Abstract
Aim
Pulmonary arterial hypertension (PAH), a deadly disorder is associated with excessive growth of human pulmonary artery endothelial (HPAECs) and smooth muscle (HPASMCs) cells. Current therapies primarily aim at promoting vasodilation, which only ameliorates clinical symptoms without a cure. 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) is an endogenous aryl hydrocarbon receptor (AhR) ligand, and mediates many cellular function including cell growth. However, the roles of ITE in human lung endothelial cells remain elusive. Herein, we tested a hypothesis that ITE inhibits growth of human pulmonary artery endothelial cells via AhR.
Materials and Methods
Immunohistochemistry was performed to localize AhR expression in human lung tissues. The crystal violet method and MTT assay were used to determine ITE’s effects on growth of HPAECs. The AhR activation in HPAECs was confirmed using Western blotting and RT-qPCR. The role of AhR in ITE-affected proliferation of HPAECs was assessed using siRNA knockdown method followed by the crystal violet method.
Results
Immunohistochemistry revealed that AhR was present in human lung tissues, primarily in endothelial and smooth muscle cells of pulmonary veins and arteries, as well as in bronchial and alveolar sac epithelia. We also found that ITE dose- and time-dependently inhibited proliferation of HPAECs with a maximum inhibition of 83% at 20 µM after 6 days of treatment. ITE rapidly decreased AhR protein levels, while it increased mRNA levels of cytochrome P450 (CYP), family 1, member A1 (CYP1A1) and B1 (CYP1B1), indicating activation of the AhR/CYP1A1 and AhR/CYP1B1 pathways in HPAECs. The AhR siRNA significantly suppressed AhR protein expression, whereas it did not significantly alter ITE-inhibited growth of HPAECs.
Conclusions
ITE suppresses growth of HPAECs independent of AhR, suggesting that ITE may play an important role in preventing excessive growth of lung endothelial cells.
Keywords: ITE, Aryl hydrocarbon receptor, Growth, Pulmonary artery endothelial cells
Introduction
Pulmonary arterial hypertension (PAH) is a deadly disorder, which is caused idiopathically or is associated with congenital heart disease and chronic obstructive pulmonary disease.[1,2] However, regardless of the cause, PAH can be characterized by progressive obliteration of small pulmonary arteries, primarily due to aberrant proliferation of human pulmonary artery endothelial (HPAECs) and smooth muscle (HPASMCs) cells, impaired vasodilation of pulmonary arteries, and in situ thrombosis, ultimately leading to right heart failure.[3,4] Current therapies aiming at promoting vasodilation can only ameliorate clinical symptoms and haemodynamics of PAH for a limited period of time without resulting in a cure.[5,6] Thus, given aberrant proliferation of HPAECs plays an important role in PAH, identifying pharmacological inhibitors for excessive proliferation of HPAECs might provide an alternative therapy for treating PAH.
The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor is well-known for its roles in mediating metabolism of environmental toxicants such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).[3–8] In addition, AhR also actively participates in mediating many essential cellular function (i.e., cell proliferation, migration, differentiation, and apoptosis),[9–14] indicating physiological importance of AhR in these essential biological processes. This notion is supported by the discovery of many endogenous AhR ligands in humans and animals. [15,16] One of endogenous AhR ligand is 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) which is likely derived from tryptophan and cysteine via a condensation reaction[7] was first identified in porcine lung[17], and was recently found to be present in human cancer cells.[18] The physiological levels of ITE have not been reported in any tissue and its roles in human lung are unknown. However, unlike TCDD, ITE has minimum side-effects on adult mice and also does not cause typical TCDD-induced toxicity in the fetus,[19,20] whom is more sensitive to TCDD than the adult.[21] Thus, ITE may be used as a therapeutic agent for AhR-related diseases as suggested.[17]
Upon binding to its ligands, AhR is capable of activating an array of downstream genes to initiate cellular function.[7,8] Enzyme cytochrome P450 (CYP), family 1, member A1 and B1 (CYP1A1 and CYP1B1) are two major target genes of AhR.[7,8] Once activation, AhR is rapidly degraded by the 26S proteasome system.[8] Therefore, after stimulation with AhR ligands, decreases in AhR protein levels and increases in CYP1A1 and CYP1B1 expression in cells generally indicate activation of AhR.
AhR has been reported to express in human pulmonary artery smooth muscle cells (HPASMCs) in vitro[11] and in human fetal lung tissues,[22] and is associated with Baicalin-inhibited proliferation of HPASMCs.[11] However, it is unknown if the ITE/AhR pathway regulates growth of HPAECs. Herein, we determined AhR expression in human lung tissues. We also tested a hypothesis that ITE inhibits growth of HPAECs via AhR in association with decreases in cell cycle progress and increases in cell apoptosis.
Materials and methods
Immunohistochemistry
Two human lung tissue microarrays were purchased from the US Biomax (cat #, LCN214, Rockville, MD). Each of the tissue microarray contained 24 cases of normal human lung tissues. Immunolocalization of AhR was visualized by indirect detection via the avidin: biotinylated-peroxidase complex method (Vector Laboratories, Burlingame, CA) as described.[23–25] Briefly, after deparaffinized and dehydrated, the microarray was boiled in a 10 mM citrate buffer solution (pH 6.0) in a microwave for 10 min for antigen retrieval. Endogenous peroxidase activity was quenched using 3% H2O2. These two tissue microarrays were run in parallel: one was probed with a rabbit AhR antibody (4 µg/mL; Thermo Fisher Scientific, Pittsburgh, PA), and another was probed with pre-immune rabbit IgG (4 µg/mL; as a negative control) for 1 hr. In the preliminary study, the similar AhR immunostaining pattern was also observed using another rabbit AhR antibody (4 µg/mL; Biomol International, Plymouth, PA). After washing in PBS containing 0.3% Triton-X100, the tissue microarrays were incubated with a biotinylated horse anti-rabbit antibody (Vector Laboratories) for 1 hr. The immunoreactivity was visualized by 3-amino-9-ethylcarbazole (Vector Laboratories). The tissue sections were counter-stained with Harris Modified Hematoxylin (Thermo Fisher Scientific).
Culture of HPAECs
Human pulmonary artery endothelial cells (Scien-Cell Research Laboratories; Carlsbad, CA) were cultured in Endothelial Cell Medium (ECM, a complete growth medium), which consists of ECM-basal (ECM-b, a basal medium without any serum; ScienCell Research Laboratories) supplemented with 5% fetal bovine serum, 1% endothelial cell growth supplement, and 1% penicillin/streptomycin.
Cell growth assays
The crystal violet method was first used to assess cell growth as we described.[10,12,14] Cells were seeded in 96-well plates (4000 cells/well). After 16 hr (Day 0) of culture in ECM, cells were serum starved in ECM-b for 24 hr. Cells were then treated with ITE at 0.01, 0.1, 1, 5, 10 and 20 µM (Tocris Bioscience, San Diego, CA) or dimethyl sulfoxide (DMSO; 0.1% v/v; the vehicle control) in ECM for 2, 4, or 6 days with a change of ECM containing ITE or DMSO every other day (5 wells/treatment). After treatment, cells were rinsed with PBS, fixed in methanol, air-dried, and stained with 0.1% (w/v) crystal violet. After staining, wells were rinsed with distilled water, and air-dried again. Once dried, cells were solubilized with 2% (w/v) sodium deoxycholate solution. Absorbance was measured at 570 nm on a Synergy HT Multi-Detection Microplate Reader (Bio-Tek Instruments, Winooski, Vermont). Wells containing known cell numbers (1250 to 40000 cells/well, 5 wells/cell density) were used to establish standard curves.
To confirm ITE’s effects on cell growth, additional cells were treated with 0.1, 1, 10, and 100 nM of TCDD (an exogenous AhR ligand; Cambridge Isotope Laboratories, Tewksbury, MA) or the vehicle (DMSO, 0.1% v/v) in ECM up to 6 days with a change of ECM containing TCDD or DMSO every other day (5 wells/treatment), followed by the crystal violet method as described.[10,12,14]
To further confirm ITE’s effects on cell growth, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT) assay was also run using the MTT kit (Cayman Chemical Company, Ann Arbor, MI).[12] Subconfluent cells (25, 000 cells/well) were seeded in 96-well plates. Cells were treated with ITE at 5, 10 and 20 µM or DMSO (0.1% v/v) in ECM for 2, 4 or 6 days with a change of ECM containing DMSO or ITE every other day (5 wells/treatment). At the end of treatment, cells were incubated with MTT reagent for 4 hr, and solubilized in crystal dissolving solution (100 µl/well) for 20 min. The absorbance was determined at 570 nm using the microplate reader (Synergy HT Multi-Detection Microplate Reader).
Cell cycle process and apoptosis assays
To examine ITE’s effects on the cell cycle process, the flow cytometry analysis was conducted as described.[14] After serum-starved in ECM-b for 24 hr, subconfluent cells were treated with ITE or DMSO in ECM for 4 days. After staining with propidium iodide (PI; 0.033 mg/ml) (cat # 195458, MP Biomedicals, Santa Ana, CA), cells were analyzed at a flow cytometer (BD FACSCalibur, Becton Dickinson, San Jose, CA).
To determine if ITE increases cell apoptosis, Western blotting[14] was performed to examine the formation of cleaved-caspase-3 proteins (indicative of apoptosis)[27] as described below.
Western blot analysis
Western blotting was conducted as described.[10,12,14] Briefly, subconfluent cells were treated with a single dose of ITE (20 µM) in ECM up to 48 hr. Proteins (20 µg for AhR and 30 µg for the cleaved caspase-3) were subjected to Western blotting. The membranes were probed with the rabbit anti-AhR antibody (1: 1000; ENZO Life Science) or the rabbit anti-cleaved caspase-3 antibody (1:500; Cell Signaling Technology), followed by reprobing with a mouse GAPDH antibody (1:20,000; Research Diagnostics, Concord, MA) or rabbit total caspase-3 (1:1000; Cell Signaling Technology). Proteins were visualized using the enhanced chemiluminescence reagent (Thermo Scientific), followed by exposure to chemiluminescence films. The immunoreactive signals were analyzed by densitometry using NIH Image-J imaging analysis software.
Real-time-quantitative PCR (RT-qPCR)
To determine the effect of ITE on expression of CYP1A1 and CYP1B1 mRNA, RT-qPCR was performed.[10,12,14] Subconfluent cells were treated with a single dose of ITE (20 µM) in ECM for 48, 24, 16, 8, 1, or 0 hr. Total RNA samples were extracted using Rneasy Plus Mini Kit (QIAGEN) and quantified using a spectrophotometer. The total RNA samples from each treatment (1 µg) were reverse transcribed to cDNA. The reverse transcription was carried out using the High Capacity cDNA Reverse transcription Kit (Applied Biosystems, Foster City, CA) for 10 min at 25 C, 120 min at 37°C, 5 min at 85°C in 20 µl reaction volume. RT-qPCR was performed using SYBR Premix Ex Taq (TaKaRa) according to the manufacturer’s instruction. The primer sequences were as follows: CYP1A1 (Sense: 5′-CACAGCACAACAAGAGACACAA–3′; Antisense: 5′–TAGCCAGGAAGAGAAAGACCTC-3′), CYP1B1 (Sense: 5′-CGGCCACTATCACTGACATC-3′; Anti-sense: 5′-CTCGAGTCTGCACATCAGGA-3′), GA PDH (Sense:5′-GCACCGTCAAGGCTGAGAAC-3′; Antisense:5′-TGGTGAAGACGCCAGTGGA-3′), β-actin (Sense:5′-CATTCCAAATATGAGATGCATTG-3′;Antisense:5′-TGCTATCACCTCCCCTGTGT-3′), and TATA BOX (Sense:5′-CATACCGTGCTGCTA TCTGG-3′; Antisense: 5′-TCCCTCAAACCAACTT GTCA-3′). All primers were synthesized at Gene Biotechnology Centre, University of Wisconsin-Madison, Madison, WI.
RT-qPCR reaction was carried out at 30 s at 95°C, and then 15 s at 95°C and 20 s at 56°C for 40 cycles. To confirm the amplification specificity, the PCR products were subjected to a melting curve analysis. Levels of mRNA were analyzed using the 2−ΔΔCT method.
Small interfering RNA (siRNA) transfection
To determine the effect of AhR on ITE-regulated growth of HPAECs, AhR was knocked down using specific human AhR siRNA (Dharmacon, Chicago, IL) as described. [10,12,14] The scrambled siRNA (siRNA controls) with 5′-Cy3 were synthesized by IDT, Coralville, IA: Sense: 5′-AGUUUGACCUGCUCUCCAUTT-3′; Antisense: 3′-TTUCAAACUGGACGAGAGGUA-5′). Subconfluent cells were transfected with the AhR siRNA or scrambled siRNA in the Lipofectamine RNAiMAX transfection reagent (Invitrogen) or treated with the transfection reagent alone (the vehicle control) for 2 and 4 days. After an optimal time was identified for AhR knockdown, additional cells were transfected and treated with ITE, followed by the crystal violet assay.
Statistics
All data were analyzed using the SigmaPlot software (Systat Software, Inc., San Jose, CA). Normality of distribution was tested by Shapiro-Wilk test with α = 0.05. Data sets containing multiple groups were analyzed by ANOVA followed by pairwise comparisons or comparisons verse the respective control using the Holm-Sidak method; Comparisons between two groups were performed with unpaired Student’s t-test. p < 0.05 was considered statistically significant.
Results
Immunolocalization of AhR in human lungs
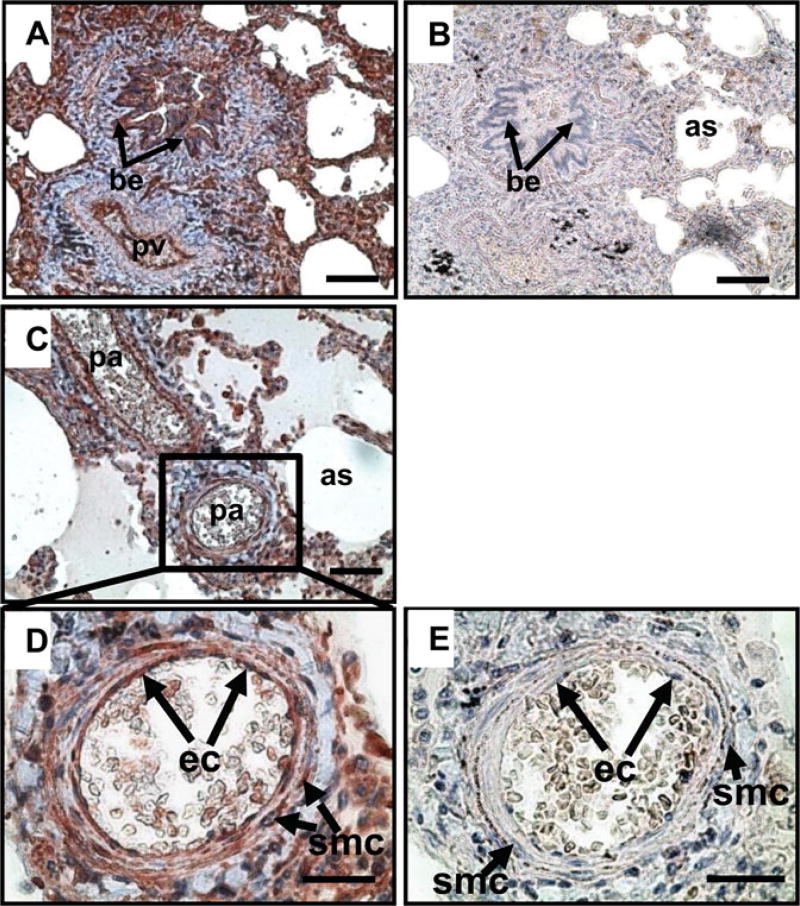
The AhR immunoreactivity was localized in all cases of human lung tissues, primarily in endothelial and smooth muscle cells of pulmonary veins (Fig. 1A) and arteries (Fig. 1C, D) as well as bronchial epithelium, alveolar sac epithelium, and stromal cells (Fig. 1A). No positive staining was observed in the preimmune rabbit IgG control (Fig. 1B, E).
Figure 1.
Immunolocalization of AhR in human lung tissues. A, C, D): The lung tissue microarray was probed with a rabbit antibody against AhR. B, E): The lung tissue microarray was probed with rabbit preimmune IgG, serving as a negative control. Reddish color indicates positive AhR staining. Representative images are shown. be: bronchial epithelium; pv: pulmonary vein; pa: pulmonary artery; as: alveolar sac; ec: endothelial cells. Bars in A-C: 80 µm; Bars in D,E: 40 µm.
ITE inhibits growth of HPAECs
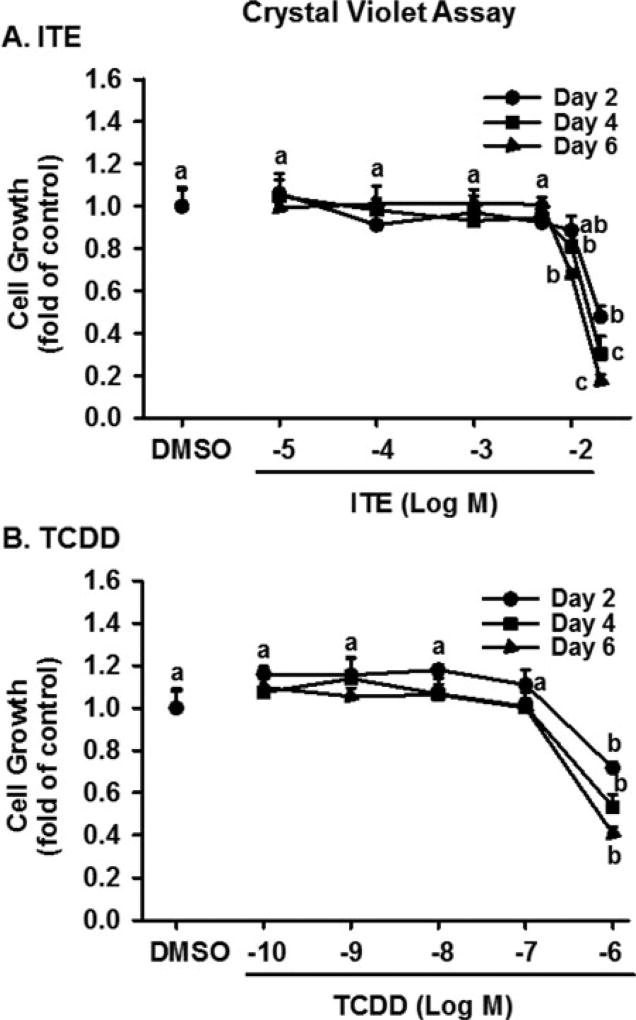
Significant effects of ITE dose, treatment day, but not their interaction on cell growth were observed. As compared with the vehicle control, ITE dose- and time-dependently inhibited (p < 0.05) growth of HPAECs induced by ECM (Fig. 2A). Treating cells with ITE at doses from 0.01 to 5 µM for 2, 4, and 6 days had no effect on cell growth. ITE at 10 µM significantly decreased (p < 0.05) cell growth by 19 and 32% on Days 4 and 6, respectively. ITE at 20 µM also decreased (p < 0.05) cell growth by 52, 60, and 83% on Days 2, 4, and 6, respectively.
Figure 2.
Effects of ITE and TCDD on growth of HPAECs. After serum starvation, cells were treated with ITE, TCDD, or DMSO (vehicle) in ECM for 2,4, or 6 days (5 wells/treatment) with a medium change every other day, followed by the crystal violet cell growth assay. Data are expressed as means ± SEM fold of cell number in DMSO (control) on each corresponding treatment day. Two-way ANOVA analysis was conducted followed by pair-wise comparisons.a-c Different letters differ within each treatment day (p< 0.05, n = 4 independent experiments).
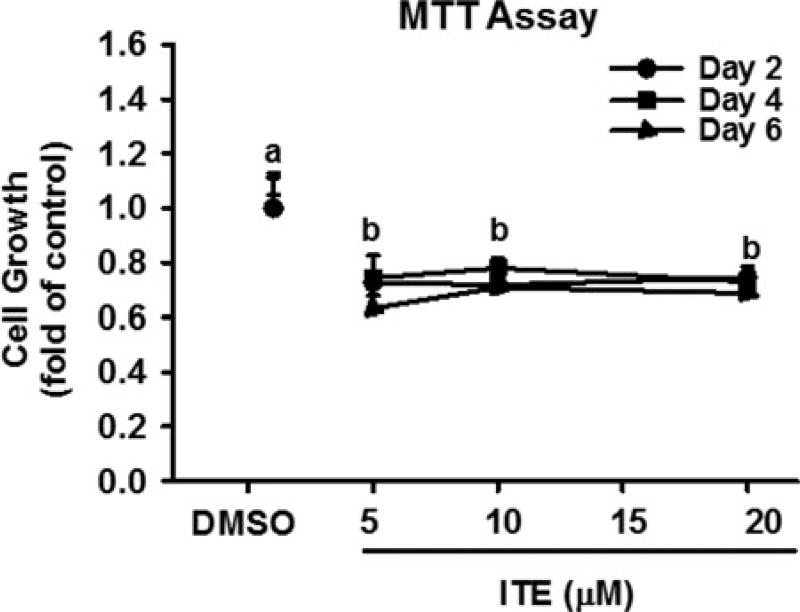
To confirm ITE’s effects on cell growth, TCDD was used. As compared with the vehicle control (DMSO), TCDD dose- and time-dependently significantly inhibited (p < 0.05) growth of HPAECs (Fig. 2B). TCDD at 100 nM significantly inhibited (p < 0.05) cell growth by 28, 47, and 60% on Days 2, 4, and 6, respectively; however, treating cells with TCDD at doses from 0.1 to 10 nM for up to 6 days did not significantly alter cell growth (Fig. 2B). To further confirm ITE’s effects on cell growth, MTT assay was also conducted. We observed that ITE at 5, 10, and 20 µM similarly decreased (p < 0.05) cell growth of HUVECs on Days 2, 4, and 6 by ∼ 27–43% (Fig. 3).
Figure 3.
Effects of ITE on growth of HPAECs. After serum starvation, cells were treated with ITE or DMSO (vehicle) in ECM for 2, 4, or 6 days with a medium change every other day, followed by the MTT cell growth assay. Data are expressed as means ± SEM fold of cell number in DMSO (control) on each corresponding treatment day. Two-way ANOVA analysis was conducted followed by pair-wise comparisons. a-bDifferent letters differ within each treatment day (p < 0.05, n = 4).
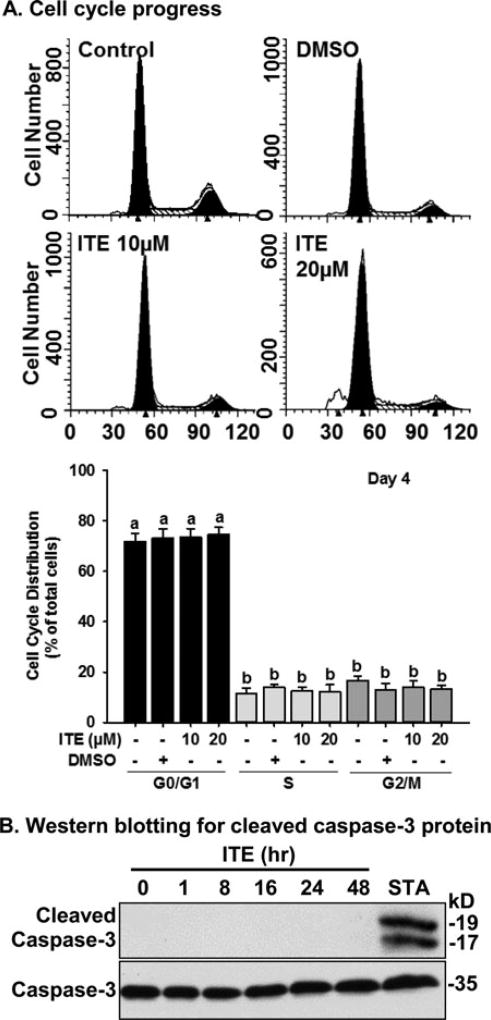
ITE does not alter cell cycle progress of HPAECs
Treating cells with 10 and 20 µM of ITE for 4 days did not alter the percentages of cells in G0/G1, S, and G2/M phases as compared with the control group (Fig. 4A), indicating that ITE did not significantly affect cell cycle progress of HPAECs.
Figure 4.
Effects of ITE on cell cycle progress and formation of cleaved caspase-3 protein in HPAECs. Cells were seeded in 60 mm culture dishes. A) Cell cycle progress: After serum starvation, cells were treated with ECM, DMSO (0.1% vol/vol; the vehicle) or ITE (10 µM or 20 µM) for 4 days with a medium change every other day. Cells were labeled with PI, and subjected to flow cytometer. Data are expressed as means ± SEM % of total cells. One-way ANOVA was conducted followed by pair-wise comparisons Holm-Sidak method. a,bDifferent letters differ (p < 0.05, n = 4) from each other. B) Cells were treated without (the time 0 control) or with ITE (20 µM) for up to 48 hr. Proteins were subjected to Western blotting (n = 3). Representative images are shown. STA: Staurosporine (200 nM, 8 hr, a positive control for apoptosis) of HPAECs.
ITE does not induce expression of cleaved caspase-3 protein in HPAECs
Treatment of ITE at 20 µM up to 48 hr did not induce formation of cleaved caspase-3, while Staurosporine clearly induced formation of two bands of caspase-3 in HUVECs (Fig. 4B), suggesting that ITE did not induce robust caspase-3-associated apoptosis in HPAECs.
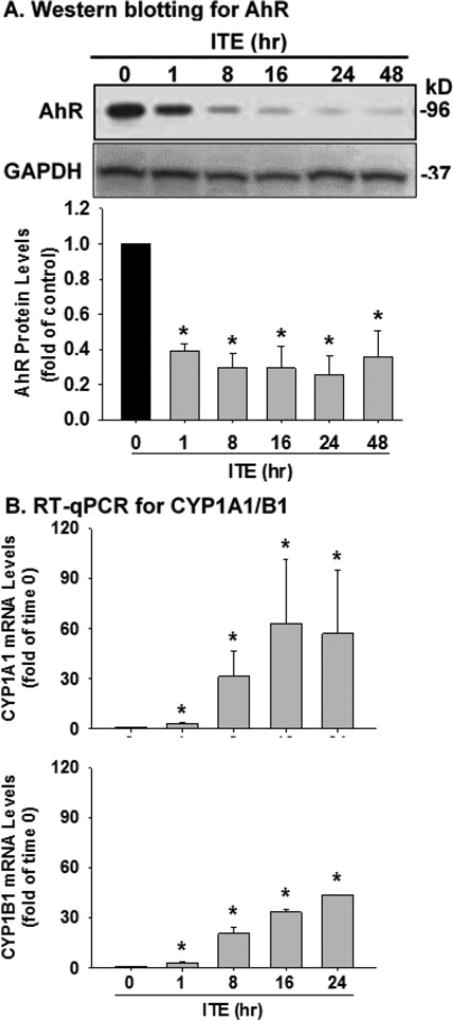
ITE increases CYP1A1 and CYP1B1 mRNA levels and decreases AhR protein levels in HPAECs
Western blotting detected a single band at ∼95kD, corresponding to the reported human AhR molecular mass.[12,14,22,25] A single dose of ITE (20 µM) rapidly decreased (p < 0.05) AhR protein levels in HPAEC by 55.9, 72.2, 70.6, 74.6, and 59.8% on 1, 8, 16, 24, and 48 hr, respectively (Fig. 5A). RT-qPCR also revealed that ITE elevated (p < 0.05) mRNA levels of CYP1A1 (by 3.0, 31.4, 62.9 and 57.0 fold at 1, 8, 16, and 24 hr, respectively) and CYP1B1 (by 3.0, 20.7, 33.4 and 43.6 fold at 1, 8, 16, and 24 hr, respectively) (Fig. 5B). These data indicated that ITE indeed activated the AhR/CYP1A1 and AhR/CYP1B1 pathways in HPAECs.
Figure 5.
Effects of ITE on AhR, CYP1A1 and CYP1B1 expression in HPAECs. A) Cells seeded in 60 mm dishes were treated without (the time 0 control) or with a single dose (20 µM) of ITE for up to 48 hr. Protein samples were subjected to Western blotting for AhR protein. B) Cells were treated with a single dose (20 µM) of ITE for up to 24 hr. Total mRNA samples were subjected to RT-qPCR for analyzing CYP1A1 and CYP1B1 mRNA levels. Data normalized to GAPDH are expressed as means ± SEM fold of the time 0 control. One-way ANOVA was conducted followed by comparisons verse the respective control. *Differ from the time 0 control (p < 0.05, n = 3).
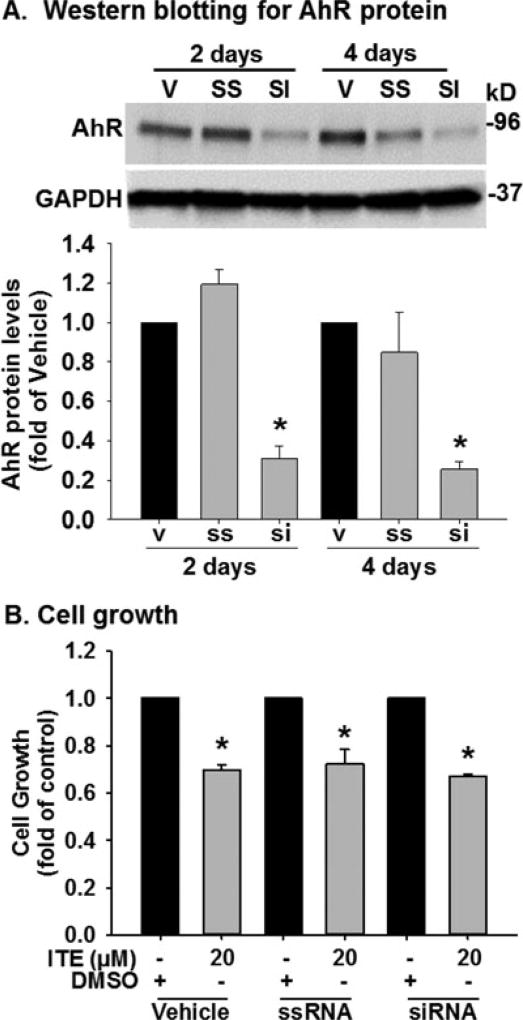
AhR knockdown does not affect ITE-suppressed growth of HPAECs
Compared with the vehicle control and the scrambled siRNA, the AhR siRNA at 20 nM decreased (p < 0.05) AhR protein levels by 69 and 75% on Days 2 and 4, respectively, in HPAECs (Fig. 6A). However, AhR knockdown did not significantly alter the ITE-suppressed growth of HPAECs (Fig. 6B).
Figure 6.
Effects of AhR knockdown on growth of HPAECs. A). Cells were treated with the transfection reagent (vehicle: V), the scrambled siRNA (ssRNA; 20 nM) or AhR siRNA (siRNA; 20 nM) for 2 and 4 days. Protein were subjected to Western blotting. Data are expressed as means ± SEM fold of the vehicle. One-way ANOVA was conducted followed by comparisons verse the respective control using the Holm-Sidak method. *Differ from the vehicle (p < 0.05, n = 3). B). After siRNA transfection for 2 days, cells were treated with DMSO (control) or ITE (20 µM) for additional for 2 days followed by the crystal violet cell growth assay. Data are expressed as means ± SEM fold of cell number in DMSO Unpaired Student’s t-test was performed. *Differ from the corresponding DMSO control (p < 0.05, n = 6).
Discussion
In the current study, we have demonstrated expression of AhR in many types of human lung cells, including endothelial cells and smooth muscle cells of pulmonary arteries and veins. More importantly, we have provided novel evidence that ITE inhibits growth of HPAECs. However, in contrast to our hypothesis, this ITE-inhibited cell growth is independent of AhR and is not associated with decreased cell cycle progress. These data suggest that ITE may attenuate the excessive growth of HPAECs, which is one major contributor to the development of PAH.[3,4]
Our current finding that the extensive expression of AhR protein in human lung tissues is consistent with our previous reports showing the presence of AhR in pulmonary arterial smooth muscle cells in vitro[11] and in human fetal lung tissues.[22] This broad distribution (endothelium, vascular smooth muscle cells, bronchial epithelium, alveolar sac epithelium, and stromal cells) of AhR in human lung tissues implies the overall importance of AhR in mediating function of different types of human lung cells.
Another major observation of the current study is that ITE decreases the growth of HPAECs at relatively high concentrations (10–20 µM), which are most likely to be pharmacological levels.[17] This ITE’s action is similar to the TCDD-induced inhibition on cell growth of HPAECs (Fig. 2B) and human umbilical vein endothelial cells (HUVECs)[14] and is in line with a previous report showing that benzo(a)pyrene (another exogenous AhR ligand) inhibited neovasculogenesis in vivo and angiogenic activity of HUVECs in vitro.[28] However, the current data are contrary to the other previous studies, [29,30] in which TCDD failed to affect growth of many human and animal primary cells and transformed cell lines (not including HPAECs). More interestingly, the ITE-inhibited growth of HPAECs is independent of AhR since knockdown of AhR did not significantly affect this inhibition, although AhR has been shown to mediate ITE-inhibited growth of ovarian cance cells.[10] To date, mechanisms underlyng this AhR-independent inhibition in HPAECs remain elusive. Nonetheless, such inhibition is not unanticipated since AhR also does not mediate TCDD-decreased growth of HUVECs,[14] and ITE-inhibited TGFβ1 signaling in human fibroblasts.[31] These data suggest that other yet-to-be identified mechanisms must underlie the ITE-suppressed growth of HPAECs.
The failure of AhR knockdown to affect the ITE-inhibited growth of HPAECs is unlikely to be due to the uncoupling ITE from the AhR/CYP1A1 and AhR/CYP1B1 pathways because ITE clearly activated both pathways in HPAECs (Fig. 4). However, since the AhR siRNA only partially (69–75%) suppressed the AhR expression in HPAECs, we cannot exclude the possibility that the residual AhR after AhR siRNA transfection is sufficient to mediate the cell growth in response to ITE in HPAECs. Our current observation that ITE did not suppress the cell cycle progression in HPAECs is in agreement with our recent findings reported in HUVECs and HUAECs;[14] however, it is in contrast to the previous study showing that 3MC arrested the cell cycle at G0/G1.[32] Moreover, we also noted that the number of cells after treatment of ITE at 10 µM up to 6 days and 20 µM up to 2 days were more (p < 0.05) than those initially seeded (4,000 cells/well). For example, the number of cells after treatment of ITE at 10 µM were 14285 ± 852 and 10251 ± 321 on Days 4 and 6, respectively suggesting that treating HPAECs with ITE at 10 µM up to 6 days attenuates cell growth of HPAECs, but does not cause significant cell death. In contrast, the number of cells after 6 days (852± 465) of ITE treatment at 20 µM were significantly lesser than those initially seeded, suggesting a relatively long-term treatment (up to 6 days) of ITE at 20 µM might induce significant cell death. This is supported by our current data from the MTT assay, which is also one of the most widely methods to detect viable cells as reduction of MTT can only occur in viable cells.[33] Thus, the decrease of MTT signals as shown in Fig. 3 indicate increases in cell death induced by ITE. To date, exact types of cell death induced by ITE in HPAECs remain unknown. However caspase-3-associated cell apoptosis appears not to be involved in the ITE-inhibited cell growth of HPAECs as treatment of ITE at 20 uM up to 6 days did not induce the formation of cleavage of caspase-3 (Fig. 4B). We speculate that increased cell necroptosis[34] may partially contribute to ITE-induced death, leading to decreases in cell growth of HPAECs.
In conclusion, we have demonstrated that ITE inhibits growth of HPAECs independent of AhR. Although further research on the mechanisms governing the ITE-inhibited growth of HPAECs is needed, these data suggest that ITE at doses that inhibit cell growth without inducing significant cell death might be potentially used to prevent excessive lung endothelial growth, which may be involved in the development of PAH.
Acknowledgments
We would like to thank Loretta A. Uttech-Hanson, Research Administration Grant Writer & Project Manager at the Office of Grant Writing & Collaborative Project Development, School of Medicine and Public Health, University of Wisconsin-Madison for English language editing.
Funding
This work was supported by the US NIH grant PO1 HD38843 to JZ, a Dept. of Ob/Gyn R&D Grant, University of Wisconsin-Madison to JZ, Science & Technology Innovation Fund of Guangdong Medical University (Grant No. STIF201116) to SAH.
Footnotes
Declaration of interest
The authors report no conflicts of interest.
References
- 1.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. PMID: 19749199. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. PMID: 20585011. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. PMID: 15194174. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. PMID: 17000921. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005;25:244–249. doi: 10.1183/09031936.05.00054804. PMID: 15684287. [DOI] [PubMed] [Google Scholar]
- 6.Provencher S, Sitbon O, Humbert M, Cabrol S, Jaı¨s X, Simonneau G. Long-term outcome with first-line bosentan therapy in idiopathic pulmonary arterial hypertension. Eur Heart J. 2006;27:589–595. doi: 10.1093/eurheartj/ehi728. PMID: 16431875. [DOI] [PubMed] [Google Scholar]
- 7.Safe S, McDougal A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers. Int J Oncol. 2002;20:1123–1128. PMID: 12011988. [PubMed] [Google Scholar]
- 8.Fujii-Kuriyama Y, Kawajiri K. Molecular mechanisms of the physiological functions of the aryl hydrocarbon (dioxin) receptor, a multifunctional regulator that senses and responds to environmental stimuli. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:40–53. doi: 10.2183/pjab.86.40. PMID: 20075607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savouret JF, Berdeaux A, Casper RF. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutr Metab Cardiovasc Dis. 2003;13:104–113. doi: 10.1016/s0939-4753(03)80026-1. PMID: 12929624. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Li Y, Jiang YZ, et al. An endogenous aryl hydrocarbon receptor ligand inhibits proliferation and migration of human ovarian cancer cells. Cancer Lett. 2013;340:63–71. doi: 10.1016/j.canlet.2013.06.026. PMID: 23851185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S, Chen P, Shui X, et al. Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J Pharm Pharmacol. 2014;66:1469–1477. doi: 10.1111/jphp.12273. PMID: 24835111. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Wang K, Jiang YZ, Chang XW, Dai CF, Zheng J. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibits human ovarian cancer cell proliferation. Cell Oncol (Dordr) 2014;37:429–437. doi: 10.1007/s13402-014-0206-4. PMID: 25404385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Chen X, Zhou Q, et al. ITE and TCDD differentially regulate the vascular remodeling of rat placenta via the activation of AhR. PLoS One. 2014;9:e86549. doi: 10.1371/journal.pone.0086549. PMID: 24475139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wang K, Zou QY, Magness RR, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin differentially suppresses angiogenic responses in human placental vein and artery endothelial cells. Toxicology. 2015;336:70–78. doi: 10.1016/j.tox.2015.08.003. PMID: 26275813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116. doi: 10.1021/tx7001965. PMID: 18076143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. PMID: 21976023. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Clagett-Dame M, Peterson RE, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. PMID: 12409613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng J, Li W, Kang B, et al. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 2015;6:7209. doi: 10.1038/ncomms8209. PMID: 26059097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys. 2006;450:67–77. doi: 10.1016/j.abb.2006.02.008. PMID: 16545771. [DOI] [PubMed] [Google Scholar]
- 20.Quintana FJ, Murugaiyan G, Farez MF, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. PMID: 21068375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. PMID: 8260069. [DOI] [PubMed] [Google Scholar]
- 22.Jiang YZ, Wang K, Fang R, Zheng J. Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J Histochem Cytochem. 2010;58:679–685. doi: 10.1369/jhc.2010.955955. PMID: 20354149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai CF, Jiang YZ, Li Y, et al. Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol. 2010;135:475–485. doi: 10.1007/s00418-011-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao YJ, Zou QY, Li Y, et al. Expression of G-protein subunit α-14 is increased in human placentas from preeclamptic pregnancies. J Histochem Cytochem. 2014;62:347–354. doi: 10.1369/0022155414521213. PMID: 24423937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zhao YJ, Zou QY, et al. Preeclampsia does not alter vascular growth and expression of CD31 and vascular endothelial cadherin in human placentas. J Histochem Cytochem. 2015;63:22–31. doi: 10.1369/0022155414558063. PMID: 25362142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang YZ, Li Y, Wang K, et al. Distinct roles of HIF1A in endothelial adaptations to physiological and ambient oxygen. Mol Cell Endocrinol. 2014;391:60–67. doi: 10.1016/j.mce.2014.04.008. PMID: 24796659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalini S, Dorstyn L, Dawar S, Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–39. doi: 10.1038/cdd.2014.216. PMID: 25526085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li CH, Cheng YW, Hsu YT, Hsu YJ, Liao PL, Kang JJ. Benzo[a]pyrene inhibits angiogenic factors-induced alphavbeta3 integrin expression, neovasculogenesis, and angiogenesis in human umbilical vein endothelial cells. Toxicol Sci. 2010;118:544–553. doi: 10.1093/toxsci/kfq279. PMID: 20876236. [DOI] [PubMed] [Google Scholar]
- 29.Beatty PW, Lembach KJ, Holscher MA, Neal RA. Efects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on mammalian cells in tissue cultures. Toxicol Appl Pharmacol. 1975;31:309–312. doi: 10.1016/0041-008X(75)90166-0. PMID: 165593. [DOI] [PubMed] [Google Scholar]
- 30.Knutson JC, Poland A. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: failure to demonstrate toxicity in twenty-three cultured cell types. Toxicol Appl Pharmacol. 1980;54:377–583. doi: 10.1016/0041-008X(80)90163-5. PMID: 7394793. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann GM, Xi X, Kulkarni AA, et al. The aryl hydrocarbon receptor ligand ITE inhibits TGFβ1-induced human myofibroblast differentiation. Am J Pathol. 2011;178:1556–1567. doi: 10.1016/j.ajpath.2010.12.025. PMID: 21406171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juan SH, Lee JL, Ho PY, Lee YH, Lee WS. Antiproliferative and antiangiogenic effects of 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, in human umbilical vascular endothelial cells. Eur J Pharmacol. 2006;530:1–8. doi: 10.1016/j.ejphar.2005.11.023. PMID: 16359657. [DOI] [PubMed] [Google Scholar]
- 33.Riss TL, Moravec RA, Niles AL, et al. Cell Viability Assays. 2013 May 1 [updated 2016 Jul 1] In: Sittampalam GS, Coussens NP, Brimacombe K, et al., editors. Assay Guidance Manual [Internet] Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004. [PubMed] [Google Scholar]
- 34.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. PMID: 24476434. [DOI] [PMC free article] [PubMed] [Google Scholar]