Abstract
Background:
Marine organisms are established to be a wealthy source of bioactive compounds with diverse chemical structures and bioactivities. Acanthostrongylophora ingens is known to be rich with pyrimidine b-carboline and manzamine-type alkaloids. The goal of the present work is to isolate and identify new alkaloids from A. ingens as well as to assess the cytotoxic potential of these metabolites towards various cancer cell lines.
Methods:
The crude MeOH extract of the sponge was separated by vacuum liquid chromatography (VLC), using n-hexane, EtOAc, and MeOH. The EtOAc fraction was chromatographed on VLC, SiO2, sephadex LH-20, and RP18 columns, affording four metabolites. Their structures were identified using infrared, ultraviolet, high-resolution mass spectrometry, and nuclear magnetic resonance spectroscopic techniques, as well as comparison with the published data.
Results:
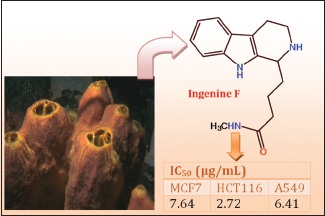
A new 1,2,3,4-tetrahydro-β-carboline (THβCs) alkaloid, ingenine F (4) and three known compounds: Annomontine (1), acanthomine A (2), and 1-oxo-1,2,3,4-THβCs (3) were isolated and identified. Ingenine F (4) exhibited cytotoxic activity toward hormone-dependent breast carcinoma (MCF7), colon carcinoma (HCT116), and lung carcinoma (A549) cell lines with IC50 values of 2.82, 1.00, and 2.37 μM, respectively, compared to doxorubicin (IC50 0.012, 0.036, and 0.102 μM, respectively).
Conclusion:
It is the first report for the isolation of THβCs alkaloids from A. ingens. The THβCs alkaloid with N-methylbutyramide unit as found in ingenine F is very rarely encountered in nature. Ingenine F may provide new promising candidates for potential cytotoxic agent.
SUMMARY
Ingenine F, a new 1,2,3,4-THβCs derivative (4) and three known alkaloids (1-3) were isolated from A. ingens. Their structures were verified by various spectroscopic analyses. Compound 4 had potent cytotoxic effect toward MCF7, HCT116, and A549 cancer cell lines.
Abbreviations used: 1D: One-dimensional; 2D: Two-dimensional; CC: Column chromatography; COSY: Correlations spectroscopy; DMSO: Dimethyl sulfoxide; HMBC: Heteronuclear multiple bond correlation experiment; HRESIMS: High resolution electrospray ionization mass spectrometry; HSQC: Heteronuclear single quantum correlation; IR: Infrared; LCQ: Liquid chromatography quadrupole; LTQ: Linear trap quadropole; NMR: Nuclear magnetic resonance; RP: Reversed phase; SiO2: Silica gel; TLC: Thin-layer chromatography; UV: Ultraviolet; VLC: Vacuum liquid chromatography.
Keywords: Acanthostrongylophora ingens, alkaloid, cytotoxic activity, Ingenine F, Tetrahydro-β-carboline
INTRODUCTION
The ability to use nature as an inspiring framework for devolving novel products is an interesting area of scientific research. In fact, large numbers of current pharmacopoeias derive directly or indirectly from natural products. Marine organisms are established to be fruitful origin of bioactive novel compounds with diverse chemical structures that are of great usefulness as new drug leads for treating various ailments. Acanthostrongylophora ingens (order Haplosclerida; family Petrosiidae) is considered to be a rich source of pyrimidine β-carboline[1,2,3] and manzamine-type alkaloids.[4,5,6,7,8] These compounds exhibit a diverse range of bioactivities, including antimalarial, antileishmanial, antituberculosis,[4,5,6] cytotoxic,[1,2,9,10,11] and anti-HIV.[4,5,6] In our further search for structurally interesting and bioactive compounds from the sponge A. ingens, a new 1,2,3,4-tetrahydro-β-carboline (THβCs) alkaloid, ingenine F (4), and three known compounds were isolated [Figure 1]. The structural identities of the isolated metabolites were proven by interpreting the spectroscopic data. Herein, the isolation, structural characterization, and cytotoxic potential of 4 were described.
Figure 1.
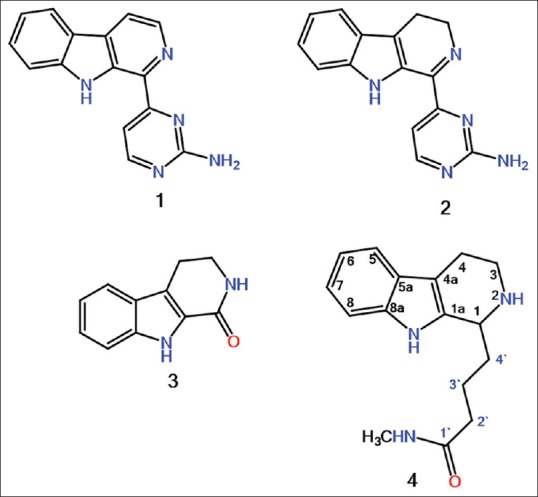
Structures of isolated compounds 1-4
MATERIALS AND METHODS
General experimental procedures
A JASCO polarimeter was utilized for measuring optical rotation (Jasco Co., Tokyo, Japan). An electrothermal-9100 digital melting point instrument was used to get the melting points (Perkin-Elmer, Waltham, MA, USA). Ultraviolet (UV) data were recorded by Hitachi-300 spectrophotometer (Kyoto, Japan). The infrared (IR) absorbance was obtained by a Shimadzu Infrared 400 spectrophotometer (Shimadzu, Kyoto, Japan). A liquid chromatography quadrupole (LCQ) DECA mass spectrometer (ThermoFinnigan, Bremen, Germany) was utilized to obtain the electrospray ionization mass spectroscopy (ESIMS) spectrum. high resolution electrospray ionization mass spectrometry (HRESIMS) was assessed by linear trap quadropole (LTQ)-Orbitrap MS spectrometer. A Bruker Avance DRX 500 MHz spectrometer was used for measuring nuclear magnetic resonance (NMR) data (Bruker BioSpin, Billerica, MA, USA). High-performance liquid chromatography (HPLC) was carried out on a C-18 column Eurospher 100 (300 mm × 8 mm, Knauer, Berlin, Germany) at 5.0 ml/min flow rate. Sephadex LH-20 (0.25–0.1 mm), silica gel (0.063–0.200 mm), and RP18 (0.04–0.063 mm) (Merck, Darmstadt, Germany) were utilized for column chromatography. For TLC, precoated plates silica gel (60 F254, 0.2 mm, Merck, Darmstadt, Germany) were used. RP18 six mL standard extraction tube (40–63 μm) (Merck, Darmstadt, Germany) was used for the purification of compounds.
Animal material
The sponge material was obtained in 2010 from Sulawesi Island by scuba diving at 12 m depth. The collected material was immediately freeze-dried after collection. A specimen (ZMAPOR. 17795-A) was preserved in the Zoological Museum (Amsterdam University).
Extraction and isolation
The dried sponge (1.3 kg) was extracted with MeOH (4 × 2 L) at room temperature (25°C). The combined extract was concentrated to get a brown residue (39.0 g). The latter was submitted to vacuum liquid chromatography (VLC), utilizing n-hexane, EtOAc, and MeOH (2 × 1.0 L, each) to get n-hexane (5.9 g), EtOAc (7.8 g), and MeOH (20.8 g) fractions. The EtOAc (7.8 g) fraction was chromatographed, using CHCl3:MeOH gradient on VLC to get 13 sufractions: EA-1 to EA-13. Fraction EA-3 (380 mg) was separated using MeOH: CHCl3 (90:10) on a sephadex LH-20 CC (50 g × 50 cm × 3 cm) to give 3 subfractions: EA-3A to EA-3C. Subfraction EA-3B (71 mg) was submitted to SiO2 CC (40 g × 50 × 3 cm), utilizing CHCl3:MeOH (95:5–80:20) to get impure 1. Purification of 1 was performed using a semi-preparative HPLC to yield 1 (10.2 mg, yellow needles). Fraction EA-4 (148 mg) was separated on a SiO2 CC (40 g × 50 cm × 2 cm), utilizing a CHCl3:MeOH gradient to give 2 subfractions: EA-4A and EA-4B. Subfraction EA-4A (69 mg) was subjected to SiO2 CC (40 g × 50 cm × 2 cm) with CHCl3:MeOH gradient to afford impure 2 and 3. Each compound was purified separately on RP18 extraction tube, using H2O: acetonitrile gradient elution to yield 2 (9.5 mg, red needles) and 3 (7.1 mg, white amorphous powder). Fractions EA-5 (65 mg) was subjected to RP18 CC (20 g × 50 cm × 2 cm) eluting with H2O: MeOH gradient to get 2 subfractions: EA-5A and EA-5B. Subfraction EA-5A (29 mg) was separated on HPLC to yield 4 (2.7 mg, pale yellow powder).
Spectral data
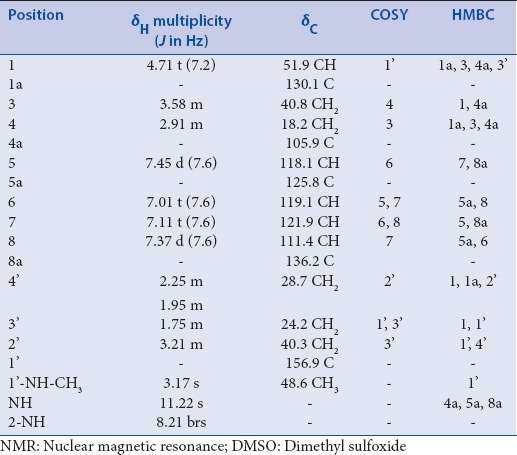
Ingenine F (4): pale yellow powder; [α]D20 + 13.9 (c 0.3, CH3OH); UV (MeOH) λmax (log ε) 226 (4.37), 300 (3.98) nm; IR (KBr) Vmax 3210, 2924, 1649, 1564, 1058 cm-1; NMR data [Table 1]; HRESIMS m/z 272.1764 (M + H) + (calcd for C16H22N3O, 272.1763).
Table 1.
NMR data of compound 4 (DMSO-d6, 500 and 125 MHz)
Cytotoxicity assay
The in vitro cytotoxic activity of 4 was assessed toward colon carcinoma (HCT116), hormone-dependent breast carcinoma (MCF7), human cervix carcinoma (Hela), lung carcinoma (A549), and brain tumor (PC12) cell lines (ATCC, Rockville, MD) as previously stated.[12,13,14,15] DMSO and doxorubicin were utilized as negative and positive standards, respectively.
RESULTS AND DISCUSSION
Compound 4 was separated as pale yellow powder and afforded positive tests for alkaloids.[16] Its molecular formula C16H21N3O was estimated by the pseudomolecular ion peak at m/z 272.1764 (M + H) + (calcd for C16H22N3O, 272.1763) in HRESIMS spectrum, requiring eight DBE. Its UV absorbances at 226, and 300 nm specified the existence of a THβC chromophore.[17,18] Its IR showed bands at 3210 (NH), 2924 (C-H), and 1649 (C = O) cm-1. The chemical shifts together with NMR experiments suggested 4 to have a C-1 substituted 1,2,3,4-tetrahydro β-carboline skeleton.[18] 13C NMR and heteronuclear single quantum correlation (HSQC) spectra of 4 exhibited 16 carbons, including 8 aromatic carbons, an amide carbonyl (δC 156.9), 5 methylenes, NH-bonded methine, and N-methyl signal (δC 48.6). The 1H NMR spectrum showed four-coupled aromatic protons at δH 7.45 (H-5), 7.01 (H-6), 7.11 (H-7), and 7.37 (H-8), suggesting the presence of an ABCD spin system characteristic for a 1,2-disubstituted benzene. They correlated to the carbons, resonating at δC 118.1, 119.1, 121.9, and 111.4, respectively, in the HSQC spectrum. In addition, signals for a NH-bonded methine at δH 4.71 (H-1)/δC 51.9 (C-1), two coupled methylenes at δH 3.58 (m, H-3)/δC 40.8 (C-3) and δH 2.91 (m, H-4)/δC 18.2 (C-4), and NH group at δH 8.21 (brs, 2-NH) were attributable to 1,2,3,4-tetrahydro-pyridine ring of the β-carboline moiety. The HMBC correlations of H-1 to C-1a, C-4a, and C-3, H-3 to C-1 and C-4a, H-4 to C-1a, C-4a, and C-3, H-5 to C-7 and C-8a, H-6 to C-5a and C-8, H-7 to C-5 and C-8a, and H-8 to C-5a and C-6 established the THβC moiety of 4 [Figure 2]. In the 1H and 13C spectra, signals for three-coupled aliphatic methylenes (δH 3.21 [m, H-2`]/δC 40.3 [C-2`], 1.75 [m, H-3`]/δC 24.2 [C-3`], and 2.25 and 1.95 [each m, H-4`]/δC 28.7 [C-4`]), and NH-bonded methyl (δH 3.17/δC 48.6), and an amide carbonyl (δC 156.9) characteristic for N-methylbutyramide moiety were observed. This moiety was assured by the cross peaks of H-2`/C-1` and C-4`, H-3`/C-1`, H-4`/C-2`, and 1`-NH-CH3/C-1` in HMBC. The connectivity of the N-methylbutyramide moiety at C-1 of the THβC moiety was assured by the HMBC relations of H-2` and H-3`/C-1, and H-4`/C-1 and C-1a. Due to the scarcity of the compound, C-1 relative configuration was assigned to be R-form based on the comparison 1H and 13C shifts and J value of H-1 as well as optical rotation of 4 with those of series of analogous compounds.[19,20] On the basis of the above evidence, 4 was identified as a THβC alkaloid substituted at C-1 by N-methylbutyramide. Thus, compound 4 was finally assigned as (R)-N-methyl-4-(2,3,4,9-tetrahydro-1H-pyrido [3,4-b] indol-1-yl) butanamide and named ingenine F.
Figure 2.
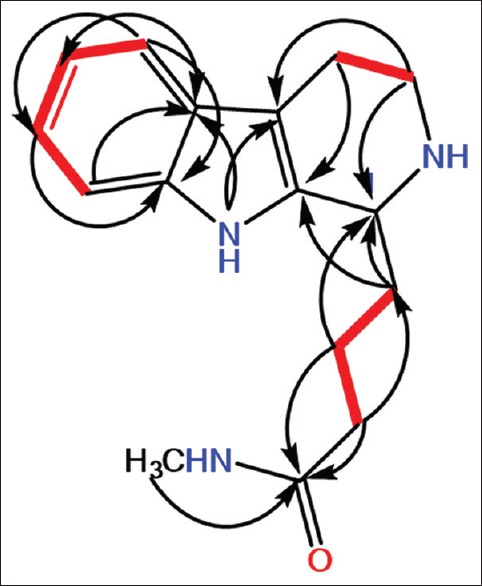
1H-1H COSY and HMBC correlations of 4
The NMR data of compounds 1-3 were in accordance with those reported previously for annomontine (1),[1,2] acanthomine A (2),[1] and 1-oxo-1,2,3,4-THβCs (3).[1]
Ingenine F (4) was assessed for its cytotoxic potential toward A549, MCF7, HCT116, PC12, and Hela cancer cell lines. It displayed cytotoxic activity against MCF7, HCT116, and A549 with IC50 values of 2.82, 1.00, and 2.37 μM, respectively, compared to doxorubicin (IC50 0.012, 0.036, and 0.102 μM, respectively). However, it was inactive toward Hela and PC12 cancer cell lines.
CONCLUSION
A new 1,2,3,4-THβCs derivative (4) and three known compounds (1-3) were separated from the Indonesian marine sponge A. ingens. Their structures were identified by the aid of comprehensive spectroscopic analysis. Compound 4 exhibited cytotoxic effect toward MCF7, HCT116, and A549 cancer cell lines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to Dr. Rob vanSoest for the identification of the sponge (Zoological Museum, University of Amsterdam).
REFERENCES
- 1.Ibrahim SR, Ebel RA, Ebel R, Proksch P. Acanthomine A, a new pyrimidine-β-carboline alkaloid from the sponge Acanthostrongylophora ingens. Nat Prod Commun. 2008;3:175–8. [Google Scholar]
- 2.Ibrahim SR, Mohamed GA, Zayed MF, Sayed HM. Ingenines A and B, two new alkaloids from the Indonesian sponge Acanthostrongylophora ingens. Drug Res (Stuttg) 2015;65:361–5. doi: 10.1055/s-0034-1384577. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim SR, Mohamed GA. Ingenine E, a new cytotoxic β-carboline alkaloid from the Indonesian sponge Acanthostrongylophora ingens. J Asian Nat Prod Res. 2017;19:504–9. doi: 10.1080/10286020.2016.1213723. [DOI] [PubMed] [Google Scholar]
- 4.Peng J, Hu JF, Kazi AB, Li Z, Avery M, Peraud O, et al. Manadomanzamines A and B: A novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J Am Chem Soc. 2003;125:13382–6. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao KV, Kasanah N, Wahyuono S, Tekwani BL, Schinazi RF, Hamann MT, et al. Three new manzamine alkaloids from a common Indonesian sponge and their activity against infectious and tropical parasitic diseases. J Nat Prod. 2004;67:1314–8. doi: 10.1021/np0400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao KV, Donia MS, Peng J, Garcia-Palomero E, Alonso D, Martinez A, et al. Manzamine B and E and ircinal A related alkaloids from an Indonesian acanthostrongylophora sponge and their activity against infectious, tropical parasitic, and Alzheimer's diseases. J Nat Prod. 2006;69:1034–40. doi: 10.1021/np0601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Desoky AH, Kato H, Eguchi K, Kawabata T, Fujiwara Y, Losung F, et al. Acantholactam and pre-neo-kauluamine, manzamine-related alkaloids from the indonesian marine sponge Acanthostrongylophora ingens. J Nat Prod. 2014;77:1536–40. doi: 10.1021/np500290a. [DOI] [PubMed] [Google Scholar]
- 8.Furusato A, Kato H, Nehira T, Eguchi K, Kawabata T, Fujiwara Y, et al. Acanthomanzamines A-E with new manzamine frameworks from the marine sponge Acanthostrongylophora ingens. Org Lett. 2014;16:3888–91. doi: 10.1021/ol5015569. [DOI] [PubMed] [Google Scholar]
- 9.Kong F, Andersen RJ. Ingenamines A and B, new cytotoxic alkaloids from the marine sponge Xestospongia ingens. Tetrahedron. 1994;50:6137–44. [Google Scholar]
- 10.Kong F, Andersen RJ. Ingenamine alkaloids isolated from the sponge Xestospongia ingens: structures and absolute configurations. Tetrahedron Lett. 1995;51:2895–906. [Google Scholar]
- 11.Andersen RJ, van Soest RW, Kong F. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 10. New York: Pergamon; 1996. pp. 301–55. [Google Scholar]
- 12.Borenfreund E, Babich H, Martin-Alguacil N. Rapid chemosensitivity assay with human normal and tumor cells in vitro. In Vitro Cell Dev Biol. 1990;26:1030–4. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed GA, Ibrahim SR, Ross SA. New ceramides and isoflavone from the Egyptian Iris germanica L. rhizomes. Phytochem Lett. 2013;6:340–4. [Google Scholar]
- 14.Ibrahim SR, Abdallah HM, Mohamed GA, Ross SA. Integracides H-J: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia. 2016;112:161–7. doi: 10.1016/j.fitote.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim SR, Elkhayat ES, Mohamed GA, Khedr AI, Fouad MA, Kotb MH, et al. Aspernolides F and G, new butyrolactones from the endophytic fungus Aspergillus terreus. Phytochem Lett. 2015;14:84–90. [Google Scholar]
- 16.Cannell RJ, editor. In: Methods in Biotechnology: Natural Products Isolation. Vol. 4. Totowa New Jersey USA: Humana Press; 1998. How to approach the isolation of a natural product; pp. 1–51. [Google Scholar]
- 17.El-Shazly A, Wink M. Tetrahydroisoquinoline and beta-carboline alkaloids from haloxylon articulatum (Cav.) bunge (Chenopodiaceae) Z Naturforsch C. 2003;58:477–80. doi: 10.1515/znc-2003-7-805. [DOI] [PubMed] [Google Scholar]
- 18.Prinsep MR, Blunt JW, Munro MH. New cytotoxic beta-carboline alkaloids from the marine bryozoan, Cribricellina cribraria. J Nat Prod. 1991;54:1068–76. doi: 10.1021/np50076a023. [DOI] [PubMed] [Google Scholar]
- 19.Megyesi R, Forró E, Fülöp F. Enzymatic strategy for the resolution of new 1-hydroxymethyl tetrahydro-β-carboline derivatives in batch and continuous-flow systems. ChemistryOpen. 2016;5:254–60. doi: 10.1002/open.201500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araźy Z, Czarnocki Z, Wojtasiewicz K, Maurin JK. Enantioselective synthesis of (1R)-1-(hydroxymethyl)-2-acetyl-1,2,3,4-tetrahydro-β-carboline from L-(+)-tartaric acid. Tetrahedron Asymmetry. 2000;11:2793–800. [Google Scholar]


