Abstract
Background
Efficient biomarkers for early prediction and diagnosis of an acutely symptomatic carotid plaque rupture event are currently lacking, impairing the ability to diagnose and treat patients with an acute plaque rupture events in a timely fashion. Resolvins are endogenous inflammation-resolving lipid mediators that are induced by inflammatory insults. We hypothesized that resolvin and other lipid profiles in sera likely mark the process towards plaque rupture.
Methods
Circulating lipids associated with plaque rupture events were quantitatively profiled via targeted mediator-lipidomics using ultraperformance liquid chromatography tandem mass spectrometry in patients with acutely symptomatic and asymptomatic carotid disease.
Results
Resolvin D1 (RvD1, 82 ± 11 pM vs. 152 ± 17 pM, p = 0.001) and docosahexaenoic acid (DHA) (0.052 ± 0.007 μM versus 0.076 ± 0.008 μM, p = 0.025) levels are decreased in the sera of patients presenting with an acutely symptomatic carotid plaque rupture event (n=21) compared to patients with asymptomatic (n=24) high-grade carotid stenosis. Circulating arachidonic acid (AA) levels, however, were higher (0.429 ± 0.046 μM versus 0.257 ± 0.035 μM, p < 0.01) in acutely symptomatic compared to asymptomatic carotid patients. ROC curve analysis demonstrates that the serum ratio AA:RvD1 (AUC 0.84, sensitivity 0.71, specificity 0.92) and AA:DHA (AUC 0.86, sensitivity 0.90, specificity 0.71) are biomarkers for the risk of atherosclerotic plaque rupture.
Conclusions
A circulating pro-inflammatory lipid profile, characterized by high AA:RvD1 and AA:DHA, is associated with acutely symptomatic carotid disease and stroke.
Keywords: lipid metabolites, DHA, EPA, RvD1, stroke, carotid, atherosclerosis
1. Introduction
Atherosclerotic plaques arise in the vessel intima and media and are thought to be a result of cholesterol deposition, hemodynamic strain, and inflammation[1]. Rupture of the fibrous cap leads to a transition from a stable to an unstable atherosclerotic plaque. This plaque rupture results in clinically relevant sequelae: in the coronary bed, an atherosclerotic plaque rupture leads to myocardial infarction and, in the carotid artery, to an ocular or cerebral ischemic event. These latter events manifest as amaurosis fugax, transient ischemic attacks, or stroke. There is a need for biomarkers that can be used by clinicians to diagnose the potential rupture of atherosclerotic plaques.
Gaps in knowledge remain in the mechanisms leading to acute symptomatic atherosclerotic plaque rupture. Increasing evidence suggests that unresolved inflammation contributes to the development of plaque rupture[2–4]. Endogenous omega-3 and omega-6 polyunsaturated fatty acids (ω3-PUFAs and ω6-PUFAs) and their metabolites likely play crucial roles in atherosclerotic rupture-associated chronic inflammation. Supplementation with ω3 docosahexaenoic acid (DHA)-rich fish oil results in fibrous cap thickening and stabilization[5]. We previously demonstrated that carotid plaques from neurologically symptomatic patients are inflammatory and have decreased intra-plaque levels of ω3 fatty acids, including DHA[4]. Consistent with this observation, resolvin D1 (RvD1, 7S, 8R, 17S-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid), a potent inflammation-resolving lipid mediator biosynthesized from DHA, was reported recently to promote plaque stability, including decreased lesion oxidative stress and necrosis, improved efferocytosis, and fibrous caps thickening[2]. Resolvins, including RvD1, are inflammation-resolving mediators initially uncovered in the resolution phase of inflammation. DHA-derived RvD1 contributes to the atherosclerotic plaque stabilization by DHA[2]. Arachidonic acid (AA) is the major ω6 PUFA in humans and animals. AA is converted to a large array of short-lived pro-inflammatory eicosanoids including thromboxane A4, leukotriene B4, and sulfido-peptide leukotrienes and generally thought to promote inflammation. We aimed to determine whether circulating serum RvD1, DHA, and AA levels represent the risk of atherosclerotic plaque rupture.
2. Materials and Methods
2.1. Study Design
Patients with ≥ 50% internal carotid artery stenosis undergoing carotid endarterectomy (CEA) were included in this study. Informed consent was obtained after approval through the Ochsner Clinic Institutional Review Board. For each enrolling patient, the age, sex, history of cardiac disease, chronic renal insufficiency (defined as a serum creatinine ≥ 1.6 mg/dL) diabetes, hypertension, and history of tobacco use were recorded, as well as current medication use (antiplatelet, oral hypoglycemic, antihypertensive, or lipid-lowering). Any previous ipsilateral ischemic symptoms, including time from onset were also taken into account. Carotid plaque stability was determined on the basis of presenting symptomatology, confirmed by imaging (magnetic resonance imaging and/or computed tomography angiography imaging of the brain). Asymptomatic patients with high-grade carotid stenosis (≥ 80% internal carotid stenosis based on duplex ultrasound imaging) were included in the stable or asymptomatic carotid atherosclerotic plaque group. Patients presenting with symptoms of temporary or partial/complete loss of vision, a transient ischemic attack, and/or an established stroke with good neurologic recovery in the index hospitalization and deemed safe to undergo CEA were included as urgent or unstable carotid plaques[6]. The cohort included 24 asymptomatic and 21 acutely symptomatic patients deemed candidates for an urgent carotid endarterectomy (CEA) during the index hospitalization[7]; mean time to intervention for acute interventions was 2.6 days (Table 1).
Table 1.
Patient demographics.
| Characteristics | Total (n=45) | Urgent (n=21) | Asymptomatic (n=24) | P value |
|---|---|---|---|---|
| Age, year | 65.53 ± 8 | 63.57 ± 6.59 | 67.25 ± 9 | 0.14 |
| Male sex | 28 | 13 | 15 | |
| BMI | 28.16 ± 5.08 | 28.13 ± 5.55 | 28.18 ± 4.75 | 0.97 |
| Total cholesterol mg/dL | 173.79 ± 52.54 | 180 ± 58.91 | 167.82 ± 46.25 | 0.45 |
| HDL mg/dL | 41.79 ± 16.24 | 42 ± 18.09 | 41.36 ± 14.69 | 0.86 |
| LDL mg/dL | 96.21 ± 42.01 | 99.63 ± 46.66 | 92.79 ± 37.7 | 0.61 |
| Triglycerides mg/dL | 184.98 ± 139.77 | 199 ± 146 | 171.32 ± 135.54 | 0.52 |
| Serum creatinine mg/dL | 1.19 ± 0.5 | 1.3 ± 0.61 | 1.09 ± 0.37 | 0.18 |
| Current (all) smoker | 15 (33) | 8 (17) | 7 (16) |
BMI, body mass index; HDL, High-density lipoprotein; LDL, low-density lipoprotein; all smoker refers to a history of prior but not active tobacco use
2.2. Lipid extraction and LC-MS/MS-based lipidomic analysis
The sera were isolated by centrifugation from the fasting peripheral blood of human patients who were identified as urgent/acutely symptomatic and asymptomatic carotid patients. The samples and associated information were de-identified after transfer from the Ochsner Clinic and handled according to the protocol approved by Institutional Review Board of Louisiana State University Health New Orleans. The extraction and LC-MS/MS-based lipid mediator analysis were performed following the protocols that others and we developed and used previously[4, 8–21] (http://www.hmdb.ca/metabolites/HMDB03733). Briefly, deuterium-labeled internal standards [2 ng of each, d4-prostaglandin D2 (d4-PGD2) and d5-DHA in 20 μl methanol] were added to each serum sample (100 μl each) at ~4°C on water-ice to determine the extraction recoveries (typically >80%) of the lipid mediators. Two volumes of ice-cold LC-MS/MS-grade methanol (EMD Millipore, MA) containing 0.005% butylated hydroxytoluene (BHT, to prevent auto-oxidation) and 0.1 mM acetic acid were added to each serum sample on ice. The mixtures were vortexed for 10 minutes. The supernatants were collected after centrifugation. The pellets were extracted two more times with 200 μl methanol:water (2:1) containing 0.005% BHT and 0.01 mM acetic acid. The extraction supernatants of each serum sample were pooled together and diluted with 10-volume water containing 0.005% BHT and 0.01 mM acetic acid. The mixture of each sample with apparent pH 4.5 was cleaned up with C18 solid phase extraction (500 mg/cartridge, Waters, Milford, MA); the cleaned extracts were reconstituted into 50% methanol and analyzed via LC-MS/MS for lipidomic analysis of eicosanoids and docosanoids.
The settings of the LC-MS/MS instrument were as follows. Xevo TQ-S triple quadruple tandem mass spectrometry equipped with Acquity I Class UPLC (Waters) was used. The UPLC was carried out with an Acquity UPLC HSS T3 column (1.8-μm particle size × 2.1 inner diameter × 50mm length). At 0.4 ml/min flowrate, the mobile phase ramped from 45% of solvent A (H2O + 0.01% acetic acid) and 55% of solvent B (methanol + 0.01% acetic acid) to 15% of solvent A and 85% of solvent B in 10 minutes, then ramped to 2% of solvent A and 98% of solvent B in 18 minutes, and then stayed at 2% of solvent A and 98% of solvent B until 25 minutes, and finally changed back to 45 % of solvent A and 55% of solvent B and re-equilibrated until 30 minutes passed. The capillary voltage was −2.5 kV. The desolvation temperature was 600°C, and the desolvation gas flowed at 1,100 L/h. The cone gas was 150 L/h, the nebulizer pressure was 7.0 Bars, and ion source temperature was 150°C. Argon collision gas was set at 0.13 mL/min and 3.8 mbar. The injection volume of the coupled autosampler was 10 μL.
LC-MS/MS data were analyzed using MassLynx version 4.1 software (Waters). The compounds in the serum samples were identified by matching the LC multiple reaction monitoring (MRM)-MS/MS ions and chromatographic retention times (within ± 0.1 min window) to those of the standards. The mixture of standards was analyzed between every 8-10 sample analyses at the same LC-MS/MS conditions as the sample analysis. The extraction recovery was determined using the deuterium-labeled internal standards. The quantities of compounds were calculated from areas of identified LC MRM-MS/MS chromatographic peaks, extraction recoveries, and calibration curves of compound standards. The concentrations (pico-molar, or pMs) of these lipids in sera (100 μL each) were then calculated. The identification of analytes was also confirmed by the full scan MS/MS spectra and LC chromatographic retention times acquired from samples via LC-full scan MS/MS after comparing with the standard compound.
RvD1, DHA, AA were purchased from caymanchem.com (Cayman Chemical, Ann Arbor, Michigan) and used as external standards for the tuning, calibration, and other optimization of LC-MS/MS equipment. Deuterium-labeled d4-prostaglandin D2 (d4-PGD2) and d5-DHA from Cayman Chemical were used as the internal standards to determine the recoveries of lipid extraction and quantities of the analytics in LC-MS/MS analysis.
2.3. Statistical analysis
All data were presented in average ± standard error of the mean (SEM) and analyzed using W ’ t tests. A p-value of ≤ 0.05 was considered statistically significant. Logistic regression was used for multivariable analysis. SPSS software (www.ibm.com) was used for the statistical analysis. Logistic regression was used for ROC analysis. Clinical factors (gender, age) do not have a significant effect on each metabolite, with p values being 0.86 (gender) and 0.9 (age) in the DHA model, 0.92 (gender) and 0.2 (age) in the AA model, and 0.54 (gender) and 0.42 (age) in the RvD1 model. Thus, these factors were excluded from the logistic models. It is important to note that for the problem of possibly over fitting the logistic model for AUC, we found that AUC for the top predictors does not drop much after applying the leave-one-out cross validation procedure (AUC changes from 0.8611 to 0.8333 for AA:DHA, from 0.8353 to 0.7758 for AA:RvD1, and from 0.8988 to 0.8651 for the sum of the Z scores of DHA:AA and the Z scores of RvD1:AA). These indicate that there was no problem with severe over fitting. Modeling and calculation of related area under curve (AUC), sensitivity/specificity, positive likelihood ratio (LR+), negative LR (LR−), and accuracy were carried out in SAS and R software packages.
3. Results
3.1. Optimization of LC-MS/MS for lipidomic analysis of patients’ sera
There were no differences between the two patient groups’ demographice (Table 1). We extracted lipid mediators using methanol and solid phase extraction (SPE) technique as descried previously[12]. SPE usually yields better recovery and cleanup of analysts than liquid-liquid extraction alone. We used LC MRM-MS/MS methodology to analyze the serum extracts because it offers high sensitivity, reproducibility, and selectivity to analyze small molecules, including the compounds targeted in this report[22–26]. Supplementary Table 1 and Supplementary Figure 1 present the optimized MRM parent ions in Q1 sector, signature daughter ions in Q3 sector, cone voltages, collision voltages, and LC MRM-MS/MS retention times for each compound that, together, yielded the best sensitivity and reproducibility in analysis of inflammation-resolving resolvin D series and lipoxin A4, as well as their respective precursors DHA and AA. The optimization provided each compound a unique set of parameters that were different from those of other compounds (Supplementary Table 1 and Supplementary Fig. 1), which allowed for the specific detection of each compound by LC MRM-MS/MS. The identification of RvD1 in sera was verified further by an MS/MS full-scan spectrum acquired from a larger volume of sera of patients (Supplementary Fig. 2), which possessed diagnostic MS/MS ions of RvD1 as showed in the MS/MS spectrum of resolving D1 standard.
3.2. Resolvin D1 and ratio of arachidonic acid:resolvin D1 or arachidonic acid:docosahexaenoic acid: potential serum biomarkers for diagnosis of acutely symptomatic carotid plaques
Following the widely-used methodology to identify small molecules, including lipid mediators in the picogram range in physiopathological specimens[22–26], we used the criterion that the compounds identified in a patient’s sera must have their LC MRM-MS/MS chromatographic retention times (within ± 0.1 min window) and signature MRM product ions match to those of the authentic standards. RvD1 was identified by this criterion (Supplementary Fig. 1 and Supplementary Fig. 2). However, other resolvins and lipoxin A4 were only detected when their amounts reached the minimal detection limits in one or two samples, thus they were not considered for biomarker potentials. DHA and AA were also identified in abundant quantities in sera (Supplementary Fig. 1).
Next, we quantified RvD1, AA, and DHA based on their LC MRM-MS/MS chromatographic peak areas, extraction recoveries, and linear calibration curves (Table 2). LC MRM-MS/MS chromatographic peak quantification of RvD1, AA, and DHA revealed that sera RvD1 concentrations were 82 ± 11 pM for acutely symptomatic (urgent) vs. 152 ± 17 pM for asymptomatic carotid patients (p = 0.001). Additionally, DHA (precursor of RvD1 biosynthesis) was lower in the sera of urgent, compared to asymptomatic carotid patients (0.052 ± 0.007 μM versus 0.076 ± 0.008 μM, p = 0.025). However, the serum concentration of AA, a major ω-6 PUFA, was higher in urgents vs. asymptomatic (0.429 ± 0.046 μM versus 0.257 ± 0.035 μM, p < 0.01). RvD1 concentration provided the strongest correlation for urgent status (p < 0.01); high levels occurred in stable plaques, while low levels occurred in the acute rupture. Note that AA and DHA are much more prominent in the sera as these are in the μM range, compared to RvD1, which is detected in the pM range.
Table 2.
Sera concentrations of select inflammation-resolving lipid mediators and omega-3 (DHA) and omega-6 (AA) PUFAs quantified by LC-MS/MS.
| Lipids | Urgent | Asymptomatic | P-value |
|---|---|---|---|
| AA (C20:4-n6) | 0.429 ± 0.046 (μM) | 0.257 ± 0.035 (μM) | 0.005 |
| DHA (C22:6-n3) | 0.052 ± 0.007 (μM) | 0.076 ± 0.008 (μM) | 0.025 |
| EPA (C20:5-n3) | 0.045 ± 0.004 (μM) | 0.056 ± 0.004 (μM) | 0.077 |
| RvD1 | 82 ±11 (pM) | 152±17(pM) | 0.001 |
Notes: Results are mean ± SEM, 21 acute patients and 24 asymptomatic patients; μM: micromolar/L serum; pM: picomolar/L serum.
Next, we investigated whether the sera concentration ratio of AA:DHA, AA:RvD1, and DHA:RVD1 could discern differences between the urgent and asymptomatic groups.
To investigate whether the concentration ratio of AA:DHA, AA:RvD1, DHA:RVD1, in the sera also could manifest differences between the acutely symptomatic and asymptomatic groups, we compared these ratios. The ratios of AA:DHA and AA:RvD1 were associated with urgent; both were higher in the sera of acutely symptomatic patients (10.3 ± 1.4 versus 4.1 ± 0.6, p < 0.01 and 7331.0 ± 1280.8 versus 2897.4 ± 932.0, p < 0.01, respectively), whereas there was no significant difference for DHA:RvD1 (Fig. 1A). Moreover, to determine whether the concentration ratio of AA:RvD1 or AA:DHA has an ability to predict the acute symptom of the carotid, the predictive values of AA:RvD1, AA:DHA, RvD1, AA, and DHA were evaluated by ROC curve analysis. The areas under the ROC curve (AUC) for predicting carotid plaque rupture were in descending order: 0.8611 (0.7525, 0.9698, 95% CI) for AA:DHA; 0.8353 (0.7097-0.9609, 95% CI) for AA:RvD1; 0.7619 (0.6178, 0.9061, 95% CI) for RvD1; 0.756 (0.6122, 0.8997, 95% CI) for AA; 0.6905 (0.5312, 0.8497, 95% CI) for DHA; and 0.5595 (0.3804, 0.7387, 95% CI) for DHA:RvD1 (Fig. 1B and 1C). The p values show that these concentrations of AA, DHA, and RvD1 as well as their concentration ratios had AUC significantly greater than 50% (classification by chance). Both AUC and accuracy show that AA:DHA and AA:RvD1 perform best. Further comparisons show that the AUCs of AA:DHA and AA:RvD1 were not significantly different (p value = 0.72), but they were significantly different from that of DHA:RvD1 (p values = 0.0121 and 0.0002). The AUCs of DHA, AA, RvD1 were not significantly different (p values: 0.6147 for AA vs DHA; 0.4636 for DHA vs RvD1). More interestingly, we found that combining two concentration ratios improved the ability of predicting carotid plaque rupture. Using the sum of the Z scores of DHA:AA and the Z scores of RvD1:AA in ROC analysis, the AUC increases to 0.90 (0.8069, 0.9907, 95% CI), which is significantly greater than 50%. This combination may provide us a powerful multi-parameter prediction tool.
Fig. 1. Potential composite biomarkers: concentration ratios of resolving lipid mediator RvD1 to omega-3 docosahexaenoic acid (DHA) or to omega-6 arachidonic acid (AA) and their receiver operating characteristic analysis.
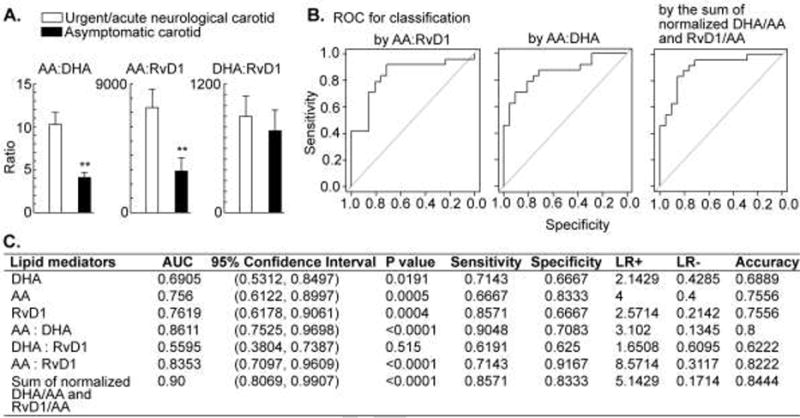
A) Concentration ratios of AA:DHA, AA:RvD1, and DHA:RvD1 (pM/pM). B) Receiver operating characteristic (ROC) curve of candidate biomarker AA:RvD1 and AA:DHA concentration ratio (pM/pM), as well as the sum of the normalized DHA/AA and RvD1/AA. C) Area under the curve (AUC) of ROC analysis and 95% confidence interval for each candidate biomarker: RvD1, DHA, AA, AA:DHA (pM:pM), DHA:RvD1 (pM/pM), AA:RvD1 (pM/pM), and the sum of the normalized DHA/AA and RvD1/AA
4. Discussion
Unresolved chronic inflammation is a major factor in atherosclerotic plaque rupture. Resolvins are endogenous inflammation-resolving lipid mediators that are triggered by inflammation, likely as a self-defense action. In instances where resolvins are not adequately expressed, a pro-inflammatory state dominates[2, 27–29]. Furthermore, in the case of atherosclerosis, inflammation is a component that is associated with the development of atherosclerotic plaque rupture [1, 2]. Here we demonstrate that circulating resolvin levels correspond to carotid plaque stability. Utilizing a translational model of plaque rupture[30], serum levels of RvD1 and DHA, a precursor of RvD1, were found to be markedly lower in patients presenting with acutely symptomatic plaque rupture events compared to patients with asymptomatic carotid disease. This correlates with our previous work demonstrating that acutely symptomatic plaques have a decreased content of anti-inflammatory lipids, such as DHA[4]. Additionally AA, the major ω6 PUFA human blood, was detected with at higher ratios of AA/RvD1 or AA/DHA in the sera of acutely symptomatic carotid patients as compared to asymptomatic carotid patients. The mechanisms underpinning this observation deserves further study in the future.
As we searched for a biomarker of plaque rupture, we chose to focus on the concentration ratio of AA:RvD1 (pM:pM) in the sera because this incorporates the AA and RvD1 differences noted between the acutely symptomatic and asymptomatic carotid patients. This was confirmed by our observation that the AA:RvD1 ratio was significantly higher for acutely symptomatic patients than asymptomatic patients (Fig. 1A). The serum concentration ratio of AA:DHA had a similar trend. These ratios are unitless and dimensionless, which could reduce the systematic errors in the analysis of the lipids. Additionally, the ratio uses one value instead of two values, which could simplify the presentation of the lipid mediators in the patient sera. To further examine the predictive values of RvD1, AA, DHA, AA:RvD1, and AA:DHA, we performed ROC curve analysis to calculate the sensitivity and specificity in the differentiation of the acutely symptomatic from the asymptomatic carotid patients. This analysis indicates that AA:RvD1, RvD1, AA:DHA, DHA, or AA has the potential to be a biomarker for diagnosis. However, AA:DHA and AA:RvD1 had the highest AUC in ROC.
A power analysis shows that at 5% significance level, our cohort of 21 acutely symptomatic and 24 asymptomatic patients has a relative high power to detect the effect size in most lipid mediators and ratios. Specifically, there is a power of: 0.92 for RvD1 samples, 0.82 for AA samples, 0.62 for DHA samples, 0.98 for the ratio of AA:DHA, 0.78 for the ratio of AA:RvD1, but low power of 0.08 for the ratio of DHA:RvD1.
Efficient biomarkers for early prediction and diagnosis of acute symptomatic plaques are lacking, which impedes the ability of physicians to promptly diagnose and treat patients presenting, with an atherosclerotic plaque rupture event. Minimizing the time between plaque rupture and treatment is critical in reducing the morbidity after a patient has sustained a stroke as select patients diagnosed within 3 hours are offered systemic thrombolysis with recombinant tissue plasminogen activator (tPA), which improves outcomes after stroke[31]. However, tPA utilization rates among acute ischemic stroke patients is less than 7%, mainly due to delay in diagnosis[7]. The novel RvD1-based biomarker candidates identified here aim to fill this gap. For example, a simple ELISA or other detection kits could be developed to quantify RvD1 in the sera and have the potential to minimize diagnostic delays, aid in patient selection for possible thrombolysis and intervention.
Furthermore, a serum biomarker predictive of plaque vulnerability and future stroke risk could also be helpful in the management of asymptomatic high-grade carotid stenosis. The stroke reduction benefit from CEA over medical therapy in patients with asymptomatic carotid disease is based on randomized controlled trials from the 1990s[32]. Since that time, however, both the safety of surgery (CEA) and medical management of carotid disease have improved[33]. Many now question which is the best therapy for the patient with asymptomatic high-grade carotid disease[33]. Identification of circulating inflammation-resolving lipid mediators could, hypothetically, be used to predict which individuals with high-grade asymptomatic carotid disease are at risk of plaque rupture and stroke. Thus, earlier identification could then be used to treat such patients with more intense medical therapy or with a prophylactic CEA.
Although our experimental results provide a preliminary demonstration of the capability of RvD1 as a biomarker for the diagnosis and prediction of acute symptomatic plaque ruptures, we need to further confirm these results on a larger population of patients. Other precursors or metabolites of resolvin biosynthesis and degradation pathways also may be unique biomarkers for this purpose, and therefore this deserves further exploration. The profiles of whole fatty acids and enzyme systems warrant a thorough study in future research; this will help decipher the underlined mechanisms for our findings in this report. The LC-MS/MS based tools and protocols established in this report provide the basis for these future studies. These studies also will provide critical data that can facilitate the exploration of cellular and molecular mechanisms underlying RvD1 deficiency in the sera of acute symptomatic plaque patients. Future studies are needed to determine how these serum lipid profiles will aid in predicting carotid plaque vulnerability and how this could be incorporated into routine clinical practice.
Supplementary Material
Summary.
Fibrous cap rupture of a carotid atherosclerotic plaque leads to a transition from a stable to an unstable atherosclerotic plaque and is the etiology of a stroke. In a translational model for plaque rupture and utilizing ultraperformance liquid chromatography tandem mass spectrometry, the sera of patients presenting acutely with a symptomatic carotid-related transient ischemic attack or stroke (n=21) were found to have lower RvD1 and DHA levels compared to patients with asymptomatic carotid disease (n=24). ROC curve analysis showed that the serum ratio AA:RvD1 and AA:DHA represent a circulating pro-inflammatory lipid profile that is associated with acutely symptomatic carotid disease and stroke. Future work with validate further if these could be biomarkers for the risk of atherosclerotic plaque rupture.
Acknowledgments
This work was supported by an Ochsner Translational Medicine Research Initiative (OTMRI) grant (to H.A. Bazan) and by R01 DK087800 grant (to S. Hong), and R01HL127092 (to T. C. Woods). This work also was supported in part by an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Fredman G, Hellmann J, Proto JD, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature communications. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazan HA, Lu Y, Thoppil D, Fitzgerald TN, Hong S, Dardik A. Diminished omega-3 fatty acids are associated with carotid plaques from neurologically symptomatic patients: Implications for carotid interventions. Vascul Pharmacol. 2009;51:331–336. doi: 10.1016/j.vph.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 6.Bazan HA, Caton G, Talebinejad S, Hoffman R, Smith TA, Vidal G, Gaines K, Sternbergh WC., 3rd A stroke/vascular neurology service increases the volume of urgent carotid endarterectomies performed in a tertiary referral center. Annals of vascular surgery. 2014;28:1172–1177. doi: 10.1016/j.avsg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Bazan HA, Zea N, Jennings B, Smith TA, Vidal G, Sternbergh WC., 3rd Urgent carotid intervention is safe after thrombolysis for minor to moderate acute ischemic stroke. Journal of vascular surgery. 2015;62:1529–1538. doi: 10.1016/j.jvs.2015.07.082. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual review of immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 9.Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50:3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Tian H, Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. Journal of lipid research. 2010;51:923–932. doi: 10.1194/jlr.M000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian H, Lu Y, Shah SP, Hong S. Novel 14S,21-dihydroxy-docosahexaenoic acid rescues wound healing and associated angiogenesis impaired by acute ethanol intoxication/exposure. J Cell Biochem. 2010;111:266–273. doi: 10.1002/jcb.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian H, Lu Y, Shah SP, Hong S. 14S,21R-Dihydroxydocosahexaenoic Acid Remedies Impaired Healing and Mesenchymal Stem Cell Functions in Diabetic Wounds. The Journal of biological chemistry. 2011;286:4443–4453. doi: 10.1074/jbc.M110.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian H, Lu Y, Shah SP, Hong S. Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions. The American journal of pathology. 2011;179:1780–1791. doi: 10.1016/j.ajpath.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong S, Alapure BV, Lu Y, Tian H, Wang Q. 12/15-Lipoxygenase deficiency reduces densities of mesenchymal stem cells in the dermis of wounded and unwounded skin. Br J Dermatol. 2014;171:30–38. doi: 10.1111/bjd.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong S, Lu Y, Tian H, Alapure BV, Wang Q, Bunnell BA, Laborde JM. Maresin-like lipid mediators are produced by leukocytes and platelets and rescue reparative function of diabetes-impaired macrophages. Chem Biol. 2014;21:1318–1329. doi: 10.1016/j.chembiol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S, Tian H, Lu Y, Laborde JM, Muhale FA, Wang Q, Alapure BV, Serhan CN, Bazan NG. Neuroprotectin/protectin D1: endogenous biosynthesis and actions on diabetic macrophages in promoting wound healing and innervation impaired by diabetes. Am J Physiol Cell Physiol. 2014;307:C1058–1067. doi: 10.1152/ajpcell.00270.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. Journal of immunology. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 18.Bazan NG, Marcheselli VL, Lu Y, Hong S, Jackson F. Lipidomic Approaches to Neuroprotection Signaling in the Retinal Pigment Epithelium. In: Fliesler SJ, Kisselev OG, editors. Signal Transduction in the Retina. CRC Press; 2007. pp. 345–374. [Google Scholar]
- 19.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods in enzymology. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 20.Musiek ES, Cha JK, Yin H, Zackert WE, Terry ES, Porter NA, Montine TJ, Morrow JD. Quantification of F-ring isoprostane-like compounds (F4-neuroprostanes) derived from docosahexaenoic acid in vivo in humans by a stable isotope dilution mass spectrometric assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:95–102. doi: 10.1016/j.jchromb.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Mas E, Michel F, Guy A, Bultel V, Falquet Y, Chardon P, Rossi JC, Cristol JP, Durand T. Quantification of urinary F2-isoprostanes with 4(RS)-F4t-neuroprostane as an internal standard using gas chromatography-mass spectrometry Application to polytraumatized patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:133–140. doi: 10.1016/j.jchromb.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Poczobutt JM, Gijon M, Amin J, Hanson D, Li H, Walker D, Weiser-Evans M, Lu X, Murphy RC, Nemenoff RA. Eicosanoid profiling in an orthotopic model of lung cancer progression by mass spectrometry demonstrates selective production of leukotrienes by inflammatory cells of the microenvironment. PloS one. 2013;8:e79633. doi: 10.1371/journal.pone.0079633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claria J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141–1149. doi: 10.1152/ajpcell.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. American journal of hematology. 2008;83:437–445. doi: 10.1002/ajh.21170. [DOI] [PubMed] [Google Scholar]
- 25.Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG. Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl Stroke Res. 2011;2:33–41. doi: 10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazan NG, Eady TN, Khoutorova L, Atkins KD, Hong S, Lu Y, Zhang C, Jun B, Obenaus A, Fredman G, Zhu M, Winkler JW, Petasis NA, Serhan CN, Belayev L. Novel aspirin-triggered neuroprotectin D1 attenuates cerebral ischemic injury after experimental stroke. Exp Neurol. 2012;236:122–130. doi: 10.1016/j.expneurol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Titos E, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Lopategi A, Dalli J, Lozano JJ, Arroyo V, Delgado S, Serhan CN, Claria J. Signaling and Immunoresolving Actions of Resolvin D1 in Inflamed Human Visceral Adipose Tissue. Journal of immunology. 2016;197:3360–3370. doi: 10.4049/jimmunol.1502522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, Sime PJ. Resolvin D1 Reduces Emphysema and Chronic Inflammation. The American journal of pathology. 2015;185:3189–3201. doi: 10.1016/j.ajpath.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, Van Eijl S, Gee LC, Bansal J, Pitrola K, Shaw C, D’Acquisto F, Colas RA, Marelli-Berg F, Dalli J, Ahluwalia A. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. The Journal of clinical investigation. 2017;127:169–182. doi: 10.1172/JCI89429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan HA, Hatfield SA, O’Malley CB, Brooks AJ, Lightell D, Jr, Woods TC. Acute Loss of miR-221 and miR-222 in the Atherosclerotic Plaque Shoulder Accompanies Plaque Rupture. Stroke; a journal of cerebral circulation. 2015;46:3285–3287. doi: 10.1161/STROKEAHA.115.010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. Jama. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 32.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA : the journal of the American Medical Association. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 33.Chaturvedi S, Chimowitz M, Brown RD, Jr, Lal BK, Meschia JF. The urgent need for contemporary clinical trials in patients with asymptomatic carotid stenosis. Neurology. 2016;87:2271–2278. doi: 10.1212/WNL.0000000000003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


