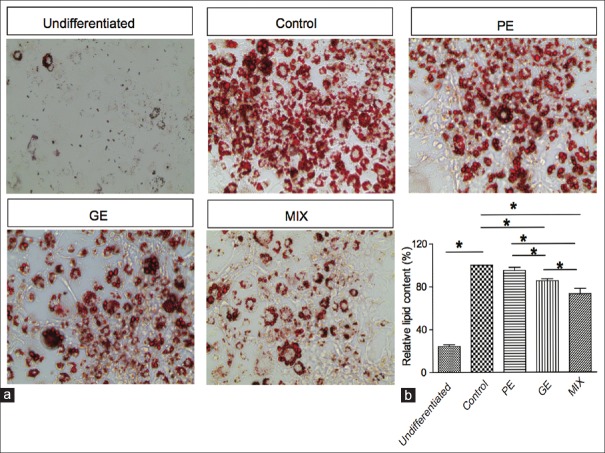
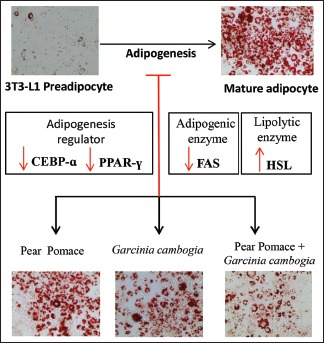
Obesity is often associated with increased lipid accumulation, which can occur by adipocyte differentiation.[4] Inhibition of adipocyte differentiation is a target for the anti-obesity therapy. Therefore, we examined the anti-adipogenic effect of the PE, GE, and MIX and the underlying mechanism using 3T3-L1 preadipocyte cells. We first examined concentration-dependent cytotoxicity of PE, GE, and MIX by MTS-PMS assay. Concentration up to 500 μg/ml PE, 1 mg/ml GE and MIX (up to 250 μg/ml PE + 500 μg/ml GE) did not affect the viability of 3T3-L1 cells [Figure 1]. Next, we examined whether PE, GE, and MIX inhibit differentiation of 3T3-L1 preadipocytes into adipocytes. In a preliminary experiment, various concentrations and compositions of PE, GE, and MIX were compared and the final concentration of PE, GE, and MIX was determined at the point where the most effective inhibition of lipid accumulation was observed without any loss of cell viability (data not shown). Preadipocytes were treated with differentiating media in the presence or absence of PE 30 μg/ml, GE 60 μg/ml, and their MIX for 8 days, and intracellular lipid accumulation was quantified. More than 90% of the cells differentiated into mature adipocytes. Cell treated with 30 μg/ml PE alone showed slight inhibition (4.1 ± 2.9% reduction) of lipid accumulation. Treatment with 60 μg/ml GE showed significant inhibition of adipocyte differentiation and lipid accumulation (14.7% ±1.8% reduction) in 3T3-L1 cells. However, cell treated with the MIX showed greater inhibition (26.9 ± 5% reduction) of lipid accumulation compared to PE and GE alone [Figure 2]. The CI value for this combination was 0.75, which suggests moderate synergism of the combination. MIX efficiently inhibited adipocyte differentiation of 3T3-L1 preadipocytes and the inhibitory effects were not due to its cytotoxicity.
Figure 1.
The viability of 3T3-L1 cells was not significantly affected by PE or GE. 3T3-L1 preadipocytes were treated with various concentrations of GE (a), PE (b), and MIX (c) for 48 h. Cell viability was determined using an MTS-PMS kit. Only cells treated with the highest concentration of PE and GE mixture (MIX, 0.5+1 mg/ml) exhibited a significantly decrease in viability. Data are presented as mean ± standard deviation (n = 5) *P < 0.05. PE: Pear pomace extract; GE: Garcinia cambogia extract
Figure 2.
Effects of PE, GE, and MIX on lipid accumulation in 3T3-L1 adipocytes. 3T3-L1 preadipocytes were differentiated into adipocytes with differentiation media for 8 days. The cells were treated with PE, GE, and MIX during the differentiation period. Lipid accumulation in the cells was analyzed by Oil Red O staining. (a) The micrographs of Oil red O-stained adipocytes. (b) Stained oil droplets were dissolved in isopropyl alcohol and quantified by reading the absorbance at 520 nm. PE: Pear pomace extract; GE: Garcinia cambogia extract
Effect on the expression of adipogenic transcription factors during adipocyte differentiation
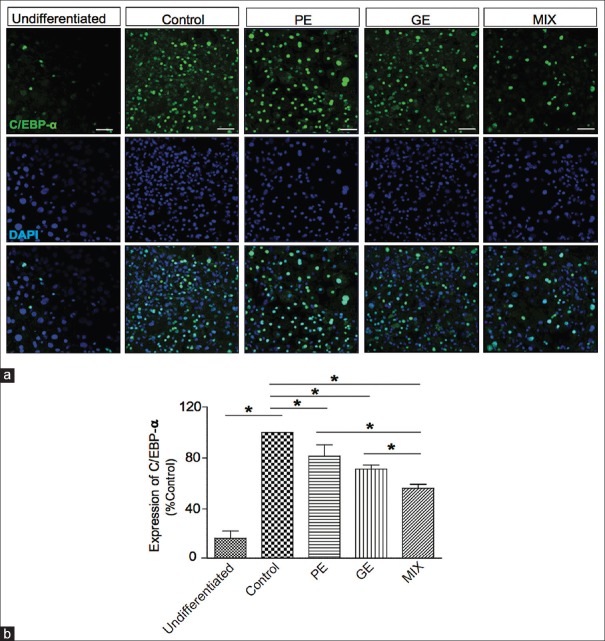
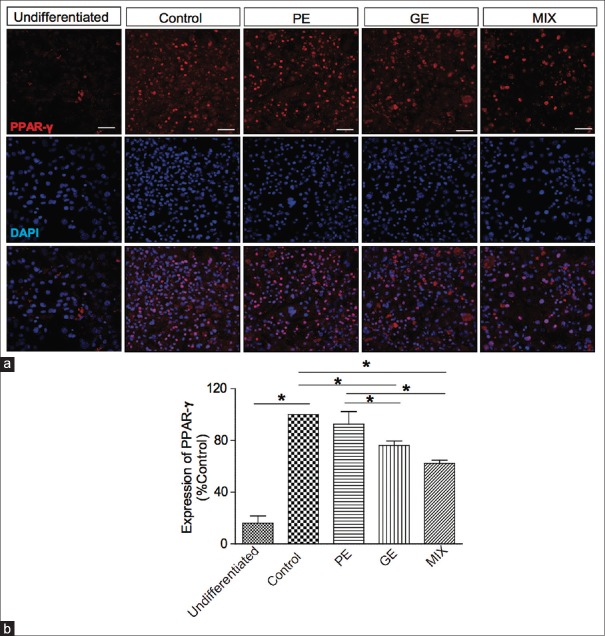
Adipocyte differentiation is accompanied by increased expression of adipogenic transcription factors including C/EBP-α and PPAR-γ.[5,6] These transcriptional factors play crucial role in promoting genes responsible for adipocyte differentiation and maturation such as FAS.[14,15] These factors are known to be regulators of the synthesis, transport, and storage of lipids during adipocyte differentiation.[16] We used immunocytochemistry analysis to examine the inhibitory effect of PE, GE, and MIX on the expression of adipogenic transcription factors during the adipocyte differentiation. C/EBP-α protein expression levels were significantly increased in differentiated adipocytes on day 8 compared to undifferentiated 3T3-L1 [Figure 3]. Treatment with PE 30 μg/ml, GE 60 μg/ml, and MIX during differentiation period significantly suppressed the expression of C/EBP-α. Among the treatment groups, MIX showed the greatest inhibitory effect on C/EBP-α expression [Figure 3, MIX 43.64% ± 3.7%, PE 18.5% ± 8.8%, GE 28% ± 3.1% inhibition]. Similarly, treatment with GE and MIX significantly reduced the expression of PPAR-γ level. The PPAR-γ expression was not influenced by PE alone. Among the treatment groups, MIX showed the strongest inhibitory effect on the expression of PPAR-γ [Figure 4, MIX 37.7% ± 2.5%, PE 7.38% ± 9.761%, and GE 23.8% ± 3.4% inhibition].
Figure 3.
Effect of PE, GE, and MIX on the expression of CCAAT/enhancer binding protein-a in 3T3-L1 adipocytes. 3T3-L1 preadipocytes were differentiated into adipocytes by incubation with isobutylmethylxanthine, dexamethasone, and insulin for 8 days. The cells were exposed to PE (30 μg/ml), GE (60 μg/ml), and MIX during the differentiation period. (a) Representative image of immunofluorescence labeled CCAAT/enhancer binding protein a. (b) Measurement of fluorescence intensity of CCAAT/enhancer binding protein a. Values are mean ± standard deviation (n = 3) *P < 0.05. PE: Pear pomace extract; GE: Garcinia cambogia extract
Figure 4.
Effect of PE, GE, and MIX on the expression of PPAR-γ in 3T3-L1 adipocytes. (a) Representative image of immunofluorescence labeled peroxisome proliferator-activated receptor gamma. (b) Graphical representation of fluorescence intensity of peroxisome proliferator-activated receptor gamma expression. Values are mean ± standard deviation (n = 3) *P < 0.05. PE: Pear pomace extract; GE: Garcinia cambogia extract
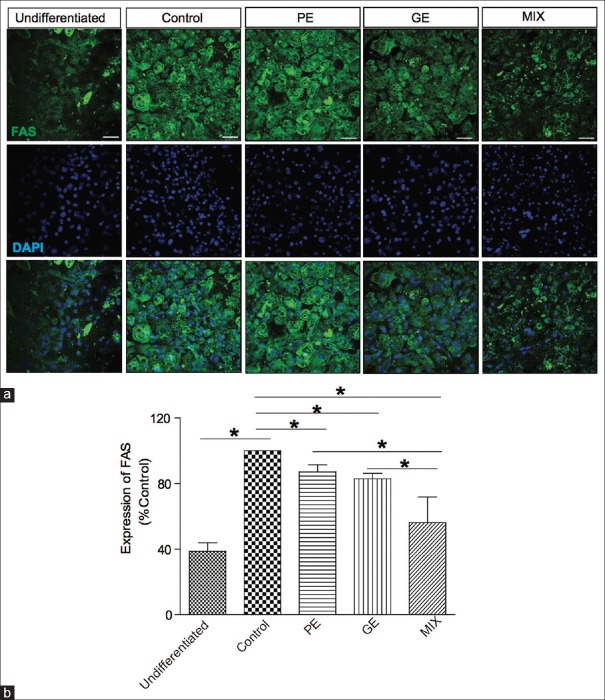
Effect on the expression of adipogenic enzyme fatty acid synthase
We further investigated the effect of PE, GE, and MIX on the expression of one of the lipogenic gene markers, FAS, which triggers the synthesis of fatty acids and triglycerides.[15] As shown in Figure 5, the expression levels of FAS dramatically increased in differentiation medium treated cells compare to undifferentiated cells. Addition of PE 30 μg/ml or GE 60 μg/ml in the differentiation media significantly decreased the FAS expression. The combination of PE and GE suppressed FAS expression even further (MIX 43.7% ± 15%, PE 12.2% ± 4.2%, and GE 17.1% ± 3.4% inhibition), suggesting that the combination is effective agent in suppressing lipid synthesis in adipose tissue.
Figure 5.
Effect of PE, GE, and MIX on the expression of FAS in 3T3-L1 adipocytes. (a) Representative image of immunofluorescence labeled FAS. (b) Graphical representation of fluorescence intensity of FAS. Values are mean ± standard deviation (n = 3) *P < 0.05. FAS: Fatty acid synthase; PE: Pear pomace extract; GE: Garcinia cambogia extract
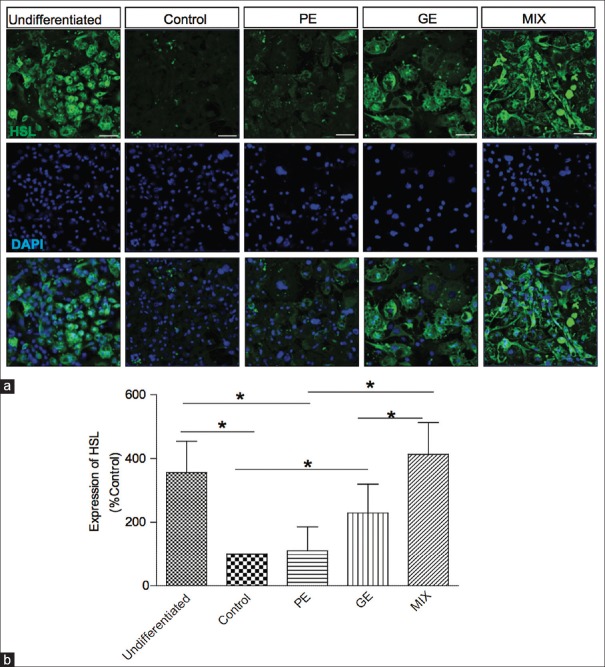
Effect on the expression of lipid degrading enzyme hormone sensitive lipase
The lipolysis pathways in adipocytes are initiated by the enzyme known as HSL.[17] It has a capacity to degrade triglyceride and is the rate-limiting enzyme of lipolysis in adipose tissue. Using immunohistochemistry, we examined the expression level of HSL in the presence and the absence of PE, GE, and MIX. Unlike the undifferentiated 3T3-L1 cells expressing high level of HSL, differentiated adipocytes expressed very low level of HSL [Figure 6]. Adding PE, GE, and MIX in the differentiation media significantly enhanced the HSL expression and MIX was the strongest enhancer of the HSL expression (MIX 313.2% ± 100.2%, PE 10% ± 75.2%, and GE 129.4% ± 90.5% increase). It suggests that MIX may be a strong promoter of lipid degradation in adipose tissue [Figure 6].
Figure 6.
Effect of PE, GE, and MIX on the expression of HSL in 3T3-L1 adipocytes. (a) Representative image of immunofluorescence labeled HSL. (b) Graphical representation of fluorescence intensity of HSL. Values are mean ± standard deviation (n = 3) *P < 0.05. PE: Pear pomace extract; GE: Garcinia cambogia extract; HSL: Hormone-sensitive lipase
DISCUSSION
Excessive fat accumulation due to energy overload causes overweight and obesity. Obesity may lead to serious diseases such as diabetes, cardiovascular diseases, chronic kidney disease, and several forms of cancer.[1] Currently used anti-obesity agents are appetite suppressants such as sibutramine and rimonabant.[18,19] Another group of drugs reduces obesity by inhibiting fat absorption, for example, orlistat. However, these drugs often show adverse effects such as insomnia, increased appetite, asthenia, nausea and anorexia (sibutramine), constipation, bladder pain, diarrhea, fever, loss of appetite, nasal congestion, and difficulty in sleeping (Orlistat) and some psychiatric issues (rimonabant).[20] Due to this undesirable adverse effect of the drugs, people are seeking an alternative method for reducing obesity. Adipogenesis, the new key target of anti-obesity drugs, is the process where mesenchymal cells differentiate into preadipocytes followed by second differentiation into lipid accumulating adipocytes. It requires sequential activation of transcription factors such as PPAR-γ and C/EBP-α and induction of adipocyte-related protein such as FAS.[21] FAS are the gene involved in lipogenesis which triggers the synthesis of fatty acids and triglycerides.[14,21] Therefore, these factors are useful markers to assess the anti-adipogenic activity of a new regimen.
Modern medicine available for the prevention and treatment of obesity tends to show adverse effect on chronic use.[22] Consequently, as convenient alternatives for the treatment and prevention of obesity, natural medicinal plants have drawn public's attention.[22,23] Anti-adipogenic activity of GE a plant extract on 3T3-L1 cell has been previously well documented. GE has significantly reduced the lipid accumulation by inhibiting adipogenic key regulators such as PPAR-γ and C/EBP-α in 3T3-L1 adipocytes.[8,24] Similarly, Rhyu et al. have demonstrated that pear pomace extracts also attenuated lipid accumulation by inhibiting PPAR-γ, C/EBP-α, and FAS expression.[11]
In this study, we have demonstrated that the combination of GE and PE effectively suppressed lipid accumulation in 3T3-L1 cells. The significantly decreased expression of C/EBP-α, PPAR-γ, and FAS indicated that the treatment inhibited adipocyte maturation and adipogenesis. Furthermore, our data demonstrated that MIX was more efficient than GE or PE alone in suppressing adipogenesis. Similarly, MIX strongly promoted the expression of HSL the lipolytic enzyme catalyzing the release of fatty acids from triacylglycerol-rich lipid storage droplets of adipocytes.[16] Taken together, our data suggest that PE and GE act synergistically in suppressing adipogenesis and promoting lipolysis and thereby, prevent lipid accumulation in adipose tissue.
CONCLUSION
The combination of PE and GE, compared to PE or GE alone, showed stronger inhibition of lipid accumulation. MIX showed effective inhibition of C/EBP-α, PPAR-γ, and FAS expression, while it enhances expression of HSL, suggesting greater anti-adipogenic and lipolytic activity. These results indicate that anti-obesity effect of PE and GE appears synergistic and the MIX may be a useful therapeutic combination for the treatment of obesity and obesity-related diseases.
Financial support and sponsorship
This work was supported by the Ministry of Trade, Industry and Energy (MOTIE), Korea Institute of Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Co-operation Region (R0004540).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–43. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Feng S, Reuss L, Wang Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules. 2016;21 doi: 10.3390/molecules21101278. pii: E1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013;92:229–36. doi: 10.1016/j.ejcb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Christy RJ, Kaestner KH, Geiman DE, Lane MD. CCAAT/enhancer binding protein gene promoter: Binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991;88:2593–7. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–55. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 7.Jung YM, Lee DS, Lee SH, Jeoung NH, Kim BJ. Antiobesity effect of mixture of black garlic and Garsinia cambogia extracts in 3T3-L1 adipocytes and L6 skeletal muscle cells. J Exp Biomed Sci. 2012;18:291–8. [Google Scholar]
- 8.Hayamizu K, Ishii Y, Kaneko I, Shen M, Okuhara Y, Shigematsu N, et al. Effects of Garcinia cambogia (Hydroxycitric acid) on visceral fat accumulation: A double-blind, randomized, placebo-controlled trial. Curr Ther Res Clin Exp. 2003;64:551–67. doi: 10.1016/j.curtheres.2003.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunsford KE, Bodzin AS, Reino DC, Wang HL, Busuttil RW. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J Gastroenterol. 2016;22:10071–6. doi: 10.3748/wjg.v22.i45.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Lee MJ, Song MY, Bose S, Shin BC, Kim HJ, et al. Efficacy and safety of mixed oriental herbal medicines for treating human obesity: A systematic review of randomized clinical trials. J Med Food. 2012;15:589–97. doi: 10.1089/jmf.2011.1982. [DOI] [PubMed] [Google Scholar]
- 11.Rhyu J, Kim MS, You MK, Bang MA, Kim HA. Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Nutr Res Pract. 2014;8:33–9. doi: 10.4162/nrp.2014.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang S, Cui X, Guo M, Tian Y, Xu W, Huang K, et al. Insoluble dietary fiber from pear pomace can prevent high-fat diet-induced obesity in rats mainly by improving the structure of the gut microbiota. J Microbiol Biotechnol. 2017;27:856–67. doi: 10.4014/jmb.1610.10058. [DOI] [PubMed] [Google Scholar]
- 13.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 14.Shon MS, Kim SK, Song JH, Lee SC, Kim GN. Anti-adipogenic activity of blue mussel (Mytilus edulis) extract by regulation of 3T3-L1 adipogenesis through Wnt/β-catenin signaling pathway. Food Sci Biotechnol. 2015;24:315–21. [Google Scholar]
- 15.Li KK, Liu CL, Shiu HT, Wong HL, Siu WS, Zhang C, et al. Cocoa tea (Camellia ptilophylla) water extract inhibits adipocyte differentiation in mouse 3T3-L1 preadipocytes. Sci Rep. 2016;6:20172. doi: 10.1038/srep20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta. 2000;1483:251–62. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 18.Luque CA, Rey JA. Sibutramine: A serotonin-norepinephrine reuptake-inhibitor for the treatment of obesity. Ann Pharmacother. 1999;33:968–78. doi: 10.1345/aph.18319. [DOI] [PubMed] [Google Scholar]
- 19.Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–84. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- 20.Seo JB, Choe SS, Jeong HW, Park SW, Shin HJ, Choi SM, et al. Anti-obesity effects of Lysimachia foenum-graecum characterized by decreased adipogenesis and regulated lipid metabolism. Exp Mol Med. 2011;43:205–15. doi: 10.3858/emm.2011.43.4.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Park HJ, Kang SN, Jang SH, Lee SJ, Ko YG, et al. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS One. 2013;8:e69925. doi: 10.1371/journal.pone.0069925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandrasekaran C, Vijayalakshmi M, Prakash K, Bansal V, Meenakshi J, Amit A. Herbal approach for obesity management. Am J Plant Sci. 2012;3:1003–14. [Google Scholar]
- 23.Esteghamati A, Mazaheri T, Vahidi Rad M, Noshad S. Complementary and alternative medicine for the treatment of obesity: A critical review. Int J Endocrinol Metab. 2015;13:e19678. doi: 10.5812/ijem.19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IH, Nam TJ. Enzyme-treated ecklonia cava extract inhibits adipogenesis through the downregulation of C/EBPα in 3T3-L1 adipocytes. Int J Mol Med. 2017;39:636–44. doi: 10.3892/ijmm.2017.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]