Abstract
Inspired by biohybrid molecules that are synthesized in Nature through post-translational modification (PTM), we have exploited a eukaryotic PTM to recombinantly synthesize lipid-polypeptide hybrid materials. By co-expressing yeast N-myristoyltransferase with an elastin-like polypeptide (ELP) fused to a short recognition sequence in E. coli, we show robust and high yield modification of the ELP with myristic acid. The ELP’s reversible phase behavior is retained upon myristoylation and can be tuned to span a 30–60 °C. Myristoylated ELPs provide a versatile platform for genetically pre-programming self-assembly into micelles of varied size and shape. Their lipid cores can be loaded with hydrophobic small molecules by passive diffusion. Encapsulated doxorubicin and paclitaxel exhibit cytotoxic effects on 4T1 and PC3-luc cells, respectively, with potencies similar to chemically conjugated counterparts, and longer plasma circulation than free drug upon intravenous injection in mice.
Keywords: Drug delivery, lipidation, myristic acid, post-translational modification, self-assembly
Recombinant biopolymers are used for a diverse array of applications including drug delivery[1] and tissue engineering[2]. Peptide polymers are particularly attractive for biomedical applications because of their biocompatibility, biodegradability, and precisely specified, genetically encoded sequence. However, compared to their synthetic counterparts, peptide polymers are comprised of the limited twenty canonical amino acids, which severely restricts their potential design space. Nature has evolved numerous strategies to diversify the proteome through post-translational modifications (PTMs) such as lipidation and glycosylation.[3] However, these PTMs have been largely unexplored in recombinantly produced polypeptides because the simplest and most established recombinant expression protocols utilize prokaryotes, which lack most PTM machinery. Motivated by this lacuna, we demonstrate herein the high yield, one-pot recombinant synthesis of a lipid-peptide polymer hybrid in a bacterial expression system (Figure 1a). These lipid-peptide biopolymer hybrids self-assemble into tunable nanoscale structures that can be used to physically encapsulate and deliver hydrophobic small molecule anti-cancer drugs such as doxorubicin (DOX) and paclitaxel (PTX) (Figure 1b).
Figure 1.
A bicistronic vector transformed into E. coli is used to co-express two genes, (1) yeast NMT and (2) a recognition sequence (rs) fused to an ELP (a). Myristoylation of ELPs creates an amphiphile that self-assembles into spherical or rod-like micelles (depending upon ELP length) whose cores can be easily loaded with hydrophobic small molecules to form a stimulus-responsive drug carrier (b).
We chose myristoylation because it is a single-enzyme catalyzed PTM that occurs at the N-terminus of a peptide substrate.[4] As a proof-of-concept for hybrid biomaterial synthesis, we selected elastin-like polypeptides (ELPs) as the peptide polymer because: 1) they can be produced at high yields in E. coli, 2) they exhibit a lower critical solution temperature (LCST) phase behavior that can simplify purification, and 3) they have been previously used for drug delivery.[5] ELPs are comprised of repeats of the monomer (VPGXG)n, where the ‘X’ can be any amino acid except proline.[6] Their LCST behavior enables them to reversibly transition from a soluble state to an insoluble coacervate by heating the solution above their transition temperature (Tt).[7] The Tt can be precisely tuned at the genetic level by manipulating the composition of ‘X’ or the number of VPGXG repeats.[8]
ELPs are post-translationally modified in situ with myristic acid via a covalent, amide bond. N-myristoyltransferase (NMT) catalyzes the reaction between the N-terminal amine group of a glycine and the activated thioester of myristoyl-CoA.[9] Successful myristoylation requires three components: 1) the NMT enzyme; 2) a peptide sequence fused to the ELP’s N-terminus that serves as the substrate for enzymatic modification; and 3) exogenous myristic acid added to the culture medium. We chose S. cerevisiae NMT as the enzyme and an 11-amino acid peptide from the natively myristoylated yeast protein, Arf2, as the substrate. This sequence has been used to generate natively myristoylated eukaryotic proteins for structure-function studies.[10] More recently, this strategy was expanded to chemoenzymatically modify heterologous proteins using unnatural NMT substrates for bioconjugation and imaging.[11] However, our report is the first example of using this methodology to recombinantly expand the chemical repertoire of repetitive peptide polymers beyond canonical amino acids with an aim to create stimuli-responsive, nanomaterials with biotechnological utility.
For proof-of-concept studies we selected a hydrophilic ELP, comprised of 120 repeats of VPGXG where X is 90% Ala and 10% Val (ELP90A,120). The 11-amino acid Arf2 recognition sequence (GLYASKLFSNL) was fused at the gene level to the ELP’s N-terminus and co-expressed with yeast NMT using a bicistronic expression vector (pETDuet-1) in BL21(DE3) cells. Myristic acid was added to the culture 10 min prior to inducing protein expression with isopropyl-β-D-thiogalactopyranoside. As a negative control, we expressed ELP90A,120 without the NMT gene, but with myristic acid. Cloning details and expression protocols can be found in Supporting Information (SI).
After expression, myristoylated ELP (M-ELP90A,120) and control (ELP90A,120) were purified using inverse transition cycling (ITC).[5a] Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) shows that the constructs could be efficiently purified with only a few rounds of ITC (Figure S2a–b). We performed reversed-phase high-performance liquid chromatography (RP-HPLC) using a hydrophobic C18 stationary phase to further demonstrate myristoylation and the high degree of purity achieved with ITC. Addition of a hydrophobic myristoyl group is expected to increase the retention time of post-translationally modified ELPs. Co-expression of the target polypeptide with NMT is necessary for lipidation, as seen by the increase in the retention time of M-ELP90A,120 to 17.6 min compared to the ELP90A,120 (grown in the absence of NMT), which elutes at 12.3 min (Figure 2a). Quantification of purified product shows that M-ELP90A,120 can be synthesized at high yield (>40 mg/L of culture), which is about half the yield of ELP90A,120 (Figure S4–5, Table S4).
Figure 2.
Physical characterization of myristoylated ELPs and their LCST phase transition. A shift in RP-HPLC elution time between ELP90A,120 grown with and without NMT (a) shows that the enzyme is necessary for myristoylation. MALDI-MS-TOF shows different experimental molecular weights (MWs) for M-ELP90A,120 versus ELP90A,120 (b), which is further supported by the 210 Da difference in the m/z of their N-terminal fragments after tryptic digestion (c), which corresponds to the MW of the myristoyl group. Myristoylation does not interfere with LCST phase behavior, but does suppress the Tt (d) and reduce its concentration dependence (e). It also drives self-assembly into nanoparticles as seen with dynamic light scattering as an increase in the Rh of M-ELPA,120 compared to the ELP90A,120 control (f).
Successful myristoylation was further verified by matrix-assisted laser desorption ionization–time of flight–mass spectrometry (MALDI-TOF-MS). Experimentally observed mass-to-charge (m/z) ratios for both constructs (Figure 2b) were in excellent agreement with the theoretical molecular weights (SI Table 1). MALDI-MS performed after digestion with trypsin confirmed myristoylation of the N-terminal Gly, as seen by a 210 Da shift between M-ELP90A,120 (849.8 Da) and ELP90A,120 (639.3 Da), which corresponds to the mass added from covalent addition of a myristoyl group (Figure 2c).
We next used UV-vis spectrophotometry to evaluate whether the lipid-ELP hybrid retained the LCST phase behavior of its parent ELP. The absorbance at 350 nm was monitored as the solution temperature was ramped up and then down at a rate of 1 °C/min. M-ELP90A,120, like its unmyristoylated controls, exhibited a reversible LCST phase transition, forming a polypeptide-dense coacervate upon heating that resolubilizes upon cooling (Figure S7). The transition temperature (Tt) is defined as the inflection point of absorbance versus temperature, calculated as the maximum of the curve’s first derivative (Figure 2d, arrow). Lipidation of an ELP suppresses the Tt by ~20°C (Figure 2d), which can be attributed to the covalent attachment of myristic acid, which increases the polypeptide’s overall hydrophobicity and also eliminates the N-terminal charge. We also showed that the recognition sequence has no appreciable impact on Tt in the absence of myristoylation (ELP90A,120 versus (-rs)-ELP90A,120, Figure 2d,e). Myristoylation also reduces the inverse dependence of Tt on concentration; when Tt is plotted versus ELP concentration on a semi-log scale, the slope is nearly flat for M-ELP90A,120, whereas the same plot for the parent ELP shows a steeper inverse log dependence of Tt on concentration (Figure 2e). This lack of concentration dependence is a hallmark of ELP self-assembly.[12]
To characterize the self-assembly of M-ELP90A,120 that is suggested by the turbidity measurements, we next performed light scattering on filtered samples in phosphate buffered saline (PBS) at 25 °C, which is well below the Tt. Dynamic light scattering (DLS) of ELP90A,120 and M-ELP90A,120 confirms that myristoylation drives self-assembly into nanoparticles with a hydrodynamic radius (Rh) of ~22 nm (Figure 2f). In contrast, ELP90A,120 has an Rh of 6.2 nm, a size that is consistent with soluble polymer chains in a Gaussian coil conformation.[13]
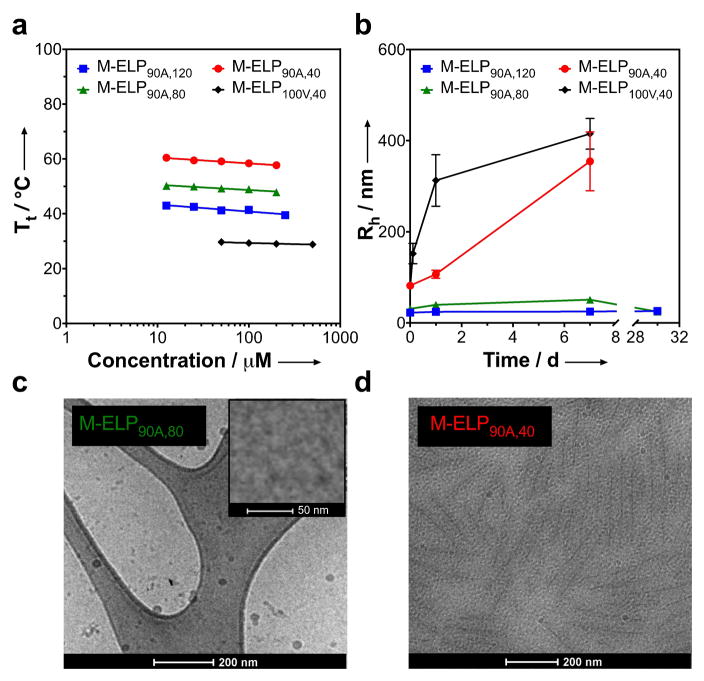
Having confirmed that we can successfully create recombinant self-assembling lipid-ELP biomaterials in one-pot, we next hypothesized that the length and hydrophilicity of the ELP could be used to control the morphology of self-assembly. We took advantage of our modular design (which allows the ELP domain to be precisely specified independently of the recognition sequence) and created three additional constructs to test this hypothesis. First, by keeping the composition constant, we systematically decreased the ELP length from 120 to 80 (M-ELP90A,80) and 40 pentapeptide repeats (M-ELP90A,40). We also synthesized a more hydrophobic construct with 40 repeats of VPGXG where X is 100% Val (M-ELP100V,40) to explore the impact of ELP composition on self-assembly. This set of ELPs was expressed in the same manner as previously described. Detailed information on the sequence, expression, and characterization of these constructs can be found in SI. Their N-terminal myristoylation, size and purity were confirmed with SDS-PAGE (Figure S2c) and MALDI-MS (Figure S3). Characterization of their LCST phase behavior showed that their Tt’s spanned 28.8 to 60.4 °C at concentrations ranging from 12.5 to 500 μM, indicating that, even within this small set of ELPs, we are able to access a wide range of LCST phase behavior (Figure 3a and Figure S8) that may be useful for biomedical applications including formation of a controlled release depot[14], intratumoral delivery,[15] or as a scaffold for tissue regeneration.[2]
Figure 3.
By varying the length and composition of the ELP, M-ELPs can be synthesized that exhibit phase transition temperatures spanning a 30 °C range (a). The ELP affects the size and kinetic stability of the self-assembled particle as seen by the Rh as a function of time (b). Cryo-TEM of self-assembled myristoylated-ELPs confirms the different size and shape of nanoparticles inferred from light scattering: spheres (c) and rods (d).
We next investigated the self-assembly of all four myristoylated constructs using DLS and static light scattering (SLS) as well as electron microscopy to determine the role of ELP length and composition in self-assembly. The critical aggregation concentration for all constructs, quantified by a pyrene assay, is in the 2–6 μM range (Figure S16). After passing freshly reconstituted lyophilized sample through a 0.22 μm filter we observed that the size of the self-assembled nanoparticles is inversely related to ELP length (Table 1); as the number of ELP repeats increased from 40 to 120, the Rh of the self-assembled M-ELPs decreased from 81.9 ± 2.9 nm to 22.7 ± 0.6 nm. In contrast, all non-myristoylated controls were unimers in solution, as evidenced by their small Rh (Figure S10).
Table 1.
Summary of light scattering characterization of M-ELPs in PBS.
| M-ELP90A,120 | M-ELP90A,80 | M-ELP90A,40 | M-ELP100V,40 | |
|---|---|---|---|---|
| Shape Factor, ρ Rg/Rh | 0.98 (± 0.03) | 1.00 (± 0.07) | 1.29 (± 0.02) | 1.30 (± 0.13) |
| Second Virial Coefficient A2 / mol dm3 g−2 | −9.23 x 10−9 (± 25.2%) | −5.58 x 10−9 (± 18.0%) | −2.30 x 10−8 (± 33.9%) | −5.10 x 109 (± 13.2%) |
| Refractive Index Increment dn/dc / cm3 g−1 | 0.1515 (± 0.0038) | 0.1660 (± 0.0010) | 0.1669 (± 0.0067) | 0.1258 (± 0.0069) |
| Aggregation Number, Nagga MWparticle/MWunimer | 29 | 48 | 142 / 1075b | 610 / 6261b |
These values were calculated using partial Zimm plots.
These valuse represent Nagg as measured after 28 days at room temperature, once the unstable 40-mer micelles have matured.
The 40-mers have a larger shape factor (ρ = Rg/Rh) and a much larger aggregation number (Nagg = MWagg/MWunimer) (Table 1), indicating that they self-assemble into a non-spherical shape. The ELP’s composition, although a very important determinant of phase behavior (Figure 3a), does not significantly impact self-assembly, as M-ELP90A,40 and M-ELP100V,40 micelles have similar structural parameters. For all constructs, the second virial coefficient (A2), a global thermodynamic measure of protein-protein interaction, was slightly negative, demonstrating an overall weak attractive interaction between the assembled particles in PBS. However, consistent with previous reports of ELP micelles, the magnitude of A2 for all constructs was very small and can safely be neglected in calculating Nagg.[16]
Interestingly, the length of the ELP also affects the kinetics of self-assembly. The larger ELPs with 80 and 120 pentapeptide repeats maintained their Rh of ~25 nm, even when left for up to a month at room temperature (Figure 3b), evidence that these are thermodynamically stable assemblies. In contrast the smaller 40-mer ELPs grew from an Rh of ~80 nm at the time of preparation to ~400 nm after one week, indicating that the morphology of these constructs evolves over time. To visualize these structures, we resuspended lyophilized material in PBS, filtered the samples, and performed cryo-transmission electron microscopy (cryo-TEM). Cryo-TEM images show that the larger MW ELPs (M-ELP90A,120 and M-ELP90A80) form spherical micelles (Figure 3c, Figure S17a) while the 40-mer ELPs assemble into rod-like micelles (Figure 3d, Figure 17b). These morphologies are consistent with the DLS and SLS results (Table 1).
A number of amphiphilic diblock ELPs with programmable self-assembly have been previously synthesized.[12,16.17] However, unlike M-ELPs, these canonical protein-based assemblies are not capable of efficient physical encapsulation of hydrophobic compounds like DOX in their protein cores, due to the high water content of ELP coacervates (Figure S20–S21). In contrast, we hypothesized that the fatty acid core of these hybrid micelles could be used to physically encapsulate hydrophobic small molecules. Because of their stability, we first selected M-ELP90A,120 and M-ELP90A,80 to encapsulate hydrophobic anti-cancer chemotherapeutics, DOX and PTX. M-ELPs were stirred overnight in a solution with excess DOX-HCl or PTX, and were then separated from free drug by ultrafiltration. Drug encapsulation did not significantly impact the size of the micelles (Figure S15). For encapsulation protocols and characterization of drug-loaded micelles, see SI Section 11.
Traditional diblock ELPs have a DOX loading capacity of < 0.7% (Figure S21). In contrast, the loading capacity of DOX in M-ELPs is 1–3% and the encapsulation efficiency is 3–5% (Figure S20). The loading capacity of these nanoparticles is slightly lower than that of liposomal formulations like Doxil® (12.5%)[18a], which is a reflection of the small, compact size of the M-ELP’s fatty acid core. Doxil® is able to achieve high drug concentrations through “remote loading” via transmembrane ion gradients[18a]. In comparison, our passive loading procedure has a lower encapsulation efficiency, but any unencapsulated drug could be easily recycled due to the gentle encapsulation process. An in vitro study of DOX encapsulated in M-ELP90A,80 and dialyzed against PBS demonstrated the formulation’s remarkable stability, retaining 50% of the drug even after a week at 37 °C, with only ~13% leaching out over the first 24 h (Figure S28).
Next, the cytotoxicity of the encapsulated drugs was compared to their free drug counterparts by an in vitro cell proliferation assay that uses the reduction of tetrazolium by metabolically active cells to determine cell viability.[19] Encapsulated DOX (DOXEnc) had a 50% inhibitory concentration (IC50) that is 4-fold higher than free DOX (DOXFree) in 4T1 cells (Figure 4a), a murine model of mammary carcinoma.[20] This loss in efficacy is an acceptable trade-off given the delivery benefits of nanoparticle formulations, as they enable higher maximal tolerated doses to be administered in vivo, have a longer circulating half-life, and accumulate to a greater extent in tumors than free drug.[21] Furthermore, this IC50 is almost identical to that of a nanoparticle formulation of DOX that uses chemical conjugation to cysteine-containing ELPs, which is a significantly more laborious and expensive process.[22] DOX was also loaded into equilibrated 40-mers. Interestingly, these rod-shaped carriers were much less cytotoxic (Figure S24a). This is consistent with reports in the literature showing that the nanocarrier’s shape can have a significant impact on cellular uptake.[23] Empty carriers did not exhibit any cytotoxicity (Figure S24b and S25).
Figure 4.
DOX and PTX encapsulated in either M-ELP90A,120 or M-ELP90A,80 show in vitro cytotoxicity towards 4T1 (a) and PC3 (b) cells. Confocal fluorescence microscopy of 4T1 cells treated for 12 h with DOXFree (c) or DOXEnc (d) shows uptake of DOXEnc, via the endosomal/lysosomal pathway, as indicated by punctate fluorescence (green arrow). Fluorescence is colored as: nucleus (blue), cell membrane (red), and DOX (green). DOXEnc has a longer plasma half-life (e) and greater distribution than DOXFree, shown by the 5-fold lower plasma concentrations 45 s post-injection (f).
PTX encapsulated into M-ELP90A,80 and M-ELP90A,120 had an IC50 of ~40 nM (20-fold higher than free PTX) in PC3-luc cells (Figure 4b), a human prostate cancer cell line. This larger difference compared to free drug could be attributed to PTX’s greater hydrophobicity than DOX. PTX has a reported octanol-water distribution coefficient (logDpH 7.5) between 3.0 and 4.0[24] which may slow its diffusion out of the lipid-ELP nanoparticles relative to the less hydrophobic DOX (logDpH 7.5 of 2.4).[25]
Confocal fluorescence microscopy of 4T1 cells treated for 12 h with free or encapsulated DOX indicates that the DOXEnc is being taken up through the endosomal/lysosomal pathway, as indicated by punctate fluorescence throughout the cell (Figure 4d, green arrow), which is less apparent in 4T1 cells treated with DOXFree (Figure 4c, Figure S26). Preliminary studies indicate that a greater amount of DOX is released from the micelles as the pH is lowered from 7.5 to 4.5 (Figure S30) —the pH in late endosomes and lysosomes[26]. We selected DOX for these studies because it is an ideal model drug due to its fluorescence and the extensive literature on its delivery with other carriers.[27] We selected M-ELP90A,80 because of its higher loading capacity. To further demonstrate the utility of encapsulating drugs in these hybrid materials, we conducted an in vivo pharmacokinetics (PK) study with DOX. Compared to DOXFree, DOXEnc in M-ELP90A,80 increased the drug’s elimination half-life (t1/2) by 6.5-fold (Figure 4e) and significantly altered its distribution, as seen by the 5-fold lower plasma concentrations than free DOX just 45 s after intravenous injection (Figure 4f).
In summary, we have successfully shown that we can recombinantly produce a range of hybrid lipid-ELP materials with molecular precision and high yield. While post-expression chemical modification of ELPs has been demonstrated[28] and solid-phase peptide synthesis (SPPS) has been used to synthesize short lipidated ELPs (length <20 residues)[29], chemical synthesis of the size of biomacromolecule shown here is not possible by SPPS. In contrast, the recombinant PTM methodology demonstrated here expands the size, complexity, and yield of lipidated peptide polymers, opening the door to a host of potential new materials and material properties not previously accessible. Furthermore, M-ELPs provide a biological complement to the exciting field of lipidated synthetic polymers and could be similarly used in combination with liposomes to create promising new drug carriers.[30]
We have demonstrated the robustness and versatility of this system, where a range of ELP lengths and compositions can be used to make M-ELPs with tunable Tt that self-assemble into micelles with pre-programmable size, shape, and stability. Spherical M-ELP micelles can be easily synthesized recombinantly and then loaded with hydrophobic small molecules simply by preferential partitioning of the drug into the lipid core. We showed that this encapsulation method is effective at enhancing the drug’s t1/2. Like Doxil®, these sub-100 nm micelles should enable passive targeting of solid tumors via the enhanced permeability and retention effect.[21]
Although Doxil® has many attractive features and is approved to treat a variety of cancers[18a], it —and the class of liposomal carriers to which it belongs— have significant issues. Some disadvantages of liposomes include scale-up, manufacturing, and stability, especially when including components that add functionality such as stealth coating or active targeting.[18b] Because our delivery vehicle is manufactured recombinantly, the M-ELP micelle’s corona could easily be functionalized for active targeted delivery by fusing a peptide or protein to the C-terminus of the ELP. Doxil® can also have severe side effects including “foot and hand syndrome” and complement activation-related pseudo-allergy (CARPA).[18] Thus, these drawbacks argue for the development of alternative nanoscale carriers like M-ELPs for the delivery of chemotherapeutics.
While DOX was selected for proof-of-principle studies, these methods could be easily applied to any hydrophobic small molecule and would be particularly useful for those that have no chemical handle for conjugation or that lose activity upon conjugation, such as camptothecin, Nutlin-3, and WP1066. Finally, recombinant myristoylation for drug delivery is not limited to ELPs. This methodology could be applied to other peptide polymers such as collagen and resilin-like polypeptides. In conclusion, this work lays the foundation for a novel class of hybrid material by recombinant lipidation of peptide polymers that can be used for diverse applications.
Experimental Section
Experimental details including sequence of proteins, expression, purification, and characterization are included in the supporting information.
Supplementary Material
Acknowledgments
The authors would like to thank the Katherine J Franz Lab in the Department of Chemistry at Duke University for allowing us use of their RP-HPLC system as well as Cedric Bouchet-Marquis at Thermo Fisher Scientific for cryo-TEM imaging of the 40-mer constructs. This work was supported by the NSF through the Research Triangle MRSEC (DMR-1121107 and by the NIH through grants R01 GM-61232 and R01 EB-00188 to A.C. K.M.L. acknowledges the support of an NSF graduate research fellowship.
References
- 1.McDaniel JR, Callahan DJ, Chilkoti A. A Drug Deliv Rev. 2010;62:1456–1467. doi: 10.1016/j.addr.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McHale MK, Setton LA, Chilkoti A. Tissue Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 3.Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr Angew Chem Int Ed. 2005;117:7508–7539. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 4.Maurer-Stroh S, Eisenhaber B, Eisenhaber F. J Mol Biol. 2002;317:523–540. doi: 10.1006/jmbi.2002.5425. [DOI] [PubMed] [Google Scholar]
- 5.a) Meyer DE, Chilkoti A. Nat Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]; b) MacEwan SR, Chilkoti A. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]; c) Chilkoti A, Dreher MR, Meyer DE. Adv Drug Deliv Rev. 2002;54:1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 6.Urry DW. J of Phys Chem B. 1997;101:11007–11028. [Google Scholar]
- 7.Cho Y, Zhang Y, Christensen T, Sagle LB, Chilkoti A, Cremer PS. J Phys Chem B. 2008;112:13765–13771. doi: 10.1021/jp8062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, Safavy A. J of the Am Chem Soc. 1991;113:4346–4348. [Google Scholar]; b) Meyer DE, Chilkoti A. Biomacromolecules. 2004;5:846–851. doi: 10.1021/bm034215n. [DOI] [PubMed] [Google Scholar]
- 9.Farazi TA, Waksman G, Gordon JI. Biochemistry. 2001;40:6335–6343. doi: 10.1021/bi0101401. [DOI] [PubMed] [Google Scholar]
- 10.a) Gluck JM, Hoffmann S, Koenig BW, Willbold D. PLoS One. 2010;5:e10081. doi: 10.1371/journal.pone.0010081. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Senin II, Zargarov AA, Alekseev AM, Gorodovikova EN, Lipkin VM, Philippov PP. Febs Lett. 1995;376:87–90. doi: 10.1016/0014-5793(95)01187-2. [DOI] [PubMed] [Google Scholar]; c) Duronio RJ, Jacksonmachelski E, Heuckeroth RO, Olins PO, Devine CS, Yonemoto W, Slice LW, Taylor SS, Gordon JI. P of the Natl Acad of Sci of the USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Linder ME, Pang IH, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]; e) Duronio RJ, Jackson-Machelski E, Heuckeroth RO, Olins PO, Devine CS, Yonemoto W, Slice LW, Taylor SS, Gordon JI. Proc Natl Acad Sci USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Heal WP, Wickramasinghe SR, Leatherbarrow RJ, Tate EW. Org Biomol Chem. 2008;6:2308–2315. doi: 10.1039/b803258k. [DOI] [PubMed] [Google Scholar]; b) Ho SH, Tirrell DA. J Am Chem Soc. 2016;138:15098–15101. doi: 10.1021/jacs.6b07067. [DOI] [PubMed] [Google Scholar]; c) Kulkarni C, Lo M, Fraseur JG, Tirrell DA, Kinzer-Ursem TL. Bioconjug Chem. 2015;26:2153–2160. doi: 10.1021/acs.bioconjchem.5b00449. [DOI] [PubMed] [Google Scholar]
- 12.McDaniel JR, Weitzhandler I, Prevost S, Vargo KB, Appavou MS, Hammer DA, Gradzielski M, Chilkoti A. Nano Lett. 2014;14:6590–6598. doi: 10.1021/nl503221p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Armstrong JK, Wenby RB, Meiselman HJ, Fisher TC. Biophys J. 2004;87:4259–4270. doi: 10.1529/biophysj.104.047746. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fluegel S, Fischer K, McDaniel JR, Chilkoti A, Schmidt M. Biomacromolecules. 2010;11:3216–3218. doi: 10.1021/bm100965y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. J Control Release. 2013;172:144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Luginbuhl KM, Schaal JL, Umstead B, Mastria EM, Li X, Banskota S, Arnold S, Feinglos M, D’Alessio D, Chilkoti A. Nat Biomed End. 2017;1:0078. doi: 10.1038/s41551-017-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Liu WG, MacKay JA, Dreher MR, Chen MN, McDaniel JR, Simnick AJ, Callahan DJ, Zalutsky MR, Chilkoti A. J Control Release. 2010;144:2–9. doi: 10.1016/j.jconrel.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Cancer Res. 2001;61:1548–1554. [PubMed] [Google Scholar]
- 16.Hassouneh W, Zhulina EB, Chilkoti A, Rubinstein M. Macromolecules. 2015;48:4183–4195. doi: 10.1021/acs.macromol.5b00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) MacEwan SR, Weitzhandler I, Hoffmann I, Genzer J, Gradzielski M, Chilkoti A. Biomacromolecules. 2017;18:599–609. doi: 10.1021/acs.biomac.6b01759. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hassouneh W, Fischer K, MacEwan SR, Branscheid R, Fu CL, Liu R, Schmidt M, Chilkoti A. Biomacromolecules. 2012;13:1598–1605. doi: 10.1021/bm300321n. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Garanger E, MacEwan SR, Sandre O, Brûlet A, Bataille L, Chilkoti A, Lecommandoux S. Macromolecules. 2015;48:6617–6627. [Google Scholar]
- 18.a) Barenholz Y. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]; b) Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 20.Pulaski BA, Ostrand-Rosenberg S. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):22. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 21.a) Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]; b) Cho K, Wang X, Nie S, Chen ZG, Shin DM. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 22.Mastria EM, Chen M, McDaniel JR, Li X, Hyun J, Dewhirst MW, Chilkoti A. J Control Release. 2015;208:52–58. doi: 10.1016/j.jconrel.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Arnida M, Janat-Amsbury M, Ray A, Peterson CM, Ghandehari H. Eur J of Pharm and Biopharm. 2011;77:417–423. doi: 10.1016/j.ejpb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang K, Fang HF, Chen ZY, Taylor JSA, Wooley KL. Bioconjugate Chem. 2008;19:1880–1887. doi: 10.1021/bc800160b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 24.a) Turunen BJ, Ge HB, Oyetunji J, Desino KE, Vasandani V, Guthe S, Himes RH, Audus KL, Seelig A, Georg GI. Bioorg Med Chem Lett. 2008;18:5971–5974. doi: 10.1016/j.bmcl.2008.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xavier FH, Gueutin C, Morais ARD, Alencar ED, do Egito EST, Vauthier C. Chromatographia. 2016;79:405–412. [Google Scholar]
- 25.Rivory LP, Avent KM, Pond SM. Cancer Chemother Pharmacol. 1996;38:439–445. doi: 10.1007/s002800050508. [DOI] [PubMed] [Google Scholar]
- 26.Sorkin A, Von Zastrow M. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 27.a) Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, Chilkoti A. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]; b) Dreis S, Rothweiler F, Michaelis M, Cinatl J, Jr, Kreuter J, Langer K. Int J Pharm. 2007;341:207–214. doi: 10.1016/j.ijpharm.2007.03.036. [DOI] [PubMed] [Google Scholar]; c) Du JZ, Du XJ, Mao CQ, Wang J. J Am Chem Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]; d) Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. J Control Release. 2001;73:255–267. doi: 10.1016/s0168-3659(01)00294-2. [DOI] [PubMed] [Google Scholar]; e) MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Yoo HS, Oh JE, Lee KH, Park TG. Pharm Res. 1999;16:1114–1118. doi: 10.1023/a:1018908421434. [DOI] [PubMed] [Google Scholar]
- 28.a) Petitdemange R, Garanger E, Bataille L, Bathany K, Garbay B, Deming TJ, Lecommandoux S. Bioconjug Chem. 2017;28:1403–1412. doi: 10.1021/acs.bioconjchem.7b00082. [DOI] [PubMed] [Google Scholar]; b) Kramer JR, Petitdemange R, Bataille L, Bathany K, Wirotius AL, Garbay B, Deming TJ, Garanger E, Lecommandoux S. ACS Macro Lett. 2015;4:1283–1286. doi: 10.1021/acsmacrolett.5b00651. [DOI] [PubMed] [Google Scholar]
- 29.Aluri S, Pastuszka MK, Moses AS, MacKay JA. Biomacromolecules. 2012;13:2645–2654. doi: 10.1021/bm300472y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.a) Le Meins JF, Schatz C, Lecommandoux S, Sandre O. Mater Today. 2013;16:397–402. [Google Scholar]; b) Liu Z, Zhang Z, Zhou C, Jiao Y. Prog in Polym Sci. 2010;35:1144–1162. [Google Scholar]; c) Lukyanov AN, Torchilin VP. Adv Drug Deliver Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.