Abstract
Transgenic poplars (Populus tremula × Populus alba) were obtained by introduction of a sense homologous transgene encoding caffeic acid O-methyltransferase (COMT) under the control either of the cauliflower mosaic virus double 35S promoter or of the eucalyptus cinnamyl alcohol dehydrogenase promoter. Although these constructs conferred a moderate overexpression of COMT in some lines, a transgenic line with the double 35S promoter was found where COMT activity in woody tissues was close to zero due to a gene-silencing phenomenon. For the first time in COMT down-regulated trees, this alteration substantially reduced lignin level in 6-month-old trees (17% decrease). Lignin structure was found to be strongly altered, with a two times higher content in condensed bonds, an almost complete lack of syringyl units, and the incorporation of 5-hydroxyguaiacyl units to the most remarkable extent reported so far. Consistent with the higher cellulose content and with the higher condensation degree of the lignin, the impact of the transformation on the kraft-pulping performances of the poplar trees positively affected the pulp yield (10% relative increase), but made lignins less amenable to industrial degradations.
Lignins are complex aromatic polymers that must be removed from wood during the chemical-pulping and bleaching steps of the papermaking process. Lignins of angiosperm woods are composed of guaiacyl (G) and syringyl (S) units linked by labile ether β-O-4 bonds and by resistant carbon-carbon linkages (Adler, 1977). The genetic alteration of lignin content and/or structure in wood is considered to be a novel route to improving our current knowledge on lignification and to facilitating the chemical-pulping and bleaching processes, thereby lowering their energy demand and environmental impact. In this context, various genes of the multi-step biosynthetic pathway leading to lignin precursors have been down-regulated by antisense or homologous-sense cosuppression strategies. The effects of these biotechnological manipulations on lignification and, in some cases, on the pulping performances of the transgenic plants have been evaluated (for reviews, see Baucher et al., 1998; Grima-Pettenati and Goffner, 1999). For many years and on the basis of in vitro experiments, O-methyltransferases have been considered as bispecific enzymes similarly involved in the methylation of G and S precursors (Higuchi, 1985; Van Doorsselaere et al., 1993). Several genes encoding poplar caffeic acid O-methyltransferase (COMT) have been cloned (Bugos et al., 1991; Dumas et al., 1992; Tsai et al., 1998) that allowed researchers to down-regulate COMT activity in poplar trees (Van Doorsselaere et al., 1995; Tsai et al., 1998). In a previous study, we have shown that a substantial reduction in COMT activity (up to 90%) through an antisense strategy did not affect the lignin content of transgenic poplar lines, but did induce a dramatic alteration of lignin structure (Lapierre et al., 1999). Similar results have been obtained recently by cosuppression in quaking aspen (Tsai et al., 1998). The main changes in the lignins of antisense COMT down-regulated poplars were an increased proportion of G units, together with an increased frequency of resistant interunit bonds, and conversely a lower frequency of S units and labile β-O-4 bonds (Lapierre et al., 1999). Another specific signature of COMT down-regulation in poplar lignins was the abnormally high level of incorporation of 5-hydroxyguaiacyl units (5-OH-G), a structural trait reminiscent of lignins from COMT-deficient maize mutants (Lapierre et al., 1988). Taken together, these results suggested that the formation of G precursors involved an alternative methylation pathway catalyzed by caffeoyl coenzyme A O-methyltransferase (CCoAOMT) (Ye and Varner, 1995). This hypothesis was supported by the observation that CCoAOMT down-regulated tobacco lines displayed a decreased frequency of lignin G units (Zhong et al., 1998). Recently, it has been proposed that CCoAOMT would mainly participate in the production of G precursors from caffeoyl-coenzyme A (CoA) intermediates, whereas COMT would more specifically contribute to the formation of S precursors from 5-hydroxyconiferaldehyde (Humphreys et al., 1999; Osakabe et al., 1999).
Consistent with the alteration of lignin structure, COMT down-regulation proved to have a detrimental impact on the pulping performances of 2-year-old transgenic poplars (Lapierre et al., 1999). This result prompted us to try the opposite strategy, namely to overexpress COMT to produce a less cross-linked S-rich lignin. Such an increased content in S units and β-O-4 structures was recently obtained through the overexpression of ferulate 5-hydroxylase (F5H) in Arabidopsis (Meyer et al., 1998; Marita et al., 1999). To achieve this goal, we introduced the entire COMT coding region in poplar under the control either of the constitutive double 35S cauliflower mosaic virus (CaMV) promoter (35S2) or of the eucalyptus cinnamyl alcohol dehydrogenase (CAD) promoter, which is specific for lignified tissue (Feuillet et al., 1995). However, the resulting poplar transgenic lines did not display any substantial or stable increase in COMT activity. Their lignin profile was not altered with the exception of one line where the introduction of the COMT gene under the control of the 35S2 promoter caused a silencing phenomenon with regard to COMT activity. This poplar line displayed a phenotype distinct from those of transgenic poplar lines with antisense COMT constructs (Van Doorsselaere et al., 1995). More importantly, and for the first time to our knowledge, a very susbtantial reduction in lignin content was obtained together with an increase in cellulose. In addition, the extent of lignin structural changes was found to be most remarkable, with an almost complete disappearance of S-lignin units. Pulping experiments were performed on 6-month-old juvenile poplars to determine whether the kraft cook efficiency would be facilitated by the lower lignin content or made more difficult due to the higher cross-linking of the lignin. In this study we report on the molecular and biochemical characterization of this line and on its pulping performances, in comparison to the control line and to three other poplar lines slightly or not altered in their lignification. This study demonstrates that the biotechnological reduction in lignin content is not necessarily a desirable goal with regard to pulping performances when accompanied by a detrimental alteration in lignin structure.
RESULTS
Production, Molecular Characterization, and COMT Activity of Transgenic Poplars
The 35S2-SOMT and EuCAD-SOMT vectors were introduced via Agrobacterium tumefaciens into the poplar clone INRA 717 1-B4. For each construct, more than 10 different transgenic lines (named 70SOMT and EuSOMT, respectively) were obtained, multiplied in vitro, and then transferred to the greenhouse. COMT activity was measured in the developing xylem of 3- to 6-month-old poplars during the summer when the COMT activity level was the highest (Bugos et al., 1991; Pilate et al., 1997). In the first series of plants, several lines displayed a moderately increased COMT activity (approximately 2-fold) and several representative lines were selected: 70SOMT-1, EuSOMT-1, and EuSOMT-2 (Table I). In contrast, a considerably reduced COMT activity (residual activity lower than 3% of the control level; Table I) was observed in one transgenic line, 70SOMT-3, which is diagnostic of a gene-silencing phenomenon. Two other series of greenhouse cultures were done to evaluate the stability of the modifications of COMT activity in these four selected lines. No difference in the growth and development of these transgenic poplars was observed relative to the control. The 70SOMT-3 line systematically displayed very low COMT activity in the three series of greenhouse cultures (data for the two first series are reported in Table I). In contrast, a more erratic behavior was observed with the other transgenic lines selected on the basis of a higher COMT activity. When determined for different trees of the same transgenic line, this activity showed large variations, which underlined the instability of the moderate COMT overexpression (Table I).
Table I.
COMT activity in the developing xylem of transgenic poplars with sense COMT constructs relative to the control level
| Culture No. | Poplar Line (COMT Activity Expressed as Percent of the Corresponding Control Level)
|
|||
|---|---|---|---|---|
| 70SOMT-1 | 70SOMT-3 | EuSOMT-1 | EuSOMT-2 | |
| First culture | 164 | 2 | 186 | 240 |
| Second culture | ||||
| Tree 1 | 102 | 0.5 | 153 | 85 |
| Tree 2 | 56 | 1 | 98 | 96 |
| Tree 3 | 83 | 3 | 186 | 40 |
Caffeic acid was used as the substrate. The assays were run in summer and for two different series of 6-month-old poplars grown in the greenhouse in 1998 and in 1999.
The COMT activity level was determined in young leaves from the selected transgenic lines. This level was very low in the control, in the EuSOMT lines and in the silenced 70SOMT-3 line. In contrast, a higher COMT activity was observed in the leaves of line 70SOMT-1, which overexpressed the COMT gene under the control of the 35S2 promoter (data not shown). These results confirmed the specificity of the eucalyptus CAD promoter with respect to lignified tissues (Feuillet et al., 1995).
The presence of T-DNA in the genome of the selected transgenic poplar lines was confirmed by Southern hybridization. In lines 70SOMT-3 and EuSOMT-2, only one T-DNA copy was evidenced, whereas 70SOMT-1 and EuSOMT-1 integrated several copies (data not shown). Hence, the silencing phenomenon observed in line 70SOMT-3 cannot be ascribed to the integration of several T-DNA copies.
Wood Coloration and Histochemical Staining of the Selected Lines
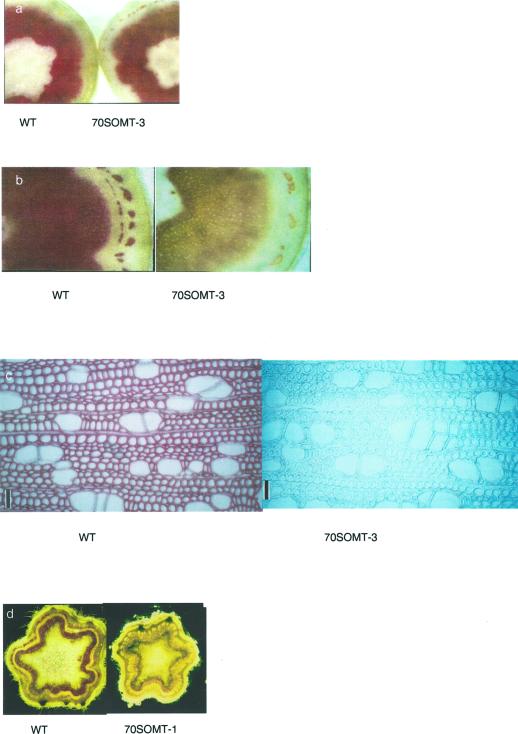
According to previous studies (Van Doorsselaere et al., 1995; Lapierre et al., 1999), the wood of transgenic poplars substantially altered in COMT activity (5%–10% residual activity relative to the control level) by the introduction of COMT antisense constructs has a pale rose coloration. The xylem of the 70SOMT-3 line showed a different brownish coloration (Fig. 1) reminiscent of transgenic aspens down-regulated for COMT activity through silencing (Tsai et al., 1998). This coloration resisted wood drying and solvent extraction. In 70SOMT-1, EuSOMT-1, and EuSOMT-2 lines, xylem was found sometimes colored but to a lower extent and with irregular patterns (Fig. 1).
Figure 1.
Phenotype of 6-month-old stems of control (WT) and transgenic poplars. 70SOMT-1, EuSOMT-1, and -2 lines overexpress COMT activity erratically, whereas this activity is severely reduced in line 70SOMT-3. The ASCADT21 line (Baucher et al., 1996) is presented for color comparison.
The cytological observation of stem cross-sections did not show any difference in shape, size or general organization of xylem and fiber cells between the control and the transgenic lines. Cross-sections of secondary stems were subjected to Wiesner and Mäule stainings. The Wiesner test (phloroglucinol-HCl staining) is considered to be specific of cinnamaldehyde end-groups, which are systematically present in native lignins, albeit in low amount (Nakano and Meshitsuka, 1992); whereas the Mäule test is specific of S units in lignins. The control and the silenced 70SOMT-3 lines exhibited similar responses to the Wiesner test (Fig. 2a), which suggests similar contents in cinnamaldehyde groups. In contrast the Mäule reaction revealed striking variations between the control and the 70SOMT-3 line (Fig. 2, b and c). Whereas the control wood exhibited the positive purple coloration diagnostic of S units, the xylem of 70SOMT-3 line displayed a light brown coloration. This observation suggests that S units are very scarce or even absent in the lignins of the 70SOMT-3 line. Consistent with the heterogeneous pattern of its native coloration, the xylem of 70SOMT-1, EuSOMT-1, and EuSOMT-2 lines reacted nonuniformly to the Mäule reagent, with both unstained and stained areas similar to the control sample (Fig. 2d). This result suggests that the deposition of S-lignin units is nonuniformly altered in these lines.
Figure 2.
Wiesner (a) and Mäule (b and c) staining of cross sections of 6-month-old secondary stems from control (WT) and 70SOMT-3 poplars; in c, bar = 50 μm. d, Mäule staining of young stems of control (WT) and 70SOMT-1 poplars.
Visible light absorption spectra under the Mäule color reaction were measured on the secondary walls of vessel elements (V-SW), the secondary walls of fibers (F-SW), and the cell corner middle lamella between fibers (FF-CC). In the control, V-SW showed weak absorption around 525 nm, whereas F-SW and FF-CC showed strong absorption around 525 nm. In the 70SOMT-3 line, no obvious absorption was found around 525 nm in V-SW, F-SW, and FF-CC (Fig. 3a).
Figure 3.
Visible light absorption spectra (a) and UV absorption spectra (b) of V-SW, F-SW, and FF-CC in the control wood and the 70SOMT-3 line.
UV absorption spectra were measured in V-SW, F-SW, and FF-CC from the differentiating xylem to the pith to trace lignification process. Between the control wood and the 70SOMT-3 line, no differences in the lignification pattern could be seen in V-SW, F-SW, and FF-CC. Spectra were averaged on the cells whose lignification was completed (Fig. 3b). V-SW exhibited the same absorption maxima (277.5 nm) in both the control and the 70SOMT-3 line. In contrast, F-SW and FF-CC in the 70SOMT-3 exhibited longer absorption maxima (277.5 nm) than in the control wood (272.5–275 nm). No difference could be observed between the control and the 70SOMT-3 line in the range of 300 to 400 nm. Overall results from microspectrophotometry suggested that the proportion of S-unit decreased in F-SW and FF-CC of the 70SOMT-3 line.
Lignin Content in Transgenic Poplars
The lignin content of the extractive-free wood was determined using the Klason method and with three different cultures of control and transgenic poplars. The lignin content of line 70SOMT-3 was systematically and substantially lower relative to the control (17% reduction in lignin content). The spectrophotometric acetyl bromide determination of the lignin level confirmed that line 70SOMT-3 had reduced lignification. The other transgenic lines displayed lignin levels similar to that of the control line (Table II). When compositional analysis of the cell wall was carried out according to the method of Jarrige (1961), it could be seen that line 70SOMT-3 has a higher content in the cellulosic fraction of the xylem (6% relative increase). To the best of our knowledge and relative to recent literature data on COMT down-regulated plants in the genus Populus (Van Doorsselaere et al., 1995; Tsai et al., 1998; Lapierre et al., 1999), line 70SOMT-3 is the first reported transgenic poplar with both reduced COMT activity and lignin content.
Table II.
Lignin content in extractive-free stem wood of 6-month-old control and transgenic poplars
| Line | First Culture | Second Culture | Third Culture |
|---|---|---|---|
| % wt | |||
| Control | 18.3 ± 0.6 (100) | 20.1 (100) | 19.2 (100) |
| 70SOMT-1 | 19.2 ± 0.5 (105) | 20.2 (100) | 18.8 (95) |
| 70SOMT-3 | 15.1 ± 0.6 (83) | 16.7 (83) | 16.0 (83) |
| EuSOMT-1 | – | 19.2 (96) | 19.2 (100) |
| EuSOMT-2 | 18.6 ± 0.6 (101) | 20.3 (101) | 18.0 (94) |
Three different cultures were analyzed. Trees were individually analyzed for the first culture (mean ± sd for triplicates). For the second and third culture several trees per line were pooled for analysis. Klason lignin content, which does not include the acid-soluble lignin, is expressed as wt percentage of the extractive-free wood. Normalized values relative to control are shown in parentheses.
Lignin Structure in Transgenic Poplars
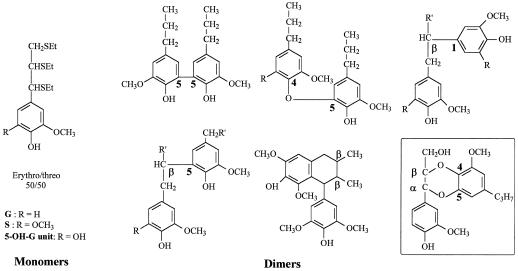
Lignin-derived monomers and dimers released by thioacidolysis of the extractive-free wood were analyzed by gas chromatography-mass spectrometry (GC-MS) of their trimethylsilyl (TMS) derivatives (Lapierre et al., 1995). Thioacidolysis is an analytical degradation method that proceeds by cleavage of labile β-O-4 bonds. G and S lignin-derived monomers (Fig. 4) are specifically generated from G and S units only involved in β-O-4 bonds, without any interference from non-lignin phenolics. When present in lignins, 5-OH-G units similarly give rise to 5-OH-G thioacidolysis monomers, whereas these units do not survive in the frequently employed nitrobenzene oxidation method (C. Lapierre, unpublished results). Therefore, the total yield in thioacidolysis monomers gives a close estimate of the lignin content in units involved in β-O-4 bonds. This structural trait is of great value with respect to kraft pulping, as the cleavage of β-O-4 bonds is the basis of efficient lignin fragmentation in the kraft cooking liquor. In addition to the G, S, and 5-OH-G monomers, lignin-derived dimers provided with resistant interunit bonds, referred to as the condensed bonds, are released by thioacidolysis (Fig. 4). In other words, the GC determination of the lignin-derived monomers and dimers provides a detailed fingerprint of the proportion of the various building units and interunit bonds in the polymer.
Figure 4.
Main lignin-derived monomers and dimers recovered from the thioacidolysis of poplar lignins. The dimers were obtained after an additional desulfurization step. The α,β-diether structure in the square is speculative (from the mass fragmentation pattern) and observed only with the 70SOMT-3 line (R = H or OCH3; R′ = H or CH2OH).
The determination of the lignin-derived monomers (Table III) revealed that lignins of most of the poplar lines had 50% to 60% units involved only in β-O-4 bonds. In contrast, this percentage was two times lower in the silenced 70SOMT-3 line. This means that lignins of the 70SOMT-3 line have a two times higher content in condensed bonds relative to the other lines. Consistent with the results obtained for antisense COMT down-regulated poplars (Van Doorsselaere et al., 1995; Lapierre et al., 1999) and with the Mäule staining observations, the relative abundance of β-O-4-linked S units was reduced 10-fold (from 60%–6% relative proportion) in the silenced 70SOMT-3 line. In addition, the 5-OH-G units, diagnostic for an alteration in the methylation of S precursors, occurred to the highest extent observed so far in transgenic or mutant angiosperm plants. Their proportion even exceeded that of S units, which was not the case of transgenic poplars with antisense COMT constructs (reported in Table III for comparison; Lapierre et al., 1999) or transgenic aspens with sense COMT constructs (Tsai et al., 1998).
Table III.
Lignin-derived monomers recovered from the thioacidolysis of 6-month-old control and transgenic poplars
| Line | Yield in G+S+5-OH-G Monomers | Relative Proportion of G/S/5-OH-G | Units Involved Only in β-O-4 |
|---|---|---|---|
| μmol g−1 KLa | % | ||
| Control | 2,720 | 34/64/2 | 68 |
| 2,290 | 33/67/Trb | 57 | |
| SOMT-1 | 2,120 | 34/66/Tr | 53 |
| 2,370 | 32/67/1 | 59 | |
| 70SOMT-3 | 1,160 | 84/5/11 | 29 |
| 1,280 | 82/6/12 | 32 | |
| EuSOMT-1 | 2,120 | 34/66/Tr | 53 |
| 2,140 | 31/68/1 | 54 | |
| EuSOMT-2 | 2,200 | 35/64/1 | 55 |
| 2,350 | 35/64/1 | 59 | |
| ASOMT2B | 1,470 | 65/29/6 | 37 |
| ASOMT10B | 1,610 | 64/31/5 | 40 |
The two series of data correspond to two different cultures. The data obtained for 6-month-old antisense COMT lines (ASOMT2B and ASOMT10B) are given for comparison (Lapierre et al., 1999). The percentage of lignin units involved only in β-O-4 bonds is calculated with the assumption that the average Mr of lignin units is 200 and the recovery yield of main thioacidolysis monomers from parent β-O-4 structures is 80%.
KL, Klason lignin.
Tr, Trace amount (<1%).
Thioacidolysis experiments were performed on permethylated samples to evaluate the frequency of free phenolic groups in β-O-4-linked units, as these groups facilitate lignin fragmentation and solubilization in the kraft cooking liquor. In contrast to CAD down-regulated poplars and in agreement with the results obtained for antisense COMT down-regulated poplars (Lapierre et al., 1999), we observed a lower amount of free phenolic groups in β-O-4-linked G units of the 70SOMT-3 line (21% versus 26% for the control line).
As the brownish coloration of the wood in transgenic aspens cosuppressed for COMT gene has been assigned recently to an increased incorporation of coniferaldehyde in the lignins (Tsai et al., 1998), the thioacidolysis monomer specifically derived from coniferaldehyde end-groups was determined by GC-MS. Consistent with the increase in conventional G units, the level of coniferaldehyde end-groups was 3-fold higher in the 70SOMT-3 lignins, relative to the control level. This result is consistent with the observation of similar Wiesner stainings for control and 70SOMT-3 lines in spite of different lignin contents. Whatever the line, the amount of coniferaldehyde end-groups remained very low (<0.5% on a molar basis). For comparison, when the same determination was run on native pure G lignins of uncolored pine or spruce woods, the recovered figure was in the 1% to 3% range (C. Lapierre, unpublished results).
Poplar lignins are esterified by p-hydroxybenzoic units (Smith, 1955). Alkaline hydrolysis of extract-free samples was performed to determine the impact of the genetic transformation on this acylation. The low-Mr phenolics released from control and 70SOMT-3 samples were identified and quantified by GC-MS of their TMS derivatives (Table IV). In agreement with previous results (Lapierre et al., 1999), it seems that the p-hydroxybenzoylation of poplar lignin units is positively related to the proportion of S units. Lignins from the 70SOMT-3 line displayed a 5-fold lower content in p-hydroxybenzoic esters relative to the control. As a further confirmation of the depletion in S compounds, 70SOMT-3 samples released trace amounts of syringaldehyde and syringic acids upon alkaline hydrolysis. Conversely, higher amounts of vanillin and vanillic acids relative to the control sample were obtained.
Table IV.
Yields of low-Mr phenolics released by alkaline hydrolysis of 6-month-old control and transgenic poplars
| Compound | Control | 70SOMT-3 |
|---|---|---|
| mg g−1 KLa | ||
| p-Hydroxybenzoic acid | 15.56 | 3.89 |
| Vanillic acid | 0.25 | 0.30 |
| Syringic acid | 0.24 | 0.02 |
| Vanillin | 1.38 | 2.28 |
| Syringaldehyde | 1.41 | 0.16 |
KL, Klason lignin.
To further delineate the structural peculiarities of lignins in 70SOMT-3 poplar line, lignin-derived dimers obtained by thioacidolysis then Raney nickel desulfurization were examined. A novel dimer could be identified in greater relative amount. Its mass fragmentation pattern strongly supported the hypothesis that this dimer corresponds to an α,β-diether structure involving a G unit and a 5-OH-G unit (Fig. 4). Such an α,β-diether bonding pattern has already been identified among the hydrogenolysis products of hardwood lignins (Hwang and Sakakibara, 1981), which demonstrates that these ether linkages may survive severe chemical treatments. This dimer may therefore be a further signature of the substantial incorporation of 5-OH-G units in the lignins of the 70SOMT-3 line. The relative frequencies of the main dimers representative of the lignin condensed bonds (Table V) further emphasized the dramatic alteration of lignin structure in the silenced 70SOMT-3 line. The main impact of the transformation, consistent with the increased proportion of G units, is the 3-fold increase in the relative importance of G 5-5 structures. The dimer profile of the 70SOMT-3 line was strikingly close to that of a pure G lignin (pine sample in Table V). The dimer profile of the antisense COMT down-regulated line ASOMT10B (Lapierre et al., 1999) was intermediate between the control and the 70SOMT-3 profiles (Table V).
Table V.
Relative frequencies (% molar) of the main dimers (Fig. 5) released by thioacidolysis of 6-month-old control and 70SOMT-3 poplars
| Line | Dimer Type
|
|||||
|---|---|---|---|---|---|---|
| 5-5 | 4-O-5 | β-5 | β-β SS | β-1 | α,β-Diether | |
| % molar | ||||||
| Control | 9.6 | 10 | 23.5 | 21.5 | 35.4 | Tra |
| 70SOMT-3 | 26.3 | 6.1 | 36.5 | Tr | 22.9 | 8.2 |
| ASOMT10B | 19.8 | 9.5 | 34.2 | 10.8 | 25.7 | Ndb |
| Pine | 33 | 6.5 | 30 | – | 28 | – |
Data obtained for the antisense COMT down-regulated poplar line (ASOMT10B, 1 year old) and for mature pine wood samples are given for comparison. The total for pine dimers does not reach 100% due to minor dimers not included in the table.
Tr, Trace amount.
Nd, Not determined.
Kraft-Pulping Assays
Laboratory-scale kraft-pulping experiments were performed for two series of 6-month-old poplars (Table VI) to determine which of the two main impacts of the very severe alteration of COMT activity in the 70SOMT-3 line would prevail with respect to kraft-pulping efficiency. As the reduction in lignin level (17% reduction) and the higher condensation degree of the lignin (two times more units involved in condensed bonds) are expected to have opposite effects on the efficiency of kraft cooking, the overall impact of the transformation could not be easily predicted. Consistent with the lower lignin amount and higher cellulose content, the pulp yield was substantially higher for the 70SOMT-3 line relative to the other lines. However, this positive effect was severely counterbalanced by a detrimentally high kappa (κ) number diagnostic for a higher residual lignin content in the pulp. The determination of cellulose degree of polymerization (DP) did not reveal any systematic difference between the various lines, which means that the susceptibility of cellulose toward kraft cook is not detrimentally affected by the transformation.
Table VI.
Kraft-cooking characteristics of 6-month-old control and transgenic poplars
| Line | Control | 70SOMT-1 | 70SOMT-3 | EuSOMT-1 |
|---|---|---|---|---|
| Pulp yield (% wt) | 47.6 | 47.5 | 54.2 | 49.2 |
| 48.9 | 49.7 | 54.8 | 51.3 | |
| κ no. | 21.3 | 21.2 | 39.4 | 21.3 |
| 21.8 | 23.5 | 31.8 | 23.2 | |
| Cellulose DP | 2,090 | 1,880 | 2,000 | 2,170 |
| 1,810 | 1,835 | 1,770 | 1,880 |
The two series of data correspond to two different cultures. Several trees of the same line were gathered for the experiment. The main stems were debarked and chipped before the cook.a
Kraft-pulping conditions: 20% active alkali, 25% sulphidity, 170°C, liquor to wood ratio = 4.
DISCUSSION
In previous studies (Van Doorsselaere et al., 1995; Lapierre et al., 1999) we have obtained, via the antisense strategy, poplar trees possessing a very low COMT activity (less than 10% of the control activity). Although the lignin level of these transgenics was not affected, lignin structure was markedly altered with an enrichment in G units and in condensed bonds. Not unexpectedly from these structural traits, these trees proved to be more resistant to kraft pulping (Lapierre et al., 1999). These results prompted us to attempt the opposite strategy, namely to overexpress COMT, via the introduction of a sense transgene, to produce more S-rich and thereby less condensed lignins, more amenable to kraft delignification.
In this context, we prepared two constructs with the entire homologous COMT coding sequence (Dumas et al., 1992), but different promoters, namely the constitutive CaMV 35S2 promoter or the CAD promoter, which is specific for lignified tissues (Feuillet et al., 1995). These vectors were introduced in a Populus tremula × Populus alba clone using the efficient procedure of Leplé et al. (1992). COMT activity in the developing xylem of a few transformants was found moderately increased (approximately 2-fold increased). This trait was unfortunately not repeatable for different trees or different cultures of the same line. In addition, such a moderate increase did not induce any change in lignin content and/or structure.
The most remarkable result obtained in the course of this biotechnological work is the production of a transgenic poplar line, line 70SOMT-3, with severe alterations both in COMT activity and lignification. Indeed, this line systematically showed a COMT activity level in the xylem of 6-month-old poplars close to zero (less than 3%). More importantly, and for the first time to our knowledge, this alteration in COMT activity induced a substantial decrease in lignin content. Cytochemical evaluation of cell walls did not show however any reduction in lignin content. This result could be explained by the distinct absorbtivity of G and S units. Past studies have reported reduction in lignin contents for COMT down-regulated tobaccos (primary transformants; Ni et al., 1994), antisense cinnamoyl CoA reductase tobaccos (Piquemal et al., 1998), antisense CAD poplars (Lapierre et al., 1999), and antisense 4-coumarate:CoA ligase poplars (Hu et al., 1999). The reduction of lignin content in poplar is only attained when COMT activity is close to zero, in contrast to 4-coumarate:CoA ligase and to cinnamoyl CoA reductase. The silenced line 70SOMT-3 line was obtained using the CaMV 35S2 promoter; we failed to detect a similar phenomenon when using the eucalyptus CAD promoter. This result further confirms the efficiency of the CaMV 35S promoter for the silencing of COMT endogenes (Atanassova et al., 1995; Tsai et al., 1998).
Wood coloration in this silenced line is similar to the coloration reported in the case of transgenic aspens where the introduction of a COMT sense construct similarly induced silencing phenomenon (Tsai et al., 1998). It is also reminiscent of the coloration of brown midrib bm3 corn mutants, similarly reduced in COMT activity, as the mutation affects the COMT gene (Barrière and Argillier, 1993; Vignols et al., 1995). This coloration is quite distinct from the pale rose coloration of previously reported COMT antisense poplars (Van Doorsselaere et al., 1995). It is probably not directly due to the accumulation of coniferaldehyde end-groups. The increased level of coniferaldehyde end-groups in the 70SOMT-3 line seems more related to the global enrichment in G units, and remains 3-fold lower than the level observed in conifer lignins, which are pure G lignins. The more pronouncedly colored phenotype of the 70SOMT-3 silenced line, relative to the previously obtained antisense lines (Lapierre et al., 1999), could be due to the higher efficiency of the sense-silencing strategy, compared to the antisense strategy (Aida et al., 1998). The severe alteration of COMT activity in the silenced 70SOMT-3 line might be responsible for major phenotypic changes that are just initiated in the lines less efficiently down-regulated by the antisense constructs. Another hypothesis is that the sense and antisense strategies proceed by different mechanisms at the molecular level, as already demonstrated by Que et al. (1998). Our present results together with past results (Baucher et al., 1996) more strongly argue for the first hypothesis.
The substantial disappearance of S units in the lignins of 70SOMT-3 poplars mainly occurs in cell corner and F-SWs. Consistent with the G type of hardwood vessel lignin (Fergus and Goring, 1970) no clear-cut reduction in S units could be evidenced in the vessels by microspectrophotometry. The depletion of S units induces important changes in the inter-unit linkages, which gives the polymer greater similarity to conifer lignins. The incorporation of 5-OH-G units in remarkably high levels could not restore the lignin amount in the 70SOMT-3 line. It seems therefore that this incorporation is less efficient than that of normal lignin units. The lack of S units in an Arabidopsis mutant altered for F5H activity has recently demonstrated that F5H is very important in controlling the flux of phenolics into S lignin units (Meyer et al., 1998). The present results demonstrate that COMT could also be a limiting factor for the production of S units. A complementation of the F5H Arabidopsis mutant with the corresponding cDNA increased the amount of S units; whereas its introduction in the wild-type line induced a silencing phenomenon (Meyer et al., 1998). Such silencing is frequently observed when ectopic copies of cDNA are introduced with the objective of enzyme overexpression (Atanassova et al., 1995; Tsai et al., 1998), which suggests that the use of heterologous transgenes might be more effective to reach this goal.
Kraft-pulping experiments were performed on the 6-month-old transgenic and control poplars. Consistent with the unchanged lignin content and structure, the pulping behavior of the transgenic lines displaying a moderate increase in COMT activity was similar to that of the control line. In contrast, the silenced line proved to be very distinct from the control line with respect to the kraft cook. We found that the 17% reduction in lignin content is effectively counterbalanced by the higher degree of condensation of the lignin. Although the former trait is beneficial to the pulp yield (10% relative increase), the alteration of lignin structure makes the polymer less amenable to industrial degradation processes. This result means that the transformation would positively affect the production of kraft pulp whenever this pulp is used without any further bleaching step. In contrast, it would be negative with respect to the production of bleached pulp, as it would induce an increased consumption of bleaching reagent.
In conclusion, this work demonstrates that an extreme reduction in O-methyltransferase activity has a major impact on lignin content and structure in poplar trees. It further establishes that the down-regulation level directly affects the characteristics of the transgenics. It clearly confirms that COMT essentially contributes to the formation of S units from 5-OH-G precursors, a role similarly and recently ascribed to F5H (Meyer et al., 1998). These two enzymes seem therefore to have a major role in designing lignin monomer composition and degree of condensation.
MATERIALS AND METHODS
Binary Vector Construction
Two binary vectors (352-SOMT and EuCAD-SOMT) were constructed as follows for the expression of the COMT cDNA under the control of the 35S2 or CAD promoters. 352-SOMT: the full-length coding sequence of the poplar (Populus deltoides × Populus trichocarpa) COMT cDNA of pPCL4 (Dumas et al., 1992) was isolated as a NcoI fragment (1.3 kb). Blunt ends were created by Klenow treatment and the cDNA was cloned in the SmaI site of pLBR19 (T. Michael, unpublished results) in the direct orientation with respect to the CaMV 35S regulatory signals. A KpnI/XbaI fragment comprising the 35S2 promoter (Kay et al., 1987), the cDNA, and the CaMV terminator sequences was introduced in the pBIB-HYG binary vector (Becker, 1990). This vector carries a hygromycin resistance gene under the control of the nopaline synthase promoter for the selection of transformed plants (Fig. 5A). EuCAD-SOMT: the same COMT blunt-ended NcoI fragment was cloned behind the pEuCAD promoter in the SmaI site (Feuillet et al., 1995). This fragment (digested with MscI, blunt-ended, and then digested with KpnI) was introduced in the pBIB-KAN binary vector and digested with HindIII-blunt ends/KpnI. This cloning strategy put the nopaline synthase terminator, already present in the binary vector, at the end of the chimeric gene. The pBIB-KAN vector (Becker, 1990) carries a kanamycin resistance gene under the control of the nopaline synthase promoter for the selection of transformed plants (Fig. 5B).
Figure 5.
Schematic outline of 352-SOMT and EuCAD-SOMT constructs. Only the T-DNA regions are shown. LB and RB are respectively left and right borders. p352, 35S CaMV promoter with a double enhancer sequence; pCAD, eucalyptus CAD promoter; nptII, coding sequence of the neomycin phosphotransferase gene conferring resistance to kanamycin; hpt, coding sequence of the hygromycin phosphotransferase gene conferring resistance to hygromycin; tNOS, terminator of the nopaline synthase gene; tCaMV, CaMV terminator; omt, coding sequence of the poplar COMT. Some restriction sites are indicated.
Plant Transformation and Regeneration
The binary vectors introduced into Agrobacterium tumefaciens strain GV301/pMP90 (Koncz and Schell, 1986) by triparental mating were used for poplar (Populus tremula × Populus alba, clone INRA 717 1-B4) transformation according to Leplé et al. (1992) using kanamycin (50 mg/L) or hygromycin (20 mg/L) for selection of transformed cells. Transformants were amplified by micropropagation in vitro before transfer into the greenhouse. Three different cultivations of the control and transgenic lines were independently performed.
Enzyme Assays and DNA Analysis
Crude protein was extracted from young leaves or from developing xylem as previously described (Van Doorsselaere et al., 1995). The protein content was determined by the method of Bradford (1976) using the Bio-Rad reagent (Bio-Rad, Hercules, CA). COMT enzyme assays were conducted with approximately 10 μg of crude protein using tritiated S-adenosyl-l-Met (NEN, Boston) and caffeic acid (Van Doorsselaere et al., 1995). The reported activity (percentage of control) is the mean of three measurements.
DNA was isolated from young leaves according to Doyle and Doyle (1990). The genomic DNA (10 μg) was digested with BamHI. DNA gel blots were performed according to standard protocols (Sambrook et al., 1989) and transferred to Biodyne B (PALL) membranes. Southern hybridization was performed according to Church and Gilbert (1984). An NcoI fragment, corresponding to the entire COMT coding region, was randomly primed, 32P-labeled, and used as a probe.
Histochemical Staining and Lignin Characterization
Fresh free-hand cross-sections of secondary stems from control and transgenic poplar lines were subjected to Wiesner and Mäule stainings according to standard protocols. Photographs were taken under a binocular microscope. Visible light absorption spectra were measured from 20-μm-thick cross-sections just after the Mäule color reaction (Yoshinaga et al., 1992) using a microspectrophotometer (Carl Zeiss MPM-800, Zeiss, Jena, Germany). UV absorption spectra were measured from 1-μm-thick cross-sections from specimens embedded in methacrylate resin (Yoshinaga et al., 1997). UV absorption was surveyed in the range of 250 to 400 nm by 2.5-nm steps for spot diameters (0.5 μm) and a band width of an illuminating monochrometer (5 nm).
Three series of lignin analysis experiments were performed on 6-month-old poplars grown in the greenhouse. The lignin content of the ground and extractive-free xylem was estimated by the standard Klason procedure (Dence, 1992). In addition, the determination of lignocellulose (wall fraction essentially composed of lignin and cellulose) was run according to Jarrige (1961) and Monties (1984). sd between independent replicates of Klason analysis was in the 0.5% to 1% range. Lignin structure was investigated using thioacidolysis and its most recent developments (Lapierre et al., 1995). The lignin-derived monomers and dimers were identified by GC-MS of their TMS derivatives. The determination of p-hydroxybenzoic esters linked to poplar lignins was done by mild alkaline hydrolysis according to a previously published procedure (Lapierre et al., 1999). sd for thioacidolysis and alkaline hydrolysis replicates was approximately 5% of the mean reported value.
Kraft-Pulping Experiments
Two series of control and transgenic poplars independently grown in the greenhouse were subjected to kraft-pulping assays as reported (Lapierre et al., 1999). The cooking conditions were 20% active alkali, 25% sulfidity, liquor to wood ratio of 4, temperature raised to 170°C during 60 min and then maintained for 60 min. The performances of the poplar lines with respect to kraft pulping were evaluated by the pulp yield, the pulp κ number and the cellulose DP according to standard procedures and as already described (Lapierre et al., 1999).
ACKNOWLEDGMENTS
The authors thank Frédéric Legée, for running the Klason lignin analysis, Valérie Ferret for the COMT assays, Gérard Vastra for the poplar cultivation in the greenhouse, and Mark Tepfer for critical reading of the manuscript.
Footnotes
This work was financially supported by the European Commission DGXII, Fishery and Agro-Industrial Research Program (TIMBER program, contract no. FAIR–CT95–0424).
LITERATURE CITED
- Adler A. Lignin chemistry: past, present and future. Wood Sci Technol. 1977;11:169–218. [Google Scholar]
- Aida R, Yoshida T, Ichimura K, Goto R, Shibata M. Extension of flower longevity in transgenic torenia plants incorporating ACC oxidase gene. Plant Sci. 1998;138:91–101. [Google Scholar]
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier M-T, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477. [Google Scholar]
- Barrière A, Argillier O. Brown-midrib mutants of maize: a review. Agronomie. 1993;13:865–876. [Google Scholar]
- Baucher M, Chabbert B, Pilate G, Van Doorselaere J, Tollier M-T, Petit-Conil M, Cornu D, Monties B, Inzé D, Jouanin L, Van Montagu M, Boerjan W. Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiol. 1996;112:1479–1490. doi: 10.1104/pp.112.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucher M, Monties B, Van Montagu M, Boerjan W. Biosynthesis and genetic engineering of lignin. Crit Rev Plant Sci. 1998;17:125–197. [Google Scholar]
- Becker D. Binary vectors which allow the exchange of plant selectable markers and reporter genes. Nucleic Acids Res. 1990;18:203. doi: 10.1093/nar/18.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudet AM. A new view of lignification. Trends Plant Sci. 1998;3:67–71. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bugos RC, Chiang VLC, Campbell WH. cDNA cloning, sequence analysis and seasonal expression of lignin-bispecific caffeic acid/5-hydroxyferulic acid O-methyltransferase of aspen. Plant Mol Biol. 1991;17:1203–1215. doi: 10.1007/BF00028736. [DOI] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dence C. Lignin determination. In: Dence C, Lin S, editors. Methods in Lignin Chemistry. Berlin: Springer-Verlag; 1992. pp. 33–61. [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissues. Focus. 1990;12:13–15. [Google Scholar]
- Dumas B, Van Doorsselaere J, Legrand M, Fritig B, Van Montagu M, Inzé D. Nucleotide sequence of a complementary DNA encoding O-methyltransferase from poplar. Plant Physiol. 1992;98:796–797. doi: 10.1104/pp.98.2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus BJ, Goring AI. The location of guaiacyl and syringyl lignins in birch xylem tissue. Holzforschung. 1970;24:113–117. [Google Scholar]
- Feuillet C, Lauvergeat V, Deswarte C, Pilate G, Boudet A, Grima-Pettinati J. Tissue- and cell-specific expression of a cinnamyl alcohol dehydrogenase promoter in transgenic poplar plants. Plant Mol Biol. 1995;27:651–667. doi: 10.1007/BF00020220. [DOI] [PubMed] [Google Scholar]
- Grima-Pettenati J, Goffner D. Lignin genetic engineering revisited. Plant Sci. 1999;145:51–65. [Google Scholar]
- Higuchi T. Biosynthesis of lignin. In: Higuchi T, editor. Biosynthesis and Biodegradation of Wood Components. Orlando, FL: Academic Press; 1985. pp. 141–160. [Google Scholar]
- Hu W-J, Harding SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai C-J, Chiang VL. Repression of lignin biosynthesis promote cellulose accumulation and growth in transgenic trees. Nat Biotechnol. 1999;17:808–812. doi: 10.1038/11758. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Hemme MR, Chapple C. New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate-5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci USA. 1999;96:10045–10050. doi: 10.1073/pnas.96.18.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BH, Sakakibara A. Hydrogenolysis of protolignin: XVIII. Isolation of a new dimeric compound with a heterocycle involving α, β-diether. Holzforschung. 1981;35:297–300. [Google Scholar]
- Jarrige R. Analyze des constituants glucidiques des plantes fourragères. Ann Biol Anim Biochim Biophys. 1961;1:163–212. [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, McPherson J. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236:1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Lapierre C, Monties B, Rolando C. Thioacidolysis of poplar lignins: identification of monomeric syringyl products and characterization of guaiacyl-syringyl rich fractions. Holzforschung. 1988;40:113–118. [Google Scholar]
- Lapierre C, Pollet B, Petit-Conil M, Toval G, Romero J, Pilate G, Leplé JC, Boerjan W, Ferret V, De Nadai V, Jouanin L. Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol. 1999;119:153–163. doi: 10.1104/pp.119.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Rolando R. New insights into the molecular architecture of hardwood lignins by chemical degradation methods. Res Chem Intermed. 1995;21:397–412. [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L. Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep. 1992;11:137–141. doi: 10.1007/BF00232166. [DOI] [PubMed] [Google Scholar]
- Marita JM, Ralph J, Hatfield RD, Chapple C. NMR characterization of lignins in Arabidopsis altered in the activity of ferulate 5-hydroxylase. Proc Natl Acad Sci USA. 1999;96:12328–12332. doi: 10.1073/pnas.96.22.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple C. Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:6619–6623. doi: 10.1073/pnas.95.12.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monties B. Dosage de la lignine insoluble en milieu acide. Agronomie. 1984;4:387–392. [Google Scholar]
- Nakano J, Meshitsuka G. The detection of lignin. In: Dence C, Lin S, editors. Methods in Lignin Chemistry. Berlin: Springer-Verlag; 1992. pp. 23–32. [Google Scholar]
- Ni W, Paiva NL, Dixon RA. Reduced lignin in transgenic plants containing a caffeic acid O-methyltransferase antisense gene. Transgenic Res. 1994;3:120–126. [Google Scholar]
- Osakabe K, Tsao CC, Li L, Popko JL, Umezawa T, Carraway DT, Smeltzer RH, Joshi CP, Chinag VL. Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angisoerms. Proc Natl Acad Sci USA. 1999;96:8955–8960. doi: 10.1073/pnas.96.16.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilate G, Leplé J-C, Baucher M, Van Doorsselaere J, Boerjan W, de Nadaï V, Jouanin L, Lapierre C. Biological Sciences Symposium, San Francisco, USA. Technological Association for Pulp and Paper Industries Proceedings. Atlanta: Tappi Press; 1997. Transgenic poplar trees with altered lignin and improved pulping characteristics; pp. 507–514. [Google Scholar]
- Piquemal J, Lapierre C, Myton K, O'Connell A, Schuch W, Grima-Petenatti J. Down-regulation of cinnamoyl-CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J. 1998;13:71–83. [Google Scholar]
- Que Q, Wang H-Y, Jorgensen RA. Distinct patterns of pigment suppression are produced by allelic sense and antisense chalcone synthase transgenes in petunia flowers. Plant J. 1998;13:401–409. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Smith DC. p-Hydroxybenzoate groups in the lignin of aspen (Populus tremula). J Chem Soc. 1955. pp. 2347–2351. [Google Scholar]
- Tsai C-J, Popko JL, Mielke MR, Hu W-J, Podila GK, Chiang VL. Suppression of O-methyltransferase gene by homologous sense transgene in quaking aspen causes red-brown wood phenotypes. Plant Physiol. 1998;117:101–112. doi: 10.1104/pp.117.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doorsselaere J, Baucher M, Chognot E, Chabbert B, Tollier MT, Petit-Conil M, Leplé J-C, Pilate G, Cornu D, Monties B, Van Montagu M, Inzé D, Boerjan W, Jouanin L. A novel lignin in poplar trees with a reduced caffeic/5-hydroxyferulic acid O-methyltransferase activity. Plant J. 1995;8:855–864. [Google Scholar]
- Van Doorsselaere J, Dumas B, Baucher M, Fritig B, Legrand M, Van Montagu M, Inzé D. One-step purification and characterization of a lignin-specific O-methyltransferase from poplar. Gene. 1993;133:213–217. doi: 10.1016/0378-1119(93)90640-o. [DOI] [PubMed] [Google Scholar]
- Vignols F, Rigau J, Torres MA, Capellades M, Puigdomnech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methytransferase. Plant Cell. 1995;7:407–416. doi: 10.1105/tpc.7.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H, Varner JE. Differential expression of two O-methyltransferases in lignin biosynthesis in Zinnia elegans. Plant Physiol. 1995;108:459–467. doi: 10.1104/pp.108.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga A, Fujita M, Saiki H. Relationships between cell evolution and lignin structural varieties in oak xylem evaluated by microscopic spectrophotometry with separated cell walls. Mokuzai Gakkaishi. 1992;38:629–637. [Google Scholar]
- Yoshinaga A, Fujita M, Saiki H. Secondary wall thickening and lignification of oak xylem components during latewood formation. Mokuzai Gakkaishi. 1997;43:377–383. [Google Scholar]
- Zhong R, Morrison IIIWH, Negrel J, Ye Z-H. Dual methylation pathways in lignin biosynthesis. Plant Cell. 1998;10:2033–2045. doi: 10.1105/tpc.10.12.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]