Abstract
Enhancement of aerobic glycolysis and suppression of mitochondrial metabolism characterize the pro-proliferative Warburg phenotype of cancer cells. High free tubulin in cancer cells closes voltage dependent anion channels (VDAC) to decrease mitochondrial membrane potential (ΔΨ), an effect antagonized by erastin, the canonical promotor of ferroptosis. Previously, we identified six compounds (X1-X6) that also block tubulin-dependent mitochondrial depolarization. Here, we hypothesized that VDAC opening after erastin and X1-X6 increases mitochondrial metabolism and reactive oxygen species (ROS) formation, leading to ROS-dependent mitochondrial dysfunction, bioenergetic failure and cell death. Accordingly, we characterized erastin and the two most potent structurally unrelated lead compounds, X1 and X4, on ROS formation, mitochondrial function and cell viability. Erastin, X1 and X4 increased ΔΨ followed closely by an increase of mitochondrial ROS generation within 30 to 60 min. Subsequently, mitochondria began to depolarize after an hour or longer indicative of mitochondrial dysfunction. N-acetylcysteine (NAC, glutathione precursor and ROS scavenger) and MitoQ (mitochondrially targeted antioxidant) blocked increased ROS formation after X1 and prevented mitochondrial dysfunction. Erastin, X1 and X4 selectively promoted cell killing in HepG2 and Huh7 human hepatocarcinoma cells compared to primary rat hepatocytes. X1 and X4-dependent cell death was blocked by NAC. These results suggest that ferroptosis induced by erastin and our erastin-like lead compounds was caused by VDAC opening, leading to increased ΔΨ, mitochondrial ROS generation and oxidative stress-induced cell death.
Keywords: Erastin, mitochondrial dysfunction, reactive oxygen species, tubulin, VDAC, Warburg metabolism
Graphical abstract

Erastin and erastin-like compounds revert tubulin-dependent VDAC inhibition to increase VDAC conductance, leading to mitochondrial hyperpolarization (↑ΔΨm), mitochondrial ROS generation (ROSm) blocked by NAC and MitoQ, mitochondrial dysfunction, and ferroptotic cell death.
1. Introduction
Aerobic glycolysis and suppression of mitochondrial oxidative phosphorylation are characteristic features of the pro-proliferative Warburg metabolic phenotype [1, 2]. Tumors generate up to 90% of cellular ATP through glycolysis with the remaining being supplied by oxidative phosphorylation. By contrast, in aerobic non-proliferative cells, mitochondria contribute as much as 95% of ATP formation [3, 4]. In several types of cancer cells, glycolytic inhibition leads to increased mitochondrial metabolism and vice versa [5–7]. Compounds that inhibit glycolysis or promote mitochondrial metabolism cause tumor cell death both in vitro and in vivo [8–10]. Most research targeting cancer metabolism has focused on inhibiting glycolytic flux and much less to enhancing mitochondrial function [11, 12].
Erastin is a small molecule that causes a type of non-apoptotic, oxidative cell death called ferroptosis in Ras/Raf-mutated cancer cell lines [13]. Ferroptosis is so named because the iron chelator, desferal, prevents erastin-induced cell killing. Indeed, desferal had been shown previously to protect after a variety of oxidative stresses, including ischemia-reperfusion, drug-induced hepatotoxicity and the addition of oxidant chemicals [14–18]. Mechanisms of action for erastin-induced ferroptosis include inhibition of the cysteine-glutamate antiporter in the plasma membrane leading to glutathione depletion and a pro-oxidant state and inhibition of glutathione peroxidase-4 [19]. Erastin also binds to isoforms of the voltage dependent anion channels (VDAC) [20, 21].
In mitochondria, Complexes I, III and IV of the respiratory chain pump protons from the mitochondrial matrix into the intermembrane space to create a protonmotive force (Δp) comprised mostly of a mitochondrial membrane potential (ΔΨ), which drives ATP synthesis through the F1FO-ATP synthase (Complex V). Flux of hydrophilic metabolites into and out of mitochondria, including ATP, ADP, Pi and respiratory substrates, occurs through a variety of inner membrane carriers, but flux of these metabolites across the outer membrane occurs through a single pathway, the voltage dependent anion channel (VDAC). VDAC closure is proposed as a regulatable ‘governator’ of mitochondrial metabolism [22]. In planar lipid bilayers, free tubulin inhibits VDAC1 and VDAC2 but not VDAC3 [23, 24]. Compared to postmitotic cells, proliferating cancer cells have high levels of free tubulin for spindle formation at metaphase. As a consequence, VDAC is in a relatively closed state, which causes a global suppression of mitochondrial metabolism. Since ΔΨ formation requires influx of respiratory substrates, a decrease of free tubulin leading to VDAC opening causes an increase of ΔΨ, whereas a decrease of tubulin leads to decreased ΔΨ [23, 25].
Recently, we showed that erastin antagonizes the inhibitory effects of tubulin on VDAC. After identifying erastin as the first known pharmacological antagonist of the inhibitory effect of free tubulin on VDAC, we identified by high content cell-based screening several erastin-like small molecules that also appear to prevent VDAC closure by high free cytosolic tubulin [26]. Here, we assess the hypothesis that increased mitochondrial metabolism after VDAC opening leads to enhanced mitochondrial generation of reactive oxygen species (ROS), mitochondrial dysfunction, bioenergetic failure and cell death. We show that erastin and two structurally dissimilar erastin-like lead compounds, X1 and X4, promoted mitochondrial hyperpolarization that was followed by mitochondrial depolarization. We also determined that erastin and X1 increased mitochondrial ROS production before onset of mitochondrial depolarization. Moreover, N-acetylcysteine (NAC, glutathione precursor and ROS scavenger) and MitoQ (mitochondrially targeted antioxidant) blocked X1-induced mitochondrial ROS formation and subsequent collapse of ΔΨ. Lastly, the selective lethality of X1 and X4 to cancer cells compared to rat liver hepatocytes was prevented by NAC, as also shown previously for erastin-induced killing of Ras/Raf-mutated cancer cells [20]. These results suggest that our erastin-like lead compounds induce non-apoptotic “ferroptotic” death of cancer cells by reversing tubulin inhibition of VDAC, leading to elevated ΔΨ, increased mitochondrial ROS generation and oxidative stress-induced necrotic cell death.
2. Materials and Methods
2.1 Materials
HepG2 cells and Eagle’s minimum essential medium were purchased from American Tissue Culture Collection (Manassas, VA); MitoSOX Red, CellROX Green and chloromethyl-2’,7’-dichlorodihydrorfluorescein diacetate (cmH2DCFDA) from Thermo Fisher Scientific (Waltham, MA); erastin, NAC, propidium iodide and tetramethylrhodamine methylester (TMRM) from Sigma (St. Louis, MO); 35-mm imaging Petri dishes from MatTek (Ashland, MA); collagenase Type I from Worthington Biochemical (Lakewood, NJ); and X1 (7997272) and X4 (5263261) from Chembridge Corporation (San Diego, CA). All other reagents were analytical grade.
2.2 Cell culture
HepG2 human hepatoma (American Type Culture Collection) and Huh7 human hepatocarcinoma cells (courtesy of Dr. Jack R. Wands, Brown University, Providence, RI) were grown in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin in 5% CO2/air at 37°C. For all experiments, cells were used 48 h after plating. Experiments involving live cells were performed in a humidified 5% CO2/air incubation at 37°C in Hank’s buffered saline solution (HBSS) containing (in mM): NaCl 137, NaHPO4 0.35, KCl 5.4, KHPO4 1, MgSO4 0.81, CaCl2 0.95, glucose 5.5, NaHCO3 25 and HEPES 20, pH 7.4, as described [23].
2.3 Confocal microscopy of TMRM
To measure changes of ΔΨ, HepG2 and Huh7 cells were loaded with TMRM, as previously described [25, 27]. Briefly, 200 nM TMRM was added to cells for 30 min before replacing with 50 nM TMRM before imaging. TMRM fluorescence was imaged by a Zeiss LSM 880 NLO inverted laser scanning confocal microscope (Thornwood, NY) using a 63X 1.4 N.A. planapochromat oil immersion lens. Fluorescence of TMRM was excited at 543 nm and detected with a Quasar multichannel spectral detector at 590–610 nm through a one Airy unit diameter pinhole. TMRM intensity quantified using Zeiss Zen and Photoshop CS4 (Adobe Systems, San Jose, CA) software after subtraction of background fluorescence measured in the nucleus.
2.4 Measurement of reactive oxygen species
To assess cellular ROS production, cells were loaded with either cm-H2DCFDA (10 µM) or CellROX Green (5 µM) for 30 min before imaging, concentrations that were maintained throughout the duration of experiments. cmH2DCFDA and CellROX Green were excited at 488 nm and emission was measured at 510–530 nm. Nonfluorescent dihydrofluoresceins react with lipid hydroperoxides and endoperoxides to generate highly fluorescent fluoresceins [28, 29]. CellRox Green (ThermoFisher) is a proprietary compound that exhibits photostable green fluorescence upon oxidation by superoxide [30]. cmDCF fluorescence was imaged exposing microscope fields only once to avoid photoactivation of the dye, whereas images of the more photostable CellROX Green images were obtained serially from the same fields before and after treatment. To assess mitochondrial superoxide production, cells were loaded with MitoSOX Red (5 µM) for 15 min before washing out [31]. MitoSOX Red was excited using a 488-nm laser and emission was measured at 560–600 nm. For all microscopy experiments, at least 4 fields were imaged at each time point in 3 independent experiments. Quantitation of fluorescence was assessed as described above for TMRM.
2.5 Cell killing
The propidium idodide (PI) cell killing assay was performed as previously described [32]. Briefly, cells plated in 24-well format were incubated in HBSS with 30 µM PI for 20 min before acquiring baseline PI fluorescence (A) using a NovoStar plate reader (BMG LABTECH GmbH, Offenburg, Germany). PI was excited at 530 nm, (25-nm band pass) and emission was measured at 590 nm (40-nm band pass). Fluorescence (X) was measured hourly. Between measurements, microtiter plates were placed in a 37°C incubator. At the end of experiments, cells were permeabilized with 100 µM digitonin for 20 min to label all nuclei with PI, and fluorescence was again measured (B). The percentage of nonviable cells (D) was calculated as D = 100(X-A)/(B-A).
2.6 Hepatocyte isolation
All animal protocols were approved by the Institutional Animal Care and Use Committee in accordance with NIH recommendations [33]. Hepatocytes were isolated from 200–300 g male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), as previously described [34]. For all experiments, hepatocyte viability was ≥90% by trypan blue exclusion. Hepatocytes were resuspended in Waymouth’s medium MB-752/1 containing 27 mM NaHCO3, 2 mM L-glutamine, 5% FBS, 100 nM insulin and 10 nM dexamethasone at pH 7.4. Hepatocytes were plated in 20 µg/well collagen-coated 96-well plates at a density of 150,000 cells/well. Hepatocytes were cultured overnight in 5% CO2/air at 37°C before experiments.
2.7 Statistics
Differences between groups were analyzed by the Student’s t-test using p < 0.05 as the criterion of significance. Data points are means ± S.E. of 3 independent experiments with at least 4 fields surveyed per experiment. Images are representative of three or more independent experiments.
3. Results
3.1 Erastin increases mitochondrial ΔΨ, promotes cellular and mitochondrial formation of reactive oxygen species and causes subsequent collapse of ΔΨ
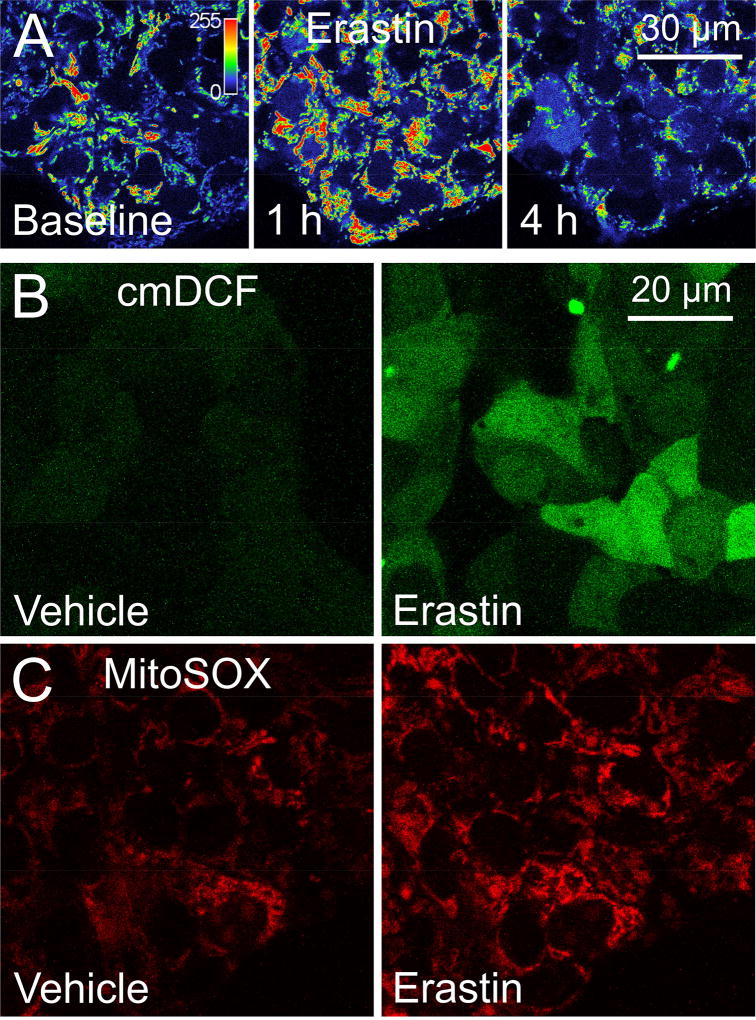
HepG2 cells were loaded with the ΔΨ indicator, TMRM, and treated with erastin (100 µM). In untreated cells, TMRM fluorescence remained unchanged for 4 h (not shown). By contrast, TMRM fluorescence increased within 1 h after treatment with erastin, signifying mitochondrial hyperpolarization, as shown previously (Fig. 1A) [23]. Subsequently after about 4 h, TMRM fluorescence began to decrease, indicating mitochondrial depolarization (Fig. 1A). In parallel experiments, HepG2 cells were loaded with the cellular ROS indicator, chloromethyl 2′,7′-dichlorodihydrofluorescein diacetate (cm-H2DCF) and the mitochondrial superoxide indicator, MitoSOX Red. After 1 h exposure to erastin, cellular cmDCF and mitochondrial MitoSOX Red fluorescence increased substantially compared to vehicle-treated cells, indicating increased cellular ROS and mitochondrial superoxide formation (Fig. 1B and C). Increased ROS formation after erastin occurred concurrently with increased ΔΨ and preceded subsequent depolarization.
Fig. 1. Erastin promotes mitochondrial hyperpolarization and formation of reactive oxygen species, which is followed by mitochondrial depolarization.
HepG2 cells were loaded with TMRM, cm-H2DCFDA or MitoSOX Red to assess changes in ΔΨ, cellular ROS and mitochondrial superoxide production respectively. In A, note an increase of TMRM fluorescence at 1 h after erastin followed by loss of fluorescence after 4 h. In B and C, note increased cellular cmDCF (B) and mitochondrial MitoSOX Red (B) fluorescence after 1 h exposure to erastin compared to vehicle treatment. In A, the indicator bar is a pseudo-color scale of the intensity of TMRM fluorescence.
3.2 X1 and X4 promote mitochondrial hyperpolarization followed by mitochondrial depolarization in hepatocarcinoma cells
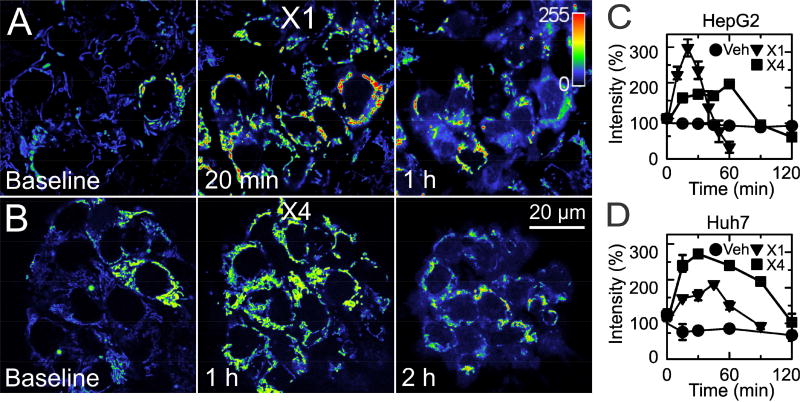
In a previous high content small molecule screening, X1 and X4 (Fig. 2) were identified as the two most potent structurally unrelated lead compounds that antagonized mitochondrial depolarization caused by high cellular free tubulin in HCC4006 lung carcinoma cells [26]. In HepG2 cells loaded with TMRM, both 10 µM X1 (Fig. 3A) and 100 µM X4 (Fig. 3B) increased mitochondrial TMRM fluorescence, which peaked after ~20 min and 1 h, respectively, and signified mitochondrial hyperpolarization. Following this increase of ΔΨ, TMRM diffused from mitochondria into the cytosol and nuclei beginning within1 h and 2 h after X1 and X4, respectively. Quantitative analysis confirmed that TMRM fluorescence first increased and then decreased in HepG2 cells after X1 and X4 (Fig. 3C). Similarly, X1 and X4 promoted mitochondrial hyperpolarization followed by depolarization in Huh7 cells (Fig. 3D and images not shown).
Fig. 2. Chemical structures of erastin, X1 and X4.
Numbers are Chembridge identifiers.
Fig. 3. X1 and X4 hyperpolarize mitochondria before collapsing ΔΨ in HepG2 and Huh7 hepatocarcinoma cells.
HepG2 cells were loaded with TMRM as described in Materials and Methods and imaged after addition of 10 µM X1 (A) or 100 µM X4 (B). Note that X1 increased mitochondrial TMRM fluorescence maximally at 20 min with release of TMRM at longer exposure. X4 increased TMRM fluorescence maximally after about an hour with subsequent release of the dye after 2 h. Indicator bar is a pseudocolor scale of TMRM fluorescence intensity. In C, plots of average TMRM fluorescence after additions of X1 and X4 to HepG2 cells are shown. TMRM fluorescence was significantly different from vehicle at 10–30 min and 60 min after X1 and at 15–60 min and 120 min after X4 (n=3/group). Panel D shows results of identical sets of experiments performed in Huh7 cells. X1 and X4 were significantly different from vehicle at all time points except 120 min (n=3/group).
3.3 The mitochondrially targeted antioxidant MitoQ decreases generation of reactive oxygen species promoted by X1 in Huh7 cells
To determine whether X1 increases cellular and mitochondrial ROS, we loaded Huh7 cells with cm-H2DCFDA. After 1 h, cmDCF fluorescence increased by ~25% in untreated cells and ~105% after X1 (Fig 4A and C). Pretreatment with the mitochondrially targeted antioxidant MitoQ (5 µM) [35] for 30 min before X1 prevented the increase in cmDCF intensity caused by X1. The increase in cmDCF fluorescence after X1 in the presence of MitoQ was nearly identical to the increase observed in vehicle-treated cells (Fig. 4A and C). To determine if X1 promoted mitochondrial superoxide formation, we loaded Huh7 cells with the mitochondrial superoxide indicator MitoSOX Red. X1 increased MitoSOX Red fluorescence intensity by more than 200% after 1 h compared to almost no increase after vehicle treatment (Fig. 4B and D).
Fig. 4. X1 increases generation of reactive oxygen species in Huh7 cells.
Huh7 cells were loaded with cm-H2DCFDA or MitoSOX Red, as described in Materials and Methods, and exposed to 10 µM X1 or vehicle. In A, note a marked increase of cm-H2DCF fluorescence after 30 min exposure to X1 that was blocked by pretreating cells with 5 µM MitoQ. Similarly in B, MitoSOX Red fluorescence increased markedly after X1 compared to vehicle. Plots of average fluorescence changes after these additions are shown in C and D. *, p<0.05 compared to vehicle and MitoQ; n=3.
3.4 N-acetylcysteine blocks X1-induced mitochondrial depolarization
To determine whether ROS contribute to mitochondrial dysfunction caused by X1, we treated cells with N-acetylcysteine (NAC, 100 µM). NAC completely blocked X1-induced mitochondrial depolarization but not the hyperpolarization (Fig. 5 A–C). Although hyperpolarization was not blocked by NAC, the progression of increased TMRM fluorescence was somewhat slower in the presence than the absence of NAC. After X1 alone, TMRM fluorescence increased maximally after about 15 min, whereas ΔΨ increased progressively to an apparent plateau at 60 min after X1 plus NAC (Fig. 5D).
Fig. 5. N-acetylcysteine prevents X1-induced mitochondrial depolarization.
Huh7 cells were loaded with TMRM to assess ΔΨ, as described in Materials and Methods, and exposed to vehicle, 10 µM X1 or X1 plus 100 µM NAC. Note that in comparison to vehicle-treated cells (A), TMRM release began within 30 min (B), which co-treatment with NAC prevented (C). Plots of average fluorescence changes after these additions are shown in D *, p<0.05 vs. X1; n=3.
3.5 N-acetylcysteine blocks mitochondrial and cytosolic generation of reactive oxygen species
To determine whether NAC blocks X1 -induced ROS formation, we loaded Huh7 cells with the oxidative stress indicator CellROX Green and treated with X1 in the presence and absence of NAC. After X1, CellROX Green fluorescence doubled after 1 h compared to vehicle treatment, which was completely prevented by NAC (Fig. 6A and C). To determine whether NAC specifically blocked mitochondrial superoxide formation, MitoSOX Red fluorescence was assessed after X1 treatment in the presence and absence of NAC. X1 alone more than doubled MitoSOX Red fluorescence after 1 h compared to vehicle, which again was abrogated by NAC (Fig. 6B and D)
Fig. 6. N-acetylcysteine abrogates increases of cellular reactive oxygen species and mitochondrial superoxide after X1.
Huh7 cells were loaded with CellROX Green (A) or MitoSOX Red (B) and treated with X1 in the presence and absence of 100 µM NAC pretreatment for 1 h. Note marked increases of CellROX Green and MitoSOX Red fluorescence after X1 that were blocked by co-treatment with NAC. Plots of average fluorescence changes after these additions are shown in C and D. *, p<0.05 vs. X1+NAC; n=3.
3.6 Cell killing by X1 and X4 is dose-dependent
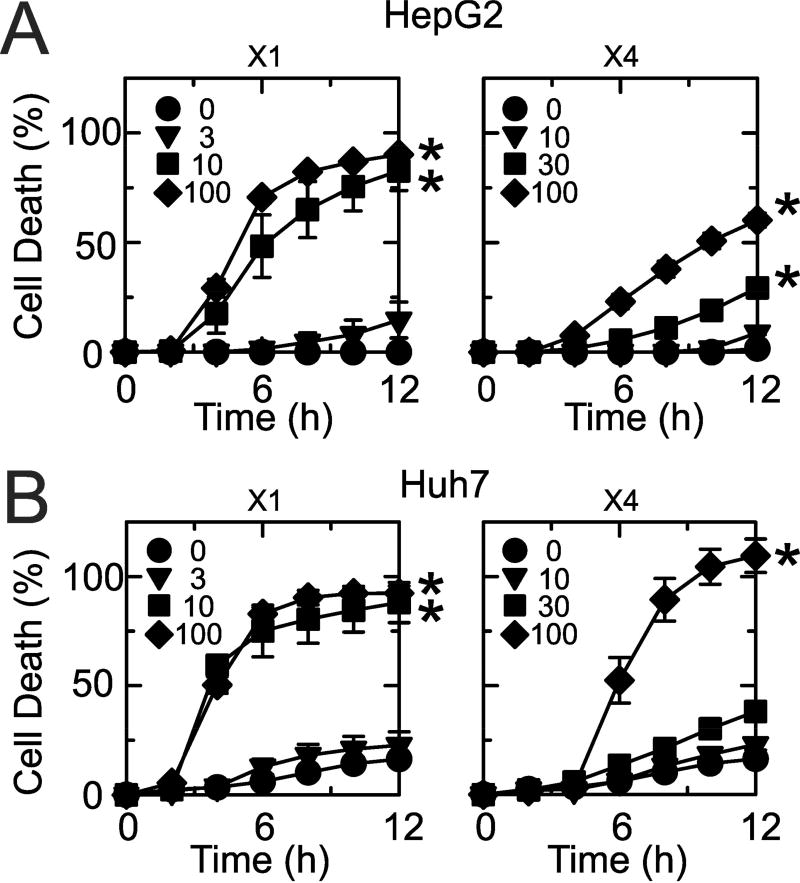
To establish whether X1 and X4 kill cancer cells, we assessed loss of cell viability caused by X1 and X4 (0–100 µM) by PI fluorometry of HepG2 and Huh7 cells incubated in HBSS. With vehicle treatment, cell death was minimal in HepG2 (1%) and Huh7 (17%) after 12 h (Fig. 7). In HepG2 cells, X1 at 10 and 100 µM caused ~90% loss of cell viability after 12 h (Fig. 7A, left panel). X4 was less potent and killed ~60% of cells at 100 µM after 12 h (Fig. 7A, right panel). Huh7 cells were also more sensitive to X1 than X4, although the difference was not as great as in HepG2 cells (Fig. 7B). Loss of viability of Huh7 cells exceeded 90% after 12 h exposure to 10 and 100 µM X1 and 100 µM X4 (Fig. 7B). X1 at ≤ 3 µM and X4 at ≤ 30 µM had minimal effect on cell killing in both HepG2 and Huh7 cells.
Fig. 7. X1 and X1 promote dose-dependent killing of HepG2 and Huh7 cells.
HepG2 (A) and Huh7 (B) cells were treated with X1 (0, 3, 10, 30 µM) and X4 (0, 10, 30, 100 µM), and cell death was assessed every hour for 12 h by PI fluorometry, as described in Materials and Methods. Cell death greater than 25% was statistically significant (p < 0.05) compared to vehicle (n=3).
3.7 N-acetylcysteine prevents cell killing by X1 and X4
To assess whether cell killing induced by X1 and X4 is ROS-dependent, we assessed loss of cell viability in the presence and absence of NAC (100 µM). In HepG2 cells, X1 (10 µM) and X4 (100 µM) alone promoted 100% and 72% cell killing, respectively, after 9 h (Fig. 8). However, pretreatment with NAC completely abolished cell killing promoted by both X1 and X4. These findings indicate that the mechanism of cell killing by X1 and X4 is ROS-dependent.
Fig. 8. N-acetylcysteine prevents X1- and X4-induced cell death.
HepG2 cells (A) and Huh7 cells (B) incubated in HBSS were treated with 10 µM X1 or 100 µM X4, and cell killing was assessed by PI fluorometry in the presence and absence of 100 µM NAC. *, p<0.05 compared to NAC; n=3.
3.8 X1 and X4 are selectively lethal to cancer cells
To determine if cell killing by X1 and X4 is selective to cancer cells, we treated rat hepatocytes and Huh7 cells incubated in HBSS with X1 (10 µM) and X4 (100 µM) and assessed cell killing as described above. X1-induced cell death exceeded 90% in Huh7 cells but was only ~25% in rat hepatocytes after 8 h exposure to X1 (Fig. 9A). Similarly, X4 promoted ~100% cell killing in Huh7 cells after 8 h and only ~20% hepatocyte killing (Fig. 9B). These findings indicate that X1 and X4 are selectively lethal to hepatocarcinoma cells compared to hepatocytes.
Fig. 9. X1 and X4 are selectively more lethal to hepatocarcinoma cells than hepatocytes.
Primary rat hepatocytes and Huh7 hepatocarcinoma cells were treated with 10 µM X1 and 100 µM X4, and cell killing was assessed by PI fluorimetry. In A, Huh7+X1 cell killing was significantly greater compared to other groups starting at 3 h. RH+X1 killing was also significant compared to RH beginning at 3 h (n=3). In B, Huh7+X1 cell killing was significantly greater compared to other groups starting at 6 h (n=3). RH+X1 killing compared to RH was not significant (p>0.05, n=3).
4. Discussion
Warburg metabolism describes a pro-proliferative phenotype where aerobic glycolysis is enhanced and mitochondrial metabolism is suppressed [1, 2]. VDAC is a dynamically modulated ‘governator’ of mitochondrial metabolism by which the open/closed state of VDAC regulates transport of metabolites into and out of mitochondria [22, 36]. Free dimeric tubulin, which is increased in proliferating cancer cells compared to non-proliferating cells, regulates VDAC opening and thus operates, at least in part, this governator of mitochondrial metabolism [23, 25]. Notably, the canonical inducer of ferroptosis, erastin, increases ΔΨ and reverses depolarization induced by free tubulin in cancer cells and also blocks tubulin-dependent inhibition of conductance of VDAC reconstituted into planar lipid bilayers [23]. Here, we show that mitochondrial hyperpolarization after VDAC opening leads to mitochondrial ROS formation, mitochondrial dysfunction and cytolethality. In this way, VDAC opening operates an anti-Warburg pro-oxidant switch that both reverts the pro-proliferative Warburg metabolic phenotype of aerobic glycolysis and stimulates mitochondrial ROS generation to culminate in oxidative stress-induced ferroptoic cell death [37, 38]. Thus, antagonism of tubulin-dependent VDAC closure is a pharmacological target to promote these events in cancer cells.
Recently, we completed a high-content, cell-based drug screen that identified small molecule lead compounds that reverse the inhibitory effect of free tubulin on ΔΨ similarly to erastin [26]. Here, we characterized the mechanism of action by which erastin and the two most potent structurally unrelated lead compounds (X1 and X4) of our previous drug screen cause cell death. To do so, we investigated the effects of erastin, X1 and X4 (Fig. 2) on mitochondrial function and ROS production in relation to loss of cell viability.
Erastin, X1 and X4 increased ΔΨ of mitochondria, as reported previously (Fig. 1 and 3) [23, 26]. Since increased ΔΨ is associated with increased mitochondrial ROS generation [39, 40], we assessed whether erastin, X1 and X4 also promoted ROS generation. Using three different fluorogenic ROS reporters, we found that erastin, X1 and X4 increased ROS generation concurrently with increased ΔΨ within 60 min or less in HepG2 and Huh7 cells (Fig. 1, 3, 4 and 6). Much of this ROS generation was mitochondrial, as indicated by the increased fluorescence of MitoSOX Red, an indicator of mitochondrial superoxide formation, and the observation that the mitochondrially targeted antioxidant (MitoQ) prevented X1-induced ROS generation (Fig. 4). However, after longer times, ΔΨ began to decrease until complete loss of mitochondrial TMRM fluorescence occurred (Fig. 1 and 3). After release from depolarized mitochondria, TMRM fluorescence transiently increased and appeared in nuclei and the cytosol due to electrostatic interaction of cationic TMRM with anionic nucleic acids and the reversal of concentration-dependent TMRM quenching inside mitochondria, as observed previously with rhodamine 123 during HgCl2-induced cell toxicity [41].
Depolarization signified onset of mitochondrial dysfunction and loss of mitochondrial membrane integrity [42]. To further investigate the relationship of ROS production and mitochondrial dysfunction by X1, we pretreated cells with NAC, a glutathione precursor and ROS-scavenging antioxidant, before adding X1. NAC at the relatively low concentration of 100 µM blocked mitochondrial depolarization and prevented ROS production after X1 (Fig. 5 and 6). Protection by NAC is consistent with the conclusion that X1-induced ROS formation was directly responsible for downstream mitochondrial dysfunction and collapse of ΔΨ. The mitochondrially targeted antioxidant MitoQ also blocked ROS generation after X1, signifying that mitochondria were the major site of ROS formation (Fig. 4). ROS generation likely triggered mitochondrial inner membrane permeabilization by opening of mitochondrial permeability transition (MPT) pores, since the MPT blocker cyclosporin A (1 µM), which delays rather than prevents oxidant-induced mitochondrial inner membrane permeabilization, also delayed cell killing (data not shown) [43, 44].
Previously, erastin and erastin-like anti-Warburg compounds were shown to antagonize the inhibitory effect of free tubulin on ΔΨ [23, 26]. Here, we propose that erastin, X1 and X4 stimulate mitochondrial ROS generation by opening VDAC and promoting flux of respiratory substrates and other metabolites across the mitochondrial outer membrane. These events increase ΔΨ and the reduction of respiratory redox components at ROS-generating sites to promote mitochondrial ROS formation (33;34;39). ROS then lead to inner membrane permeabilization (MPT), collapse of ΔΨ, mitochondrial failure and cell death. Protection against mitochondrial depolarization by antioxidants like NAC and MitoQ implicates ROS and specifically mitochondrial ROS in this cascade leading to mitochondrial dysfunction and cell death.
Erastin, X1 and X4 caused loss of cell viability exceeding 65% after 8 h in a dose-dependent fashion in both HepG2 and Huh7 cells (Fig. 7). Huh7 cells were somewhat more sensitive than HepG2 cells, particularly when treated with X1, but NAC completely protected against both X1- and X4-induced cell death (Fig. 8). Thus, ROS generation after X1 and X4 was ultimately responsible for the cell killing. Previously, erastin was shown to be selectively lethal to cancer cells, promoting an oxidative stress-dependent form of non-apoptotic killing of Ras/Raf-mutated cancer cell lines called ferroptosis [13, 20]. Similarly, X1- and X4-induced cell killing was selective to hepatocarcinoma cells as compared to primary rat hepatocytes (Fig. 9). Erastin-induced lethality is greater in human tumor cell lines harboring mutations in HRAS, KRAS or BRAF [20]. HepG2 cells have an NRAS mutation, but Huh7 cells do not have mutations in these oncogenes [45]. Nonetheless, HepG2 and Huh7 cells were about equally vulnerable to cytotoxicity from X1 and X4 (Fig. 7). Cell death was necrotic rather than apoptotic, since pilot studies showed that plasma membrane labeling with fluorescently tagged annexin and nuclear staining with PI occurred simultaneously, which is characteristic of necrosis (data not shown). In apoptosis by contrast, annexin labeling occurs without PI nuclear staining.
In conclusion, we characterized a novel mechanism of cell killing caused by erastin and erastin-like lead compounds in which reversal of tubulin-dependent VDAC inhibition promotes mitochondrial hyperpolarization and oxidative stress, leading to mitochondrial dysfunction, collapse of ΔΨ, and cell death. This proposed mechanism is relatively selective for cancer cells, since proliferating cancer cells require a large pool of free tubulin for spindle formation at metaphase compared to post-mitotic cells like primary hepatocytes [25]. This novel mechanism may underlie, at least in part, ferroptotic cell death. Ferroptosis after erastin has previously been attributed to inhibition of glutamate/cystine exchange leading to depletion of the antioxidant glutathione and inhibition of glutathione peroxidase-4 [13, 21]. However, cystine was not present in our cell death assay medium, which makes unlikely that cystine uptake by cystine/glutamate exchange was occurring. Future studies will be needed to determine the relative contributions of these different mechanisms to ferroptotic cell death and whether our new erastin-like lead compounds inhibit glutamate/cystine exchange or glutathione peroxidase-4. Other studies will also be needed to assess how lead compounds affect oxygen consumption, ATP/ADP ratios, and the redox status of mitochondrial NAD(P) and to determine the chemotherapeutic efficacy of VDAC openers like X1 and X4 in in vivo models of hepatic and other types of malignancy.
Acknowledgments
This work is in partial fulfillment of the requirements for the degree of Doctor of Philosophy to DND and was supported, in part, by Grants T32DK083262 to DND and JJL; DK073336, DK037034 and 14.Z50.31.0028 to JJL; and R01 CA18445601A1, P50 CA058187, ACS 13-043-01 and COBRE Investigator P20GM103542 to ENM. Imaging facilities were supported, in part, by P30 CA138313.
Abbreviations
- ΔΨ
mitochondrial membrane potential
- cmDCF
chloromethyl 2′,7′-dichlorodihydro-fluorescein
- cm-H2DCFDA
chloromethyl-2’,7’-dichlorodihydrorfluorescein diacetate
- MitoQ, HBSS
Hank’s buffered saline solution; mitochondria-targeted ubiquinone
- NAC
N-acetylcysteine
- PI
propidium idodide
- ROS
reactive oxygen species
- TMRM
tetramethylrhodamine methylester
- X1 and X4
small molecules identified by drug screening
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work contained in this manuscript most closely fits into the category of “Antibiotics and Chemotherapeutics.” More specifically, the research involves chemotherapeutic drug discovery and development.
Portions of this work were presented at the 58th Annual Meeting of the Biophysical Society, San Francisco, CA, February 15–19, 2014, and the 66th Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA, November 14–17, 2015.
References
- 1.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J.Gen.Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat.Rev.Cancer. 2002;2(9):683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima RA, Paggi MG, Pedersen PL. Contributions of glycolysis and oxidative phosphorylation to adenosine 5’-triphosphate production in AS-30D hepatoma cells. Cancer Research. 1984;44(12 Pt 1):5702–5706. [PubMed] [Google Scholar]
- 5.Robinson GL, Dinsdale D, Macfarlane M, Cain K. Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene. 2012;31(48):4996–5006. doi: 10.1038/onc.2012.13. [DOI] [PubMed] [Google Scholar]
- 6.Smolkova K, Bellance N, Scandurra F, Genot E, Gnaiger E, Plecita-Hlavata L, Jezek P, Rossignol R. Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J Bioenerg Biomembr. 2010;42(1):55–67. doi: 10.1007/s10863-009-9267-x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Enriquez S, Carreno-Fuentes L, Gallardo-Perez JC, Saavedra E, Quezada H, Vega A, Marin-Hernandez A, Olin-Sandoval V, Torres-Marquez ME, Moreno-Sanchez R. Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. Int J Biochem Cell Biol. 2010;42(10):1744–51. doi: 10.1016/j.biocel.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the "Warburg Effect" and a pivotal target for effective therapy. Semin.Cancer Biol. 2009;19(1):17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the "Warburg Effect",i.e., elevated glycolysis in the presence of oxygen. Journal of Bioenergetics and Biomembranes. 2007;39(3):211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 11.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633–46. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 13.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farber JL. Mechanisms of cell injury by activated oxygen species. Environmental health perspectives. 1994;102(Suppl 10):17–24. doi: 10.1289/ehp.94102s1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, Lemasters JJ. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48(5):1644–54. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Wang JH, Lemasters JJ. Mitochondrial permeability transition in rat hepatocytes after anoxia/reoxygenation: role of Ca2+-dependent mitochondrial formation of reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2012;302(7):G723–31. doi: 10.1152/ajpgi.00082.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am.J.Physiol. 1997;272(4 Pt 1):C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am.J.Physiol Heart Circ.Physiol. 2006;290(5):H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 19.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447(7146):864–8. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochim Biophys Acta. 2006;1762(2):181–90. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK, Lemasters JJ. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem. 2013;288(17):11920–9. doi: 10.1074/jbc.M112.433847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc.Natl.Acad.Sci.U.S.A. 2008;105(48):18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maldonado EN, Patnaik J, Mullins MR, Lemasters JJ. Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 2010;70(24):10192–201. doi: 10.1158/0008-5472.CAN-10-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeHart DN, Lemasters JJ, Maldonado EN. Erastin-Like Anti-Warburg Agents Prevent Mitochondrial Depolarization Induced by Free Tubulin and Decrease Lactate Formation in Cancer Cells. SLAS discovery : advancing life sciences R & D. 2017 doi: 10.1177/2472555217731556. 2472555217731556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemasters JJ, Ramshesh VK. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods in cell biology. 2007;80:283–95. doi: 10.1016/S0091-679X(06)80014-2. [DOI] [PubMed] [Google Scholar]
- 28.Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Analytical Biochemistry. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Lemasters JJ. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic Biol Med. 2013;63:243–53. doi: 10.1016/j.freeradbiomed.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McBee ME, Chionh YH, Sharaf ML, Ho P, Cai MW, Dedon PC. Production of Superoxide in Bacteria Is Stress- and Cell State-Dependent: A Gating-Optimized Flow Cytometry Method that Minimizes ROS Measurement Artifacts with Fluorescent Dyes. Frontiers in microbiology. 2017;8:459. doi: 10.3389/fmicb.2017.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochemica Biophysica Research Commun. 2007;358(1):203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieminen AL, Merrick P, Harper R, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxcity screening assay using multi-well fluorescence scanner: correlation with LDH release and Draize eye scores. Johns Hopkins Center for Alternatives to Animal Testing, 10th Aniversary symposiu. 1992 [Google Scholar]
- 33.Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. The Physiologist. 1996;39(4):199, 208–11. [PubMed] [Google Scholar]
- 34.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am.J.Physiol. 1997;273(6 Pt 1):C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 35.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276(7):4588–96. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado EN, Lemasters JJ. Warburg revisited: regulation of mitochondrial metabolism by voltage-dependent anion channels in cancer cells. J Pharmacol Exp Ther. 2012;342(3):637–41. doi: 10.1124/jpet.112.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maldonado EN. VDAC-Tubulin, an Anti-Warburg Pro-Oxidant Switch. Frontiers in oncology. 2017;7:4. doi: 10.3389/fonc.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemasters JJ. Evolution of voltage-dependent anion channgel function: From molecular sieve to governator to actuator of ferroptosis. Frontiers in oncology. 2017;10:1–4. doi: 10.3389/fonc.2017.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15–8. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 40.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86(5):1101–7. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 41.Nieminen AL, Gores GJ, Dawson TL, Herman B, Lemasters JJ. Toxic injury from mercuric chloride in rat hepatocytes. Journal of Biological Chemistry. 1990;265(4):2399–2408. [PubMed] [Google Scholar]
- 42.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy 134. Biochimica et Biophysica Acta. 1998;1366(1–2):177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 43.Broekemeier KM, Pfeiffer DR. Cyclosporin A-sensitive and insensitive mechanisms produce the permeability transition in mitochondria. Biochemica Biophysica Research Commun. 1989;163:561–566. doi: 10.1016/0006-291x(89)92174-8. [DOI] [PubMed] [Google Scholar]
- 44.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Letter. 2002;512(1–3):1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 45.Ewald F, Norz D, Grottke A, Bach J, Herzberger C, Hofmann BT, Nashan B, Jucker M. Vertical targeting of AKT and mTOR as well as dual targeting of AKT and MEK signaling Is synergistic in hepatocellular carcinoma. Journal of Cancer. 2015;6(12):1195–205. doi: 10.7150/jca.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]