Abstract
Episodic memory undergoes dramatic improvement in early childhood; the reason for this is poorly understood. In adults, episodic memory relies on a distributed neural network. Key brain regions that supporting these processes include the hippocampus, portions of the parietal cortex, and portions of prefrontal cortex, each of which shows different developmental profiles. Here we asked whether developmental differences in the axonal pathways connecting these regions may account for the robust gains in episodic memory in young children. Using diffusion weighted imaging, we examined whether white matter connectivity between brain regions implicated in episodic memory differed with age, and were associated with memory performance differences in 4- and 6-year-old children. Results revealed that white matter connecting the hippocampus to the inferior parietal lobule significantly predicted children’s performance on episodic memory tasks. In contrast, variation in the white matter connecting the hippocampus to the medial prefrontal cortex did not relate to memory performance. These findings suggest that structural connectivity between the hippocampus and lateral parietal regions is relevant to the development of episodic memory.
Keywords: White matter, Memory development, Episodic memory, Diffusion weighted imaging
1. Introduction
Remembering a past event and the specific spatiotemporal context in which the event occurred is a hallmark of episodic memory. Early childhood marks an important developmental period for episodic memory, as substantial growth in this ability is observed. Many studies have shown robust age differences between 4- and 6-year-old children, with 4-year-olds performing worse than 6-year-olds on tasks that require relational memory, i.e., memory linking multiple items (Drummey and Newcombe, 2002, Lloyd et al., 2009, Newcombe et al., 2014, Ngo et al., 2017, Sluzenski et al., 2006), or memory for contextual details (Bauer et al., 2012, Riggins, 2014, Riggins et al., 2015, Riggins and Rollins, 2015). The enhancement in episodic memory during childhood is thought to rely, at least in part, on complex and dynamic developmental changes in the brain, in an interplay with social and other cognitive factors (Riggins, 2012). Understanding the neural bases of episodic memory development requires investigation of the relation among key regions of episodic memory, including the hippocampus, the parietal cortex, and the prefrontal cortex. The goal of our study was to better understand this interaction by examining the structural connectivity among these brain areas via white matter pathways.
In the last two decades, there have been substantial efforts in characterizing the developmental profiles of white matter pathways in the brain. Convergent findings from cross-sectional (e.g., Bonekamp et al., 2007, Lebel et al., 2008, Loenneker et al., 2011, Moon et al., 2011, Qiu et al., 2010, Rollins et al., 2010, Sadeghi et al., 2014) and longitudinal studies (e.g., Krogsrud et al., 2016, Lebel and Beaulieu, 2011, Simmonds et al., 2014) show a protracted timeline of white matter development from early childhood until adulthood, with differential maturational rate across white matter tracts (reviewed in Lebel et al., 2017). It is believed that the information transmission properties of any given white matter tract can be predicted by the function of the gray matter regions that it connects (Maunsell and van Essen, 1983, Passingham et al., 2002). Thus, it is likely that specific white matter pathways connecting brain regions implicated in episodic memory should play a role in age-related improvements in memory performance in children. The focus of this paper is to examine such relations.
An essential role of the hippocampus is to construct relational memories by binding together multiple elements of an event to form a cohesive episode (Backus et al., 2016; Cohen and Eichenbaum, 1993; Horner and Doeller, 2017). Developmental changes in hippocampal structure and function relate to improvement in episodic memory in school-aged children (e.g., DeMaster et al., 2013, DeMaster and Ghetti, 2013, Ofen et al., 2007; reviewed in Ghetti and Bunge, 2012). Gray matter volume of the hippocampal head predicts children’s ability to recall contexts in which events occur, but this relation only exists in 6-year-olds, not in 4-year-old children (Riggins et al., 2015). A recent study using resting state functional connectivity in 4- and 6-year-olds showed that the hippocampal-cortical network supporting episodic memory varies with age, such that with age, the hippocampus becomes more functionally integrated with cortical regions associated with the adult-like memory network (Riggins et al., 2016). Thus, age-related differences in the hippocampus and its functional connectivity with cortical regions contribute to the rapid memory improvements exhibited in young children. However, the role of structural connectivity has not been investigated.
1.1. Memory-related cortical regions
The inferior parietal lobe (IPL) has been strongly linked to episodic memory in adults, yet its precise role remains controversial. A large number of fMRI studies have reported activations in the IPL during episodic memory retrieval. For instance, it is more active during retrieval of studied, versus unstudied items, and during source, as compared to item memory judgments (reviewed in Cabeza et al., 2008). Despite the consistency of neuroimaging findings, evidence from patients with lesions to the IPL suggests that its role in episodic memory is quite nuanced. Patients with bilateral IPL lesions are not amnesic; rather, they exhibit normal performance on many episodic memory tasks (Berryhill et al., 2009, Haramati et al., 2008, Simons et al., 2010). However, these same patients show diminished detail, and vividness of recollection when recalling autobiographical memories based on a cue (Berryhill et al., 2007). They also consistently show decreases in subjective aspects of recollection (Drowos et al., 2010, Hower et al., 2014, Simons et al., 2010). Most recently, it was reported that unilateral IPL lesions can cause deficits in cued recall (Ben-Zvi et al., 2015).
The medial prefrontal cortex (mPFC) is also believed to play an important role in episodic memory. In rodents, an axonal pathway connecting the mPFC to the hippocampus is critical for several forms of memory including the classic Morris water maze (Goto and Grace, 2008; Wang and Cai, 2008). This evidence has led to the proposal that the mPFC takes inputs from the hippocampus about the past and combines this with information about the current context to predict adaptive responses (reviewed in Euston et al., 2012). Less is known about the functional significance of hippocampal-mPFC structural connectivity in humans, although it is known that such connectivity exists. Theories about the frontal lobe in episodic memory have focused on its role in retrieval strategy and control. For instance, functional connectivity between the hippocampus and PFC has been related to mnemonic control in adults (Benoit and Anderson, 2012). It has been proposed that age-related improvements in episodic memory depend on the development of strategic processes mediated by portions of the prefrontal cortex (DeMaster and Ghetti, 2013, Shing et al., 2008). However, little is known about whether structural connectivity between the hippocampus and mPFC relates to the improvements of episodic memory in early childhood.
Taken together, the interactions between the hippocampus and the IPL, as well as between the hippocampus and the mPFC, are likely to play a key role in the development of episodic memory in young children. To better understand the interplay among these regions, it is important to examine the underlying structural connectivity among these regions, given that developmental changes in white matter connectivity are crucial aspects of cognitive development (reviewed in Ghetti and Bunge, 2012). To our knowledge, no study has linked age-related changes in white matter connectivity and memory performance during early childhood, an imperative developmental period for episodic memory development.
1.2. Current study
The goal of the current study was to examine the relation between white matter connectivity of the hippocampus and specific cortical regions hypothesized to be related to episodic memory enhancement during early childhood. Specifically, we focused on the children ages four and six, which marks a critical transition from fragile to robust episodic memory (Lloyd et al., 2009, Riggins, 2014, Sluzenski et al., 2006). The currently study had two aims: (1) to test age-related differences in the macrostructure and microstructure of white matter connectivity among brain regions implicated in episodic memory in four- and six-year-olds; and (2) to relate variations in hippocampal-cortical white matter connectivity to episodic memory performance.
We administered the Children’s Memory Scale (CMS; Cohen, 1997), as well as an Episodic Memory task developed to test young children (Riggins et al., 2015, Riggins and Rollins, 2015). The CMS is a standardized and well-known measure of episodic memory (e.g., Willford et al., 2004, Jack et al., 2009), which provides a “gross” measure of episodic memory. The Episodic memory task is a lab-based task designed to specifically probe context details surrounding an event, tapping memory for what happened and where it happened. We collected diffusion-weighted imaging data in the same group of children and employed probabilistic tractography to examine macro- and microstructural properties of white matter connecting key brain regions shown to support episodic memory. These regions included the hippocampus, the inferior parietal lobule, and the medial prefrontal cortex. In addition, we delineated a control tract (hippocampus – primary visual cortex), which should not be associated with memory functions.
Furthermore, we conducted an exploratory analysis to examine whether memory performance related to two major limbic pathways: the fornix and the uncinate fasciculus, both of which have been implicated in memory functions (fornix: reviewed in Douet and Chang, 2014; uncinate fasciculus: reviewed in Olson et al., 2015). The fornix is the largest efferent pathway from the hippocampus and projects from the posterior hippocampus to the septal area, mammillary bodies, and portions of the hypothalamus, and has long been linked to episodic memory (e.g., Metzler-Baddeley et al., 2011, Mielke et al., 2012, Oishi et al., 2011, Sexton et al., 2010, Tsivilis et al., 2008; Zhuang et al., 2013, reviewed in Douet and Chang, 2014). The uncinate fasciculus connects the anterior temporal lobe, as well as perirhinal and entrorhinal cortex and possibly portions of the anterior hippocampus to lateral and orbitofrontal prefrontal cortex. It has also been linked to memory functions in older children (ages 7–11: Wendelken et al., 2015) and adults (Alm et al., 2016; reviewed in Von Der Heide et al., 2013 and Olson et al., 2015). Given these findings, we tested whether variations in the macrostructure or microstructure of the fornix and uncinate relates to episodic memory performance using probabilistic tractography.
To preview, we found that, although no age differences emerged across the white matter connectivity measures, the microstructure of the white matter connecting the hippocampus to the inferior parietal lobule predicted children’s episodic memory performance. All other tracts examined did not relate to memory performance.
2. Methods
2.1. Participants
The sample in this report included 29 4-year-old (19 females; Mmonth = 53.14 ± 3.73; range = 48.00–59.00) and 23 6-year-old children (14 females; Mmonth = 77.35 ± 3.19; range = 73.00–83.00). Of these, DTI data from 5 children were excluded due to incomplete scans (n = 4) and excessive head motion (n = 1). The final sample included 47 (24 4-year-old and 23 6-year-old children). The racial break down was as follow: 53.84% Caucasian, 9.62% African American, 3.85% Native American or Native Alaskan, and 32.69% undisclosed/unreported or wished to not disclose. The majority of the children’s families had high SES: 73.08% of the families earned more than $75,000/year. This study was a part of a larger study, such that additional children (n = 23) were tested but were not included in the present report due to not completing the memory assessments of interest, DTI assessment or both (see Riggins et al., 2015 for report on the same sample).
2.2. Memory tasks
2.2.1. Children’s Memory Scale (Cohen, 1997)
At encoding, children were told that the experimenter would read them some short stories, and that they should listen carefully and try to remember as much as they could so that they could retell the story at a later time. The experimenter read the stories aloud. Each story included 7 short sentences.
2.2.1.1. Immediate recall
Immediately after each story was read, children were asked to retell the story without leaving out details. After the recall of the second story, children were told to remember both stories because they would be asked to retell the stories at a later time point. The proportion of correctly recalled details (out of 57 pre-determined content details) was calculated for each of the two stories, which were then averaged for each child.
2.2.1.2. CMS delayed recall
After approximately an hour, children were asked to recall each story, and were prompted with the general topic of the story (e.g., “Remember the story I read to you about the cat? I want you to tell me the story one more time.”).
2.2.1.3. CMS delayed recognition
After the delayed recall, children were given a yes/no recognition test consisting of 15 items for each story. The questions asked details about the story. Mean proportion of correct trials was calculated for each child.
2.2.2. Episodic Memory Task (Riggins et al., 2015)
At encoding, children were shown 36 object toys in two different rooms (18 toys/room). The rooms were made to be engaging to children and perceptually distinct from one another. Children were instructed to interact with each item by carrying out one of the three actions (put it on your head, beat on it like a drum, or hug it). The experimenter first carried out the action and asked the child to imitate the action. The order of rooms visited was randomized across children. After a one-hour delay, children were tested on 54 toy items, presented sequentially. Among 54 items, 36 were old and 18 were novel toy items, presented in a randomized order across children. Children were asked to identify each item as either old or new. For the items identified as old, children were then asked to recall which action was associated with those items, and the location in which they were encountered. The proportion of correctly recalled contextual details was averaged and used as an index of context memory for each child. Five training trials were administered to ensure all children understood the task at encoding and retrieval.
2.2.3. Kaufman brief intelligence test (KBIT)
The verbal and nonverbal subtests of the KBIT were administered to each child as an assessment of general intelligence. This measure was used as a control variable in our statistical analyses. In the KBIT Verbal test, the experimenter showed the child a page consisting of 6 color images. The experimenter read aloud names (e.g., “socks”) to the child and asked the child to point to the object images corresponding to each name. The total number of correct trials was used to compute a KBIT verbal score. In the KBIT Nonverbal test (Riddles), the experimenter showed the child a page consisting of 6 color images and read aloud a verbal description of an item/concept “what hops, eats carrots, and has long ears?” The child was asked to point to the image that corresponded to the verbal description. The first 8 items were with images, the later items required verbal responses. Administration was discontinued after 4 consecutive incorrect responses.
3. DWI acquisition and analyses
3.1. Image acquisition
Images were collected on a 12-channel head coil on a Siemens 3T scanner (MAGNETOM TrioTim) at the University of Maryland. Image acquisition included one T1-weighted 3D MPRAGE sequence (176 contiguous sagittal slices, voxel size = 1.0 × 1.0 × 1.0 mm, TR/TE/inversion time = 1900 ms/2.52 ms/900 ms; flip angle = 90, pixel matrix = 256 × 256). Diffusion-weighted images included three non-diffusion-weighted volumes (b = 0) and 30 non-collinear gradient directions (b = 1000 s/mm2 with 3 sequence repetitions) at 128 × 128 resolution and voxel size of 1.8 × 1.8 × 4 mm3.
3.2. Diffusion weighted imaging preprocessing
Diffusion-weighted images were preprocessed and analyzed using FSL (Smith et al., 2004). Preprocessing included correction for head movements and eddy current distortions. Similar to previous research (e.g., Westlye et al., 2010), we averaged the three acquisitions and removed non-brain tissue. Non-brain tissue was removed using FSL’s automated brain extraction tool (BET).
3.3. Selection of regions of interest
The neural regions of interest (ROIs) in this study included: hippocampus (HC), inferior parietal lobule (IPL), medial prefrontal cortex (mPFC), and primary visual cortex (V1). V1 was included as a control brain region. Bilateral hippocampal ROIs and mPFC ROIs were obtained from the Harvard-Oxford Atlas. Because the IPL and V1 masks were not available in the Harvard-Oxford Atlas, bilateral IPL ROIs and V1 ROIs were obtained from Juelich Histological Atlas. Other ROIs used to create exclusion masks included the left and right hemisphere, brain stem, cerebellum, and four lobes from the MNI Structural Atlas, again, given that these ROIs were not available from either the Harvard-Oxford or the Juelich Histology Atlases. All aforementioned atlases were available through FSL tools. It is worth noting that these atlases are based on adult brain templates. However, they have been used successfully in children in previous studies (e.g., Chaddock-Heyman et al., 2013, Krogsrud et al., 2016, Wendelken et al., 2015).
3.4. Probabilistic tractography
Probabilistic tractography models the anisotropic movement of water molecules in restricted compartments, such as axons, to infer the presence of white matter fibers. Virtual reconstruction of white matter pathways and their associated diffusion properties are derived from diffusion data.
Tractography analyses were performed in participants’ native anatomical space and the results were output in Montreal Neurological Institute (MNI) standard space according to transformation parameters. First, the FA image was registered to each subject’s T1-weighted image using six degrees of freedom and a mutual information cost function. Next, the T1-weighted image was registered to the 2 × 2 × 2 mm3 MNI template using a nonlinear warping algorithm. These transformation parameters were then used as a conversion matrix to transform from diffusion space to MNI space.
We used BEDPOSTX to build the probability distributions of diffusion parameters at each voxel in the brain to model crossing fibers within each voxel. Then, we employed “seeded” probabilistic tractography to delineate tracts connecting the hippocampus to cortical ROIs using the FMRIB Diffusion Toolbox (FDT, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT) with a partial volume model (Behrens et al., 2003), allowing for up to two fiber directions in each voxel. The model estimates the probability distribution of the diffusion parameters to determine the most likely location of a pathway that connects the assigned seed and target ROIs (Behrens et al., 2007). The connectivity distribution between seed and target ROIs were generated using 5000 streamline samples that travelled along the probability function at each voxel (curvature threshold = 0.2, step length = 0.5, maximum steps = 2000) (Behrens et al., 2007). The hippocampus was assigned as the seed ROI – the departure location of subsequent tractography, with the cortical ROIs (IPL and mPFC) assigned as targets for each individual tractography. To assess specificity of findings, a control tract between the hippocampus and V1, a region that should not be involved in episodic memory, was examined and correlated with performance.
All tractographies were performed separately for the left and right hemispheres. Exclusion masks were used for each tractography such that lobes that did not include either the seed or target ROI were excluded. For example, for the tractography between left hippocampus and mPFC, exclusion masks were placed on the following regions of non-interest: brain stem, cerebellum, occipital lobe, and parietal lobe. In addition, the brain hemisphere contralateral to the tractography was also excluded to ensure modeled tracts were fully lateralized (see Fig. 1A and B).
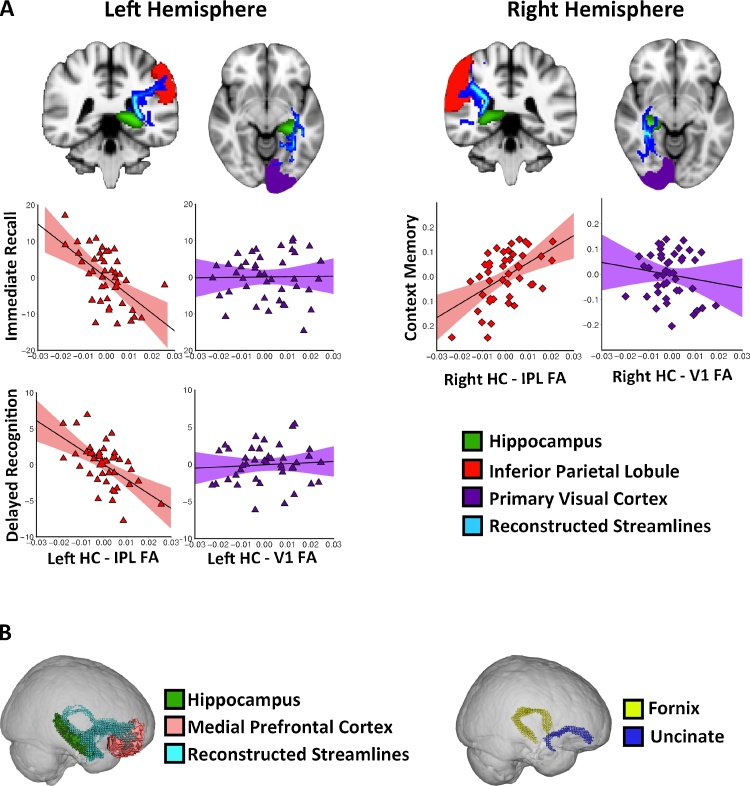
Fig. 1.
(A) Examples of reconstructed streamlines of hippocampus – IPL connectivity and hippocampus – V1 connectivity in the left and right hemispheres. Scatterplots of (red) or control tract (purple) and memory performances. (B) An example of reconstructed streamlines of the hippocampus – mPFC connectivity (left), and reconstructed streamlines of the fornix and uncinate fasciculus (right).
3.5. Exploratory analysis: tractography of major limbic tracts
We used Wake Forest Atlas to generate 10 mm spheres surrounding the x, y, z coordinates that mark the starting and end points of both the fornix and uncinate fasciculus based on the FSL atlas. For the fornix, seed, waypoint, and target ROIs encompassed the left and right anterior pillars, body, and left and right fimbria of the fornix, respectively. The coordinates were determined based on the white matter fornix ROI from the Juelich Histological Atlas (see Table 1). For the uncinate, we performed separate tractography for the left and right hemispheres. Seeds, waypoints, and targets were determined based on the white matter left and right uncinate fasciculus ROI from the John Hopkins University White Matter Atlas (see Table 1). To ensure that probabilistic tractography delineates specific limbic tracts of interest, we created customized exclusion masks in FSL to ensure that tractography was only performed for voxels that belong to a given tract. We visually inspected the reconstructed streamlines for each participant to ensure tractography was successful and acceptable for further analyses (see Fig. 1B).
Table 1.
X, Y, Z coordinates of seed, waypoint, and target ROIs generated.
| Tracts | Seeds |
Waypoints |
Targets |
|||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| Fornix | −35; 10, −36 | 35, 10, −36 | 0, −15, 24 (midline) | −6, 9, −16 | 6, 9, −16 | |
| Uncinate | −16, 54, −8 | 12, 54, −12 | −20, 26, −2 | 20, 26, −2 | −38, 10, −26 | 34, 0, −16 |
3.6. DWI analysis
Following tractography, we extracted white matter fractional anisotropy (FA), a measure of microstructure and white matter volume, a measure of macrostructure, for each delineated tract. We calculated FA to assess white matter microstructural properties of specific tracts and the whole brain. FA quantifies the dispersion of water molecules in a given voxel, such that voxels within which water molecules diffuse in a similar direction yield higher FA values. FA values ranges from 0 (isotropic diffusion) to 1 (highly anisotropic diffusion). Estimates of FA were calculated using the following equation: , where λ1, λ2, and λ3 represent each of the three eigenvalues. FA depends on several factors including axonal packing, membrane thickness, myelination, and crossing fibers (Beaulieu, 2002, Jones et al., 2013). We used the average FA within specific delineated tracts to index white matter microstructure. It is worth noting that there are other diffusivity parameters such as mean diffusivity, radial diffusivity, and axial diffusivity that can be measured. To minimize multiple comparisons, we only calculated FA, given that it is the most commonly used diffusivity index in the DWI literature.
To examine whether memory performance specifically correlates with white matter connectivity among regions of interest as opposed to global changes in white matter in the brain, we calculated whole-brain FA for each participant as a control variable. Structural segmentation from T1-weighted images was performed using FAST (Zhang et al., 2001) to create separate partial volume maps for gray matter, white matter, and cerebrospinal fluid. Partial volume maps for white matter were used as a mask to extract mean FA isolated to white matter tissues in the whole brain.
In addition to microstructure, we measured white matter macrostructure of each delineated tract by calculating the number of voxels of the reconstructed streamlines. In addition, we used partial volume maps for white matter as a mask to extract the mean number of voxels of white matter tissue in the whole brain for each participant.
3.7. Statistical analyses
Statistical analyses were performed using SPSS (Version 24.0). Hierarchical linear regression analyses were conducted to examine the specificity of the relation between white matter and memory performance. Separate regression models were conducted for white matter macrostructure (volume) and microstructure (FA) for each tract of interest to avoid multicollinearity. In Step 1, we included five variables: age, whole-brain FA/volume, KBIT score, and FA of the bilateral control tract (hippocampus – V1). In Step 2, we entered two additional predictors: the left and right FA values of a given tract of interest. Regression models were constructed to predict mean performances on 3 dependent variables from the Children’s Memory Scale test: Immediate Recall, Delayed Recall, and Delayed Recognition, and 1 dependent variable from the Episodic Memory test: context memory. To control for multiple comparisons, we applied Bonferroni correction in Step 1 (corrected α = 0.01, with 5 predictors), and Step 2 (corrected α = 0.007, with 7 predictors) for all regression analyses.
4. Results
4.1. Behavioral tasks
-
(1)
Children’s Memory Scale (CMS)
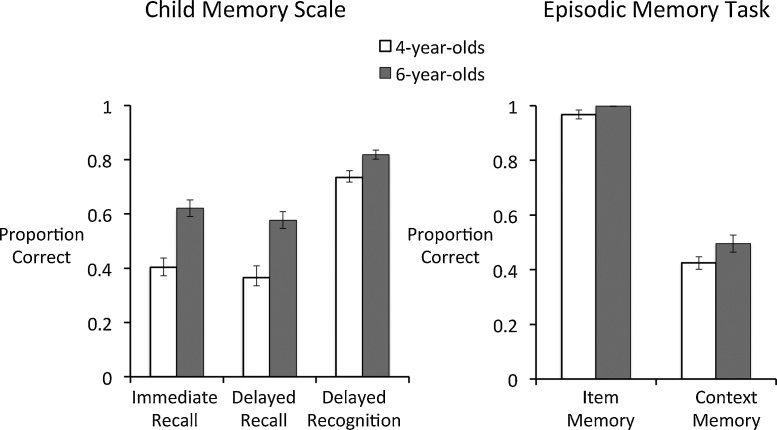
On the Immediate Recall portion of the CMS task, six-year-olds performed better than four-year-olds, (M = 0.62, SE = 0.03 vs. M = 0.40, SE = 0.03, t(49) = −4.58, p < 0.001, d = 1.30). A similar result was found on the Delayed Recall portion of the CMS task with six-year-olds out-performing four-year-olds (M = 0.58, SE = 0.03 vs. M = 0.37, SE = 0.04; t(46) = −3.91, p < 0.001, d = 1.30). Last, on the Delayed Recognition task, six-year-olds again performed better than four-year-olds (M = 0.82, SE = 0.02 vs. M = 0.73, SE = 0.02; t(48) = −2.72, p < 0.01, d = 0.79) (see Fig. 2 Left). Male and female children performed similarly on all CMS tests (all p’s > 0.47).
-
(2)
Episodic Memory Task
Fig. 2.
Mean proportion correct in the immediate recall, delayed recall, and delayed recognition tests of the Children’s Memory Scale (Left), and mean proportion correct in the item recognition and context memory recall tests of the Episodic Memory task (Right) in 4- and 6-year-olds.
As previously reported in Riggins et al. (2015), four- and six-year-olds performed similarly (and near ceiling) on the item memory task, t(50) = −1.68, p = 0.10. Given the restricted range of performance, we did not examine the relation between item memory and white matter indices in the subsequent analyses. Six-year-olds recalled numerically more context details than four-year-olds; however this difference failed to meet the conventional threshold for statistical significance (M = 0.50, SE = 0.03 vs. M = 0.42, SE = 0.02; t(50) = −1.86, p = 0.07, d = 0.51 (see Fig. 2 Right). Male and female children performed similarly on both tasks (all p’s > 0.71). Collinearity statistics showed that the assumption of collinearity was not violated (all VIF values ranged from 1.07 to 2.45).
-
(3)
KBIT
Four- and six-year-olds did not differ on KBIT standardized scores (M = 116.90, SE = 1.80 vs. M = 115.74, SE = 2.62; t(48) = 0.38, p = 0.052, d = 0.52). However, six-year-olds scored higher on the KBIT nonverbal portions than four-year-olds (M = 111.78, SE = 3.88 vs. M = 107.07, SE = 2.37; t(48) = −1.08, p = 0.02, d = 1.47). No sex differences were found, all p’s > 0.10.
4.2. Diffusion-weighted imaging
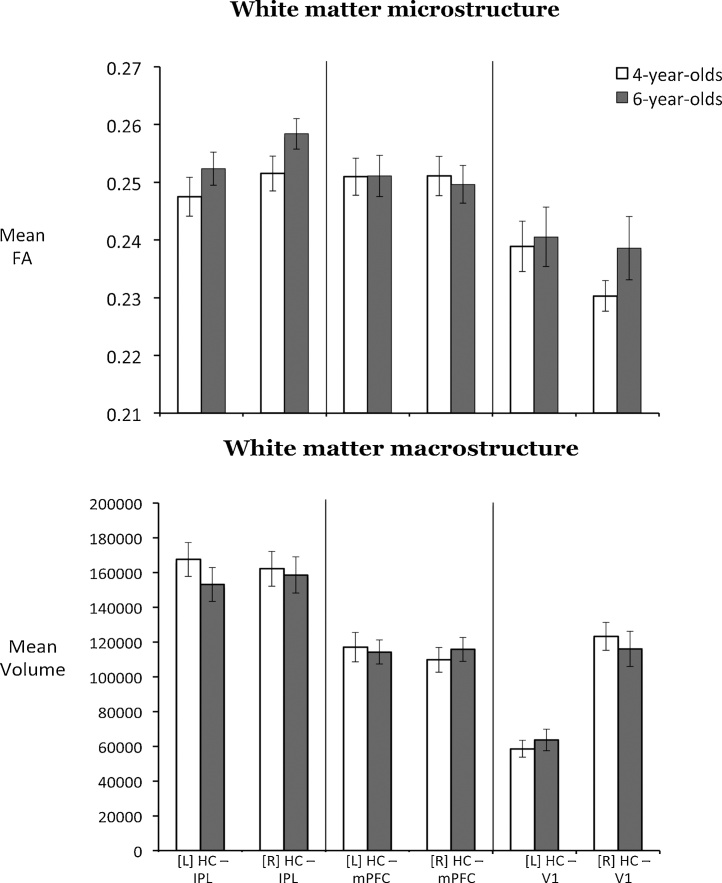
Mean FA and volume of each tract as a function of age group are presented in Fig. 3. Mean FA and volume did not differ between four- and six-year-olds in any of the white matter tracts examined (all p’s > 1.00). Moreover there were no statistically significant age differences in either whole-brain FA or whole-brain white matter (all p’s > 0.60). Last, no sex differences were found in any DWI measure (all p’s > 0.06).
Fig. 3.
Mean FA (top) and volume (bottom) of tracts of interest: bilateral hippocampus – inferior parietal lobule, bilateral hippocampus – medial prefrontal cortex, and the control tract: bilateral hippocampus – primary visual cortex in each age group. Mean FA and volume did not differ between four- and six-year-olds in any of the white matter tracts examined.
Hemispheric differences in macro- and microstructure for each tract were tested for four- and six-year-olds separately. For macrostructure, hippocampus – V1 volume was significantly greater for the right than the left hemisphere in 4-year-olds (M = 58573.67, SE = 4912.83 vs. M = 123170.33, SE = 7981.29; t(23) = −8.88, p < 0.001), and in 6-year-olds, (M = 636300.61, SE = 6188.62 vs. M = 115979.48, SE = 10130.40; t(22) = −6.85, p < 0.001). No hemispheric differences were found for the other two tracts, all p’s > 0.14. For microstructure, in four-year-olds, hippocampus – V1 FA was significantly higher in left than in right hemisphere (M = 0.24, SE = 0.004 vs. M = 0.23, SE = 0.002; t(23) = 2.25, p = 0.03). No differences were found for the other two tracts. In six-year-olds, hippocampus – IPL FA was significantly greater in the right than in the left hemisphere (M = 0.25, SE = 0.003 vs. M = 0.26, SE = 0.002; t(23) = −2.62, p = 0.02), with no hemispheric differences found for the other two tracts (see Fig. 3).
4.3. White matter microstructure – behavioral performance relations
Pearson correlations of behavioral performances and the white matter microstructure are presented Table 2. Our goal was to examine the unique variances of memory performance accounted for by each tract of interest, thus we focused on the results of the regression analyses of hippocampus – IPL, and hippocampus – mPFC connectivity predicting memory performance, presented in Table 3, Table 4, respectively. We conducted hierarchical regression models for each tract of interest’s microstructure and macrostructure separately to predict each memory performance index.
Table 2.
Pearson correlation statistics of the variables examined. *** p < 0.001; ** p < 0.01; * p < 0.01 (uncorrected for multiple comparisons).
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | |||||||||||||||
| 2. KBIT Verbal SS | −0.05 | – | ||||||||||||||
| 3. KBIT Nonverbal SS | 0.19 | 0.34* | – | |||||||||||||
| 4. CMS Immediate Recall | 0.51** | 0.34* | −0.04 | – | ||||||||||||
| 5. CMS Delayed Recall | 0.45** | 0.36 | 0.04 | 0.86** | – | |||||||||||
| 6. CMS Delayed Recognition | 0.29 | 0.09 | −0.003 | 0.53** | 0.28 | – | ||||||||||
| 7. Context Memory | 0.11 | 0.20 | 0.27 | 0.08 | −0.04 | 0.36* | – | |||||||||
| 8. [L] HC − IPL FA | 0.31* | −0.02 | 0.14 | 0.01 | −0.004 | −0.15 | 0.24 | – | ||||||||
| 9. [R] HC − IPL FA | 0.33* | 0.16 | 0.23 | 0.44** | 0.36* | 0.20 | 0.30* | 0.61*** | – | |||||||
| 10. [L] HC −mPFC FA | 0.05 | −0.004 | 0.05 | 0.05 | 0.11 | 0.06 | 0.26 | .54*** | 0.29 | – | ||||||
| 11. [R] HC −mPFC FA | 0.003 | 0.18 | 0.16 | 0.13 | 0.11 | 0.16 | 0.30* | 0.26 | 0.41** | 0.65*** | – | |||||
| 12. [L] HC − V1 FA | 0.04 | −0.09 | −0.16 | 0.01 | −0.02 | 0.09 | 0.18 | 0.48** | 0.11 | 0.33* | −0.04 | – | ||||
| 13. [R] HC − V1 FA | 0.17 | −0.04 | −0.08 | 0.23 | 0.16 | 0.30 | 0.07 | 0.58*** | 0.46** | 0.34* | 0.05 | 0.66*** | – | |||
| 14. Fornix FA | −0.11 | 0.19 | 0.05 | 0.05 | 0.12 | 0.21 | 0.12 | 34* | 0.06 | 0.57*** | 0.34* | 0.42** | 0.56** | – | ||
| 15. [L] Uncinate FA | 0.003 | −0.11 | 0.14 | −0.11 | −0.10 | 0.09 | 0.20 | 0.36* | 0.16 | 0.39* | 0.40* | 0.23 | 0.27 | 0.27 | – | |
| 16. [R] Uncinate FA | 0.14 | 0.25 | 0.32* | 0.25 | 0.17 | 0.16 | 0.27 | 0.33* | 0.31* | 0.36* | 0.40* | 0.36* | 0.26 | 0.29 | 0.39* | – |
| 17. Whole brain FA | 0.06 | −0.05 | −0.02 | 0.13 | 0.07 | 0.18 | 0.18 | 0.42** | 0.30 | 0.63*** | 0.70*** | 0.27 | 0.25 | 0.60*** | 0.39* | 0.43** |
Table 3.
Summary of hierarchical linear regression models of white matter connectivity between hippocampus and inferior parietal lobe microstructure (FA) predicting memory performances.
| Dependent variable | Predictors | β | t-value | F | ΔF | R2 | ΔR2 | |
|---|---|---|---|---|---|---|---|---|
| Children’s Memory Scale | ||||||||
| Immediate Recall | ||||||||
| Step 1 | 6.29*** | 0.46 | ||||||
| Age | 0.49 | 4.00** | ||||||
| Whole-brain FA | 0.10 | 0.78 | ||||||
| KBIT Verbal SS | 0.39 | 3.17* | ||||||
| [L] HC − V1 FA | −0.20 | −1.22 | ||||||
| [R] HC − V1 FA | 0.03 | 1.84 | ||||||
| Step 2 | 9.36** | 0.19 | ||||||
| Age | 0.52 | 4.83*** | ||||||
| Whole-brain FA | 0.16 | 1.41 | ||||||
| KBIT Verbal SS | 0.33 | 3.18* | ||||||
| [L] HC − V1 FA | 0.05 | 0.34 | ||||||
| [R] HC − V1 FA | 0.27 | 1.77 | ||||||
| [L] HC − IPL FA | −0.61 | −4.05** | ||||||
| [R] HC − IPL FA | 0.44 | 3.19* | ||||||
| Delayed Recall | ||||||||
| Step 1 | 4.44** | 0.40 | ||||||
| Age | 0.44 | 3.26* | ||||||
| Whole-brain FA | 0.08 | 0.59 | ||||||
| KBIT Verbal SS | 0.41 | 3.03* | ||||||
| [L] HC − V1 FA | −0.18 | −1.00 | ||||||
| [R] HC − V1 FA | 0.26 | 1.42 | ||||||
| Step 2 | 4.20* | 0.13 | ||||||
| Age | 0.47 | 3.63** | ||||||
| Whole-brain FA | 0.13 | 0.93 | ||||||
| KBIT Verbal SS | 0.37 | 2.94* | ||||||
| [L] HC − V1 FA | 0.03 | 0.16 | ||||||
| [R] HC − V1 FA | 0.22 | 1.26 | ||||||
| [L] HC − IPL FA | −0.50 | −2.62 | ||||||
| [R] HC − IPL FA | 0.36 | 12.16 | ||||||
| Delayed Recognition | ||||||||
| Step 1 | 1.82 | 0.20 | ||||||
| Age | 0.25 | 1.64 | ||||||
| Whole-brain FA | 0.14 | 0.87 | ||||||
| KBIT Verbal SS | 0.12 | 0.80 | ||||||
| [L] HC − V1 FA | −0.21 | −1.02 | ||||||
| [R] HC − V1 FA | 0.38 | 1.88 | ||||||
| Step 2 | 8.61** | 0.27 | ||||||
| Age | 0.35 | 2.63 | ||||||
| Whole-brain FA | 0.28 | 2.03 | ||||||
| KBIT Verbal SS | 0.10 | 0.76 | ||||||
| [L] HC − V1 FA | 0.03 | 0.13 | ||||||
| [R] HC − V1 FA | 0.50 | 2.63 | ||||||
| [L] HC − IPL FA | −0.77 | −4.12** | ||||||
| [R] HC − IPL FA | 0.24 | 1.39 | ||||||
| Episodic Memory | ||||||||
| Context Memory | ||||||||
| Step 1 | 1.45 | 0.16 | ||||||
| Age | 0.18 | 1.79 | ||||||
| Whole-brain FA | 0.18 | 1.23 | ||||||
| KBIT Nonverbal SS | −0.14 | −1.52 | ||||||
| [L] HC − V1 FA | −0.31 | −1.62 | ||||||
| [R] HC − V1 FA | 0.33 | 1.66 | ||||||
| Step 2 | 6.62 | 0.23 | ||||||
| Age | 0.06 | 0.40 | ||||||
| Whole-brain FA | 0.04 | 0.29 | ||||||
| KBIT Nonverbal SS | −0.27 | −1.90 | ||||||
| [L] HC − V1 FA | −0.14 | −0.70 | ||||||
| [R] HC − V1 FA | −0.03 | −0.16 | ||||||
| [L] HC − IPL FA | 0.03 | 0.16 | ||||||
| [R] HC − IPL FA | 0.60 | 3.19* | ||||||
FA: fractional anisotropy, β: standardized regression coefficient, HC – mPFC: white matter connecting hippocampus and medial prefrontal cortex, HC – V1: white matter connecting hippocampus and primary visual cortex.
ΔF: the change in F values between models 1 and 2. ΔR2: the change in R2 between models 1 and 2.
*** p < 0.001; ** p < 0.01; * p < 0.01 Bonferroni corrected (corrected α= 0.01 for Model 1 and corrected α= 0.007 for Model 2).
Table 4.
Summary of hierarchical linear regression models of white matter connectivity between hippocampus and medial prefrontal cortex microstructure (FA) predicting memory performances.
| Dependent variable | Predictors | β | t-value | F | ΔF | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|
| Children’s Memory Scale | |||||||
| Immediate Recall | |||||||
| Step 1 | 5.83*** | 0.43 | |||||
| Age | 0.48 | 3.88** | |||||
| Whole-brain FA | 0.12 | 0.93 | |||||
| KBIT Verbal SS | 0.36 | 2.92* | |||||
| [L] HC − V1 FA | −0.13 | −0.76 | |||||
| [R] HC − V1 FA | 0.31 | 1.78 | |||||
| Step 2 | 0.64** | 0.02 | |||||
| Age | 0.49 | 3.94** | |||||
| Whole-brain FA | 0.04 | 0.19 | |||||
| KBIT Verbal SS | 0.33 | 2.60 | |||||
| [L] HC − V1 FA | −0.07 | −0.41 | |||||
| [R] HC − V1 FA | 0.34 | 1.94 | |||||
| [L] HC − mPFC FA | −0.18 | −0.93 | |||||
| [R] HC − mPFC FA | 0.23 | 1.03 | |||||
| Delayed Recall | |||||||
| Step 1 | 4.00** | 0.36 | |||||
| Age | 0.42 | 3.13* | |||||
| Whole-brain FA | 0.10 | 0.72 | |||||
| KBIT Verbal SS | 0.38 | 2.78 | |||||
| [L] HC − V1 FA | −0.10 | −0.55 | |||||
| [R] HC − V1 FA | 0.26 | 1.37 | |||||
| Step 2 | 0.27 | 0.01 | |||||
| Age | 0.43 | 3.10* | |||||
| Whole-brain FA | −0.03 | −0.13 | |||||
| KBIT Verbal SS | 0.36 | 2.51 | |||||
| [L] HC − V1 FA | −0.08 | −0.37 | |||||
| [R] HC − V1 FA | 0.25 | 1.30 | |||||
| [L] HC − mPFC FA | 0.02 | 0.09 | |||||
| [R] HC − mPFC FA | 0.15 | 0.57 | |||||
| Delayed Recognition | |||||||
| Step 1 | 1.95 | 0.20 | |||||
| Age | 0.26 | 1.77 | |||||
| Whole-brain FA | 0.14 | 0.89 | |||||
| KBIT Verbal SS | 0.12 | 0.81 | |||||
| [L] HC − V1 FA | −0.23 | −1.08 | |||||
| [R] HC − V1 FA | 0.39 | 1.92 | |||||
| Step 2 | 1.00 | 0.04 | |||||
| Age | 0.28 | 1.89 | |||||
| Whole-brain FA | 0.08 | 0.32 | |||||
| KBIT Verbal SS | 0.09 | 0.58 | |||||
| [L] HC − V1 FA | −0.14 | −0.65 | |||||
| [R] HC − V1 FA | 0.45 | 2.14 | |||||
| [L] HC − mPFC FA | −0.31 | −1.33 | |||||
| [R] HC − mPFC FA | 0.29 | 1.09 | |||||
| Episodic Memory | |||||||
| Context Memory | |||||||
| Step 1 | 1.45 | 0.15 | |||||
| Age | 0.22 | 1.48 | |||||
| Whole-brain FA | 0.17 | 1.12 | |||||
| KBIT Nonverbal SS | −0.12 | −0.77 | |||||
| [L] HC − V1 FA | −0.25 | −1.21 | |||||
| [R] HC − V1 FA | 0.30 | 1.46 | |||||
| Step 2 | 0.52 | 0.02 | |||||
| Age | 0.24 | 1.55 | |||||
| Whole-brain FA | 0.10 | 0.44 | |||||
| KBIT Nonverbal SS | −0.14 | −0.88 | |||||
| [L] HC − V1 FA | −0.18 | −0.82 | |||||
| [R] HC − V1 FA | 0.31 | 1.51 | |||||
| [L] HC − mPFC FA | −0.19 | −0.84 | |||||
| [R] HC − mPFC FA | 0.23 | 0.90 | |||||
FA: fractional anisotropy, β: standardized regression coefficient, HC – mPFC: white matter connecting hippocampus and medial prefrontal cortex, HC – V1: white matter connecting hippocampus and primary visual cortex.
ΔF: the change in F values between models 1 and 2. ΔR2: the change in R2 between models 1 and 2.
*** p < 0.001; ** p < 0.01; * p < 0.01 Bonferroni corrected (corrected α = 0.01 for Step 1 and corrected α = 0.007 for Step 2).
4.4. Hippocampus – inferior parietal lobule (see Table 3)
4.4.1. CMS Immediate Recall
At Step 1, all control variables were entered including age, whole-brain FA, KBIT Verbal standardized score, and bilateral hippocampus – V1 FA. The model was significant, F(5, 37) = 6.29, R2 = 0.46, p = 0.001. Age, β = 0.49, t(44) = 4.00, p < 0.001, and KBIT verbal, β = 0.39, t(44) = 3.17, p = 0.02, were significant predictors. In Step 2, introducing bilateral hippocampus – IPL FA explained an additional 19% of the variance, and this change in R2 was significant, ΔF(2, 35) = 9.36, p = 0.001. Both left, β = −0.61, t(44) = −4.05, p = 0.002, and right, β = 0.44, t(44) = 3.19, p = 0.02, hippocampus – IPL FA significantly predicted CMS immediate recall. The left and right hippocampus – IPL FA accounted for 32% and 23% of variance, respectively. Importantly, using Steiger’s Z tests, we found that the effect was significantly greater in the left hippocampus – IPL (R2 = 0.32) than in its respective control tract, the left hippocampus – V1 (R2 = 0.003), z = 2.60, p = 0.009 (see Fig. 1A). However, the effect in the right hippocampus – IPL (R2 = 0.23) was not significantly greater than that in the right hippocampus – V1 (R2 = 0.08), Z = 0.96, p = 0.34. These findings suggest that connectivity between the left hippocampus and left IPL microstructure significantly predicts performance on the CMS immediate recall above and beyond age, global FA, and verbal intelligence. There was no multicollinearity violation (all VIFs < 2.33).
4.4.2. CMS delayed recall
When all 5 control variables were included in Step 1, the model significantly predicted performance on the CMS delayed recall, F(5, 34) = 4.44, R2 = 0.40, p = 0.003. Age, β = 0.44, t(40) = 3.26, p = 0.01, and KBIT verbal, β = 0.41, t(40) = 3.03, p = 0.02, significantly predicted CMS delayed recall performance. Adding bilateral hippocampus – IPL FA in Step 2 explained an additional 13% of the variance, ΔF(2, 32) = 4.20, p = 0.02. Neither the left, t(40) = −2.72, p = 0.07, or right, t(40) = 2.16, p = 0.28 tract, when taken alone significantly predicted CMS delayed recall performance. Again, no violation of multicollinearity was detected (all VIFs < 2.29).
4.4.3. CMS delayed recognition
The regression model in Step 1 did not predict CMS delayed recognition accuracy, F(5, 36) = 1.82, R2 = 0.20, p = 0.14. None of the control variables significantly predicted CMS Delayed Recognition, all p’s > 0.34. However, introducing bilateral hippocampus – IPL FA explained an additional 27% of the variance, ΔF(2, 34) = 8.61, p = 0.001. Left hippocampus – IPL FA was the only significant predictor of CMS delayed recognition, β = −0.77, t(42) = −4.12, p = 0.002, accounting for 33% of the variances. Importantly, the effect was significantly greater in the left hippocampus – IPL (R2 = 0.33) than in its respective control tract, the left hippocampus – V1 (R2 = 0.00), Z = 2.80, p = 0.005 (see Fig. 1A). These results suggest that the left hippocampus – IPL white matter microstructure significantly predicts performance on the CMS delayed recognition above and beyond age, global FA, and verbal intelligence. In addition, our data did not violate the assumption of collinearity (all VIFs < 2.34).
4.4.4. Context memory
Entering five control variables (age, whole-brain FA, and KBIT nonverbal, bilateral hippocampus – V1 FA) in Step 1 did not significantly predict context memory accuracy, F(5, 38) = 1.45, R2 = 0.16, p = 0.23. None of the control variables significantly predicted context memory, all p’s > 0.41. In Step 2, entering bilateral hippocampus – IPL FA explained an additional 23% o the variance, ΔF(2, 36) = 6.62, p = 0.004. Right hippocampus – IPL FA was the only significant predictor, β = 0.60, t(44) = 3.19, p = 0.02, accounting for 22% of the variance. Importantly, the effect was significantly greater in the right hippocampus – IPL (R2 = 0.22) than in its respective control tract, the right hippocampus – V1 (R2 = 0.00), Z = 2.18, p = 0.03 (see Fig. 1A). White matter connecting the right hippocampus and right IPL microstructure significantly predicts context memory accuracy and beyond age, global FA, and nonverbal intelligence (see Fig. 1A). Furthermore, no violation of the assumption of collinearity was found (all VIFs < 2.45).
4.4.5. KBIT
To examine whether bilateral hippocampal – IPL FA predict behavioral variable of non-interest, i.e., verbal and nonverbal intelligence, we conducted hierarchical regression models predicting KBIT Verbal and Nonverbal standardized scores separately. For KBIT Verbal, entering variables including age, whole-brain FA, bilateral hippocampus – V1 FA in Step 1 did not predict children’s scores on the KBIT Verbal test, F(4, 40) = 0.14, R2 = 0.01, p = 0.97. Adding bilateral hippocampus – IPL FA in Step 2 only explained an additional 6% of the variance, and this change in R2 was not significant, F(2, 38) = 1.15, p = 0.82. The results on the KBIT Nonverbal were similar. The control variables entered in Step 1 significantly predicted KBIT nonverbal, F(4, 40) = 0.70, R2 = 0.07, p = 0.60. Only an additional 7% of the variance was accounted for by adding bilateral hippocampus – IPL FA to the model at Step 2, F(2, 38) = 1.54, p = 0.45. These results suggest that the white matter tract connecting the hippocampus and IPL relates to performance on various memory tasks, but not to performance on tasks with a low declarative memory demand. Again, multicollinearity was not an issue, all VIFs < 2.40.
4.5. Hippocampus – medial prefrontal cortex (see Table 4)
Unlike the HC – IPL microstructure, neither the left nor right hippocampus – mPFC FA predicted performances on any of the CMS tests or Episodic Memory tasks, all p’s > 0.99.
4.6. White matter macrostructure– behavioral performance relations
The same statistical approach was conducted for the white matter macrostructure of each tract predicting each memory performance. In Step 1, the control variables included age, whole-brain white matter volume, KBIT standardized score, and bilateral control tract (hippocampus – V1) volume; and bilateral tract of interest volumes were entered in Step 2. Neither the left nor right hippocampus – IPL volume predicted any of the CMS tests, all p’s > 0.56, or context memory on the Episodic Memory task, all p’s > 0.99. Similarly, the hippocampus – mPFC volume did not predict performance on any of the CMS or context memory on the Episodic Memory task, all p’s > 0.99.
4.7. Exploratory analyses: limbic white matter pathways
It is possible that although the hippocampus – mPFC connectivity did not correlate with memory performance, an analysis with higher granularity might unveil a relation between medial temporal-frontal connectivity and the memory of young children. Visual inspection of the hippocampus mPFC tractography revealed that this white matter pathway includes portions of the fornix and the uncinate fasciculus (see Fig. 1B).
Neither the Fornix FA nor volume differed between 4 and 6-year-olds (all p’s > 0.16). To examine the whether variations in the fornix microstructure and macrostructure relate to memory, we conducted hierarchical regression analyses with five control variables (same as described above) entered in Step 1, and fornix FA/volume entered in Step 2. Separate regression models were conducted for microstructure and macrostructure. Our results showed that entering fornix FA to the model did not explain a significant additional amount of variance in any of the memory tests (ΔR2 = 0.00–0.04, all p’s > 0.13).
Similar results were found for the uncinate fasciculus. No age differences were found for either the left or right uncinate fasciculus FA or volume (all p’s = 0.40). In the hierarchical regression models, adding bilateral uncinate FA or volume did not explain a significant additional amount of variances on any of the memory tests (ΔR2 = 0.00–0.02, all p’s > 0.66).
In sum, we found no age effect in the fornix and uncinate micro- and macrostructure between 4- and 6-year-old children. Furthermore, variations in the micro- and macrostructure of the fornix and uncinate fasciculus did not relate to any of the memory measures. These findings corroborate our findings on the hippocampus – mPFC connectivity, suggesting that neither of the specific major fronto-temporal white matter tracts – fornix and uncinate – related to memory performance in young children (for results summary, see Table 5).
Table 5.
Results summary of white matter microstructure of the tracts examined and their relations with each memory task.
| Memory Tasks | HC–IPL |
HC–mPFC |
Fornix | UF |
|||
|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | ||
| Children’s Memory Scale | |||||||
| Immediate Recall | ✓* | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Delayed Recall | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Delayed Recognition | ✓* | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Episodic Memory | |||||||
| Context Memory | ✗ | ✓* | ✗ | ✗ | ✗ | ✗ | ✗ |
✓: Significant predictor.
*: Significantly greater than that of the respective control tract (left/right hippocampus – V1 FA).
5. General discussion
Early childhood, particularly between the ages of four and six, is marked by important and robust growth in episodic memory (e.g., Newcombe et al., 2014; Ngo et al., 2017, Lloyd et al., 2009, Riggins, 2014). Gains in episodic memory performance may partly reflect maturation of brain networks essential for episodic memory (reviewed in Olson and Newcombe, 2014). These networks mature along several dimensions: in terms of gray matter volume (Riggins et al., 2015), functional connectivity within the memory networks (Riggins et al., 2016), and in terms of age-related differences in white matter macro and microstructure. Importantly, different brain regions mature at different rates. Thus the dynamic interplay of maturational processes both within and outside of the hippocampus will affect episodic memory functioning (reviewed in Olson and Newcombe, 2014).
Specifically, we examined age-related differences in white matter tracts that support episodic memory systems. We assessed episodic memory using the Children’s Memory Scale standardized test – a verbal recall memory paradigm, and an episodic memory test that taps memory for single objects, as well as memory for context – a hallmark of episodic memory. We delineated two main white matter tracts of interests: one connecting the hippocampus to the inferior parietal lobule, and one connecting the hippocampus to the medial prefrontal cortex. We found that the microstructural properties of the white matter pathway connecting the hippocampus to the inferior parietal lobule significantly correlated with performance on several memory tasks.
Specifically, the left hippocampus – IPL microstructure predicted children’s performance on the CMS immediate recall and delayed recognition, whereas the right hippocampus – IPL microstructure predicted children’s ability to recall contextual details in the Episodic Memory task. The hemispheric effect aligns with the nature of these tasks: CMS is a verbal memory task in which children recalled stories, whereas the context memory contains less verbal demand (recalling the location and action associated with an object). White matter microstructure of this pathway explained significant amounts of variance (10%–26%), above and beyond age, whole-brain FA, verbal/nonverbal intelligence, and a bilateral control white matter tracts that should not be implicated in episodic memory.
5.1. Age-related differences in white matter
Although previous studies have found a general increase in white matter volume, as well as FA, throughout development (e.g., Lebel and Beaulieu, 2011, Westlye et al., 2010; reviewed in Lebel et al., 2017), we found that white matter macrostructure and microstructure did not differ between 4- and 6-year-olds. However, our sample had a severely restricted age range. We suspect that including a wider age range may yield an age effect – a potential future direction of this work. In addition, higher resolution imaging, such as HARDI imaging, may reveal more subtle age-related white matter changes.
5.2. The role of the inferior parietal lobe in episodic memory
It is believed that the information transmission properties of any given white matter tract can be predicted by the function of the gray matter regions that it connects (Maunsell and van Essen, 1983, Passingham et al., 2002). For this reason, we chose the hippocampus as our seed region, as its role in episodic memory is well established from decades of research across a range of species. One question that must be asked is whether our findings of hippocampal-IPL structural connectivity have any support from the neuroanatomy literature, especially those studies using techniques that are more precise than diffusion imaging. Studies in monkeys using injected radiotracers have identified several monosynaptic axonal pathways between the IPL and medial temporal lobe. The cingulum bundle, which begins in the medial temporal lobe and circles through the cingulate cortex, connects lateral and medial regions of the posterior IPL to the parahippocampal gyrus (Seltzer and Pandya, 1984) and connections exist between Area 7 in the IPL and entorhinal cortex (Insausti and Amaral, 2008, Wellman and Rockland, 1997). Most interestingly, there are connections between hippocampal area CA1, in the anterior hippocampus, and Area 7a and 7b of the IPL (Clower et al., 2001, Rockland and Van Hoesen, 1999). Connections have also been identified between the presubiculum and Area 7a of the IPL (Cavada and Goldman-Rakic, 1989, Ding et al., 2000). Thus it is likely that the tractography results from the current work closely reflect the ground-truth evidence from gross anatomical dissection.
A second question that must be asked is what is the nature and manner of IPL involvement in episodic memory? As noted in the introduction, fMRI studies in adults have consistently linked the inferior parietal cortex to memory retrieval accompanied by recollection (reviewed in Wagner et al., 2005). For example, studies have reported that IPL activity is greater for items recognized with recollection judgments than those with familiarity judgments (Cansino et al., 2002, Henson et al., 1999, Dobbins et al., 2003, Hutchinson et al., 2014, Wheeler and Buckner, 2004; reviewed in Cabeza et al., 2012), and when retrieval is supported by recollection as opposed to familiarity (Dobbins et al., 2003, Dobbins et al., 2002, Dobbins and Wagner, 2005; reviewed in Wagner et al., 2005). Corroborating this view, patients with bilateral parietal lobe lesions report few details when recalling autobiographical memories compared to healthy controls (Berryhill et al., 2007), and have reduced certainty in their memories, as indexed by reduced subjective confidence (Hower et al., 2014, Simons et al., 2010).
Several theories have been proposed to explain this relationship, ranging from theories relating the IPL to a mnemonic accumulator, tracking memory signal strength to help make old/new decisions, and decisions related to subjective aspects of memory (Ally et al., 2008, Hower et al., 2014, Simons et al., 2010), to theories linking this region to “internal attention”, essential for retrieval (reviewed in Cabeza et al., 2008). Our findings cannot adjudicate between these different views.
However, our findings do add to the growing literature linking the IPL to episodic memory function. Our findings are consistent with the literature implicating the IPL in recollection, but cannot speak to findings on subjective aspects of memory since young children lack the meta-cognitive abilities required to report on such things. Importantly, our findings highlight the role of structural connectivity between the hippocampus and IPL in episodic memory development, using recall and recognition accuracy as measures of interest.
5.3. Frontal connectivity
In contrast to the hippocampus – IPL connectivity, structural connectivity between the hippocampus and mPFC did not relate to any of the memory measures. The same results were found when we delineated specific medial temporal-frontal pathways, the fornix and uncinate fasciculus. At first glance, these findings may appear to contradict findings showing involvement of the prefrontal cortex in episodic memory (reviewed in Raj and Bell, 2010) and neuropsychological findings showing that damage to the PFC impedes source memory retrieval (e.g., Ciaramelli and Spanoil, 2009, Duarte et al., 2005), increases false recognition (Curran et al., 1997), and increases susceptibility to interference (Shimamura et al., 1995).
Several speculations can be made about our null results in regards to hippocampal-mPFC connectivity. First, one possibility is that the age groups examined in this study are quite young and the mPFC is still undergoing regional maturation (Gogtay et al., 2004). If true, regional changes in the mPFC should relate to memory development (Ofen et al., 2007). Second, it is possible that the white matter connectivity between the hippocampus and the mPFC is underdeveloped and has a long to way to reach adult-like form. White matter volume gradually increases around the age of 2 until adulthood (Groeschel et al., 2010), with frontal-temporal connections showing the most prolonged development (Lebel and Beaulieu, 2011). If true, we should expect that age-related differences in this white matter would closely track differences in memory performance. These hypotheses merit further investigations, possibly at a later stage in development.
5.4. Laterality and directionality
The direction of the left hippocampus – IPL FA and CMS memory performance and the right hippocampus – IPL FA and context memory are in the opposite directions. Lower left hippocampus – IPL FA was associated with higher performances on the CMS tests, whereas higher right hippocampus – IPL FA was associated with higher context memory recall. Interestingly, diffusion-weighted imaging studies in adults have found similar patterns in young adults (e.g., Alm et al., 2016, Nugiel et al., 2016, Metoki et al., 2017). It is a possibility that the relation of the hippocampus – IPL connectivity and memory differs depending on the nature of the stimuli in the memory tasks. The fact that CMS is a verbal task, whereas context memory is primarily visual, may influence the directionality of effects. Furthermore, the extent of myelination may have different effects on signal transduction depending on different brain areas. Although these patterns of findings have been reported in other studies, the issue of directionality and white matter indices merits further investigation.
5.5. Specificity and generality of findings
The level of neural specificity of any brain-behavior effect is critical for its interpretation. We used several levels of control to assess specificity. First, we used white matter connecting the hippocampus to V1 as a control fiber pathway. As expected, we found no reliable relation between this tract and memory. Second, we carefully controlled for several variables, including whole brain white matter and IQ, reasoning that these variables could potentially explain differences in behavior, microstructure or both. Indeed, IQ was related to memory performance; however, it did not correlate with microstructural differences in our pathways of interest. Therefore, there seems to be some specificity to our findings.
It is also important to consider how the findings of any given study generalize to other tasks and populations. In our study, episodic memory was indexed by immediate and delayed verbal recall tasks (CMS) and a delayed context recall memory task (Episodic Memory tasks). Our delay intervals were half an hour and one hour. Future studies should examine whether these results would generalize to memory tasks with longer delay such as 24 h or a one-week delay. In addition, episodic memory tasks vary in the complexity of relational structure. In this study, the episodic memory task required children to bind a toy to specific action and a specific context. Other episodic memory tasks used in previous studies employed a non-overlapping AB-CD associations (e.g., Lloyd et al., 2009, Sluzenski et al., 2006), or differential extents of overlapping elements (e.g., AB-AC associations: Ngo et al., 2017, Darby and Sloutsky, 2015, Yim et al., 2013; AB-ABr: Yim et al., 2013). Relational memory for different extents of overlapping features may result in differential demands on retrieval strategies – potentially recruited from frontal regions – to minimize potential inference. Thus, our findings may not generalize to other variants of episodic memory tasks. Lastly, given that both white matter connectivity and episodic memory change drastically in early development, different patterns in the relation between white matter and memory performance may be observed with different age ranges, particularly in later development.
5.6. Limitations
One limitation of the study is its cross-sectional design, which allowed us a glimpse into brain development. However, brain maturation accompanying memory development is a complex process, encompassing the dynamic interplay among many key players. A longitudinal design would be ideal. In addition, due to our sample size, we limited our investigation of hippocampal connectivity to a subset of cortical target regions. With larger sample sizes, future studies should explore other white matter tracts that may also be important for the development of episodic memory.
Another limitation of the current work lies in the technical challenge of diffusion weighted imaging as indirect measurements of white matter tissue based on estimates of water diffusivity. Diffusion weighted imaging techniques only provide computational models of WM tissue with many assumptions about the underlying processes and structures. Thus, the success of diffusion-weighted imaging in delineating white matter pathways in the brain is highly dependent on data quality, the chosen diffusion model, and the analysis pipeline (Jones et al., 2013). In this study, we acquired diffusion tensor imaging with three repetitions, hence increasing the signal-to-noise ratio in our data. In addition, we employed the dual-fiber model, which accounts for crossing fibers in the brain, as opposed to the a single tensor model with a deterministic tracing algorithm, which only calculates a single principle diffusion direction in each voxel. Nevertheless, one must be wary of technical limitations and pitfalls in interpretation of white matter connectivity using diffusion weighted imaging (Jbabdi et al., 2015, Jones et al., 2013), as there is unlikely to be a one-to-one correspondence between diffusion parameter and the underlying tissue structure (Assaf et al., 2017).
6. Conclusions
Typical episodic memory functioning relies on the operations of a far-flung yet exquisitely orchestrated network of brain regions. The “wires” connecting the nodes of this network are axons, bundled into tracts that can be measured with diffusion imaging. However, white matter matures slowly and variably, making its measurement critical for understanding the emergence of cognition over development. Our study was among the first to identify and characterize the relation between white matter connectivity and episodic memory in young children, ages four and six. Our results show that hippocampal-inferior parietal lobe white matter is a key variable in predicting episodic memory performance in this age range.
Conflict of Interest
None
Acknowledgement
We would like to thank the members of the Neurocognitive Development Lab and Developmental Social Cognitive Neuroscience Lab at the University of Maryland, especially, Elizabeth Redcay, Sarah Blankenship and Katherine Rice for their contribution to this work. We additionally thank the families who participated in this study and the Maryland Neuroimaging Center for their support in data acquisition. Portions of these data were presented at the Society for Research in Child Development (Austin, TX) 2017). This work was supported by a National Institutes of Health (RO1 MH091113 to I.R.O and F31HD090872 to C.T.N), a National Science Foundation (SBE-1041707 to N.S.N), and by a Maryland Neuroimaging Center seed grant, the Behavioral and Social Sciences Dean’s Research Initiative, the University of Maryland Department of Psychology and an National Institutes of Health (R01HD079518A to T.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors declare no competing or conflicting financial interests.
References
- Ally B.A., Simons J.S., McKeever J.D., Peers P.V., Budson A.E. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46(7):1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm K.H., Rolheiser T., Olson I.R. Inter-individual variation in fronto-temporal connectivity predicts the ability to learn different types of associations. Neuroimage. 2016;132:213–224. doi: 10.1016/j.neuroimage.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y., Johansen-Berg, Schotten M.T. The role of diffusion MRI in neuroscience. NMR Biomed. 2017 doi: 10.1002/nbm.3762. [DOI] [PubMed] [Google Scholar]
- Backus A.R., Bosch S.E., Ekman M., Grabovetsky A.V., Doeller C.F. Mnemonic convergence in the human hippocampus. Nat. Commun. 2016 doi: 10.1038/ncomms11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J., Doydum A.O., Pathman T., Larkina M., Guler O.E., Burch M. It’s all about location, location, location: children’s memory for the where of personally experienced events. J. Exp. Child Psychol. 2012;133(4):510–522. doi: 10.1016/j.jecp.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Johansen-Berg H., Woolrich M.W., Smith S.M., Wheeler-Kingshott C.A., Boulby P.A., Barker G.J., Sillery E.L., Sheehan K., Ciccarelli O., Thompson A.J., Brady J.M., Matthews P.M. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi S., Soroker N., Levy D.A. Parietal lesion effects on cued recall following pair associate learning. Neuropsychologia. 2015;73:176–194. doi: 10.1016/j.neuropsychologia.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Benoit R.G., Anderson M.C. Opposing mechanisms supporting the voluntary forgetting of unwanted memories. Neuron. 2012;76(2):450–460. doi: 10.1016/j.neuron.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill M.E., Phuong L., Picasso L., Cabeza R., Olson I.R. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill M.E., Drowos D.B., Olson I.R. Bilateral parietal cortex damage does not impair associative memory for paired stimuli. Cognit. Neuropsychol. 2009;26(7):606–619. doi: 10.1080/02643290903534150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp D., Nagae L.M., Degaonkar M., Matson M., Abdalla W.M., Peter B. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cognit. Sci. 2012;16(6):338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S., Maquet P., Dolan R., Rugg M. Brain activity underlying encoding and retrieval of source memory. Cereb. Cortex. 2002;12(10):1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cavada C., Goldman-Rakic P.S. Posterior parietal cortex in rhesus monkey: i. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chaddock-Heyman L., Erickson K., Voss M.W., Powers J.P., Knecht A., Hillman C.H., Kramer A.F. White matter microstructure is associated with cognitive control in children. Biol. Psychol. 2013;94(1):109–115. doi: 10.1016/j.biopsycho.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E., Spanoil J. Ventromedial prefrontal damage and memory for context: perceptual versus semantic features. Neuropsychology. 2009;23(5):649–657. doi: 10.1037/a0015937. [DOI] [PubMed] [Google Scholar]
- Clower D.M., West R.A., Lynch J.C., Strick P.L. The inferior parietal lobule is the target output from the superior colliculus, hippocampus, and cerebellum. J. Neurosci. 2001;21(16):6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N.J., Eichenbaum H. MIT Press; Cambridge, MA: 1993. Memory, Amnesia, and the Hippocampal System. [Google Scholar]
- Cohen M.J. Harcourt Brace and Company; San Antonio, TX: 1997. Children’s Memory Scale Manual The Psychological Corporation. [Google Scholar]
- Curran T., Schacter D.L., Norman K.A., Galluccio L. False recognition after a right frontal lobe infarction: memory for general and specific information. Neuropsychologia. 1997;35(7):1035–1049. doi: 10.1016/s0028-3932(97)00029-8. [DOI] [PubMed] [Google Scholar]
- Darby K.P., Sloutsky V.M. The cost of learning: interference effects in memory development. J. Exp. Psychol. Gen. 2015;144:410–431. doi: 10.1037/xge0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D.M., Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2013;49(6):1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Pathman T., Lee J., Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior-posterior axis. Cereb. Cortex. 2013;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Ding S.L., Van Hoessen G., Rockland K.S. Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. J. Comp. Neurol. 2000;425(4):510–530. doi: 10.1002/1096-9861(20001002)425:4<510::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Wagner A.D. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb. Cortex. 2005;15(11):1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Foley H., Schacter D., Wagner A.D. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35(5):989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Rice H.J., Wagner A.D., Schacter D.L. Memory orientation and success: separable cognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Douet V., Chang L. Fornix as an imaging marker for episodic memory deficits in healthy changing and in various neurological disorders. Front. Aging Neurosci. 2014;6:343. doi: 10.3389/fnagi.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drowos D.B., Berryhill M., André J.M., Olson I.R. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24(4):465–475. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummey A.B., Newcombe N.S. Developmental changes in source memory. Dev. Sci. 2002;5(4):502–513. [Google Scholar]
- Duarte A., Ranganath C., Knight R.T. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. J. Neurosci. 2005;25(36):8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76(6):1057–1070. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., Bunge S.A. Neural changes underlying the development of episodic memory during middle childhood. Dev. Cognit. Neurosci. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., III, Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Grace A.A. Dopamine modulation of the hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb. Cortex. 2008;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeschel S., Vollmer B., King M.D., Connelly A. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int. J. Dev. Neurosci. 2010;6:481–489. doi: 10.1016/j.ijdevneu.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Haramati S., Soroker S., Dudai Y., Levy D.A. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2008;46(7):1756–1766. doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Henson R.N., Rugg M.D., Shallice T., Joshephs O., Dolan R.J. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J. Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hower K.H., Wixted J., Berryhill M.E., Olson I.R. Impaired perception of mnemonic oldness, but not mnemonic newness: after parietal lobe damage. Neuropsychologia. 2014;56:409–417. doi: 10.1016/j.neuropsychologia.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson B., Uncapher M., Weiner Bressler D., Silver M., Preston A., Wagner A. Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb. Cortex. 2014;24(1):49–66. doi: 10.1093/cercor/bhs278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R., Amaral D.G. Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. J. Comp. Neurol. 2008;509:608–641. doi: 10.1002/cne.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack F., MacDonald S., Reese E., Hayne H. Maternal reminiscing style during early childhood predicts the age of adolescents’ earliest memories. Child Dev. 2009;80(2):496–505. doi: 10.1111/j.1467-8624.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- Jbabdi S., Sotiropoulos S.N., Haber S.N., Van Essen D.C., Behrens T.E. Measuring macroscopic brain connections in vivo. Nat. Neurosci. 2015;18(11):1546–1555. doi: 10.1038/nn.4134. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Krogsrud S.K., Fjell A., Tamnes C.K., Grydeland J., Mork L., Due-Tonnessen P. Changes in white matter microstructure in the developing brain – a longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage. 2016;124:473–486. doi: 10.1016/j.neuroimage.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Treit S., Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2017 doi: 10.1002/nbm.3778. [DOI] [PubMed] [Google Scholar]
- Lloyd M.E., Doydum A.O., Newcombe N.S. Memory binding in early childhood: evidence for a retrieval deficit. Child Dev. 2009;80(5):1321–1328. doi: 10.1111/j.1467-8624.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Loenneker T., Klaver P., Bucher K., Lichtensteiger J., Imfeld A., Martin E. Microstructural development: organizational differences of the fiber architecture between children and adults in dorsal and ventral visual streams. Hum. Brain Mapp. 2011;32(6):935–946. doi: 10.1002/hbm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell J.H., van Essen D.C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 1983;3(12):2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metoki, A., Alm, K., Wang, Y., Ngo, C., Olson, I.R., (2017). Never forget a name: white matter connectivity predicts person memory. [DOI] [PMC free article] [PubMed]
- Metzler-Baddeley C., Jones D.K., Belaroussi B., Aggleton J., O’Sullivan M.J. Frontotemporal connections in episodic memory and aging: a diffusion MRI tractography study. J. Neurosci. 2011;31(37):13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M.M., Okonkwo O.C., Oishi K., Mori S., Tighe S., Miller M.I., Ceritoglu C., Brown T., Albert M., Lyketsos C.G. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer’s Dementia. 2012;8(2):105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon W.J., Provenzale J.M., Sarikaya B., Ihn Y.K., Morlese J., Chen S. Diffusion tensor imaging assessment of white matter maturation in childhood and adolescence. Am. J. Roentgenol. 2011;193(3):704–712. doi: 10.2214/AJR.10.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe N.S., Balcomb F., Ferrara K., Hansen M., Koski J. Two rooms, two representations. Episodic-like memory in toddlers and preschoolers. Dev. Sci. 2014;17(5):743–756. doi: 10.1111/desc.12162. [DOI] [PubMed] [Google Scholar]
- Ngo C.T., Newcombe N.S., Olson I.R. The ontogeny of relational memory and pattern separation. Dev. Sci. 2017 doi: 10.1111/desc.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugiel T., Alm K.H., Olson I.R. Individual differences in white matter microstructure predict semantic control. Cognit. Affect. Behav. Neurosci. 2016;16(6):1003–1016. doi: 10.3758/s13415-016-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N., Kao Y., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J.D.E. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Oishi K., Mielke M.M., Albert M., Lyketsos C.G., Mori S. The fornix sign: a potential sign for Alzheimer’s disease based on diffusion tensor imaging. Neuroimage. 2011;22(4):365–374. doi: 10.1111/j.1552-6569.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Newcombe N.S. Binding together the elements of episodes: relational memory and the developmental trajectory of the hippocampus. Patricia J. Bauer and Robyn Fivush; 2014. [Google Scholar]
- Olson I.R., Von Der Heide R.J., Ahm K., Vyas G. Development of the uncinate fasciculus: implications for theory and developmental disorder. Dev. Cognit. Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R.E., Stephan K.E., Kötter R. The anatomical basis of functional localizer in the cortex. Nat. Rev. Neurosci. 2002;3:606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Qiu M.G., Li Q.Y., Liu G.J., Xie B., Wang J.A. Voxel-based analysis of white matter during adolescence and young adulthood. Brain Dev. 2010;32(7):531–537. doi: 10.1016/j.braindev.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Raj V., Bell M.A. Cognitive processes supporting episodic memory formation in childhood. The role of source memory, binding, and executive function. Dev. Rev. 2010;30:384–402. [Google Scholar]
- Riggins T., Rollins L. Developmental differences in recollection and familiarity during early childhood: insights from event-related potentials. Child Dev. 2015;86(3):889–902. doi: 10.1111/cdev.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Blankenship S., Mulligan E., Rice K., Redcay E. Developmental differences in relations between episodic memory and hippocampal subregion volume during early childhood. Child Dev. 2015;86(6):1710–1718. doi: 10.1111/cdev.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Geng F., Blankenship S., Redcay E. Hippocampal functional connectivity and episodic memory in early childhood. Dev. Cognit. Neurosci. 2016;19:58–69. doi: 10.1016/j.dcn.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T. Building blocks of recollection. In: Ghetti, Bauer P.J., editors. Origins and Development of Recollection: Perspectives from Psychology and Neuroscience. Oxford University Press; New York, NY: 2012. pp. 42–72. [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals qualitative differences between item memory and binding. Dev. Psychol. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockland K.S., Van Hoesen G.W. Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cereb. Cortex. 1999;9:232–237. doi: 10.1093/cercor/9.3.232. [DOI] [PubMed] [Google Scholar]
- Rollins N.K., Glasier P., Seo Y., Morriss M.C., Chua J., Wang Z.Y. Age-related variations in white matter anisotropy in school-age children. Pediatr. Radiol. 2010;40(12):1918–1930. doi: 10.1007/s00247-010-1744-1. [DOI] [PubMed] [Google Scholar]
- Sadeghi N., Nayak A., Walker L., Irganoglu Okan, Albert M., Pierpaoli P.S., Brain Development Cooperative Group C. Analysis of the contribution of experimental bias, experimental noise, and inter-subject biological variability on the assessment of developmental trajectories in diffusion MRI studies of the brain. Neuroimage. 2014;109:480–492. doi: 10.1016/j.neuroimage.2014.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B., Pandya D.N. Further observations on parieto-temporal connections in the rhesus monkey. Exp. Brain Res. 1984;55(2):301–312. doi: 10.1007/BF00237280. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., Mackay C.E., Lonie J.A., Bastin M.E., Terriere E., O’Carroll R.E., Ebmeier K.P. MRI correlates of episodic memory in Alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Res.: Neuroimaging. 2010;184(1):57–62. doi: 10.1016/j.pscychresns.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Jurica P., Mangels J.A., Gershberg F.B., Knight R. Susceptibility to memory interference effects following frontal lobe damage: findings from tests of paired-associate learning. J. Cognit. Neurosci. 1995;7(2):144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- Shing Y.L., Werkle-Bergner M., Li S., Lindenberger U. Associative and strategic components of episodic memory: a life-span dissociation. J. Exp. Psychol. Gen. 2008;137(3):495–513. doi: 10.1037/0096-3445.137.3.495. [DOI] [PubMed] [Google Scholar]
- Simmonds D.J., Hallquist M.N., Asato M., Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J.S., Peers P.V., Mazuz Y.S., Berryhill M.E., Olson I.R. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb. Cortex. 2010;20(2):479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluzenski J., Newcombe N.S., Kovacs S.L. Binding, relational memory: and recall of naturalistic events: a developmental perspective. J. Exp. Psychol. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tsivilis D., Vann S.D., Denby C., Roberts N., Mayes A.R., Montaldi D., Aggleton J.P. A disproportionate role of the fornix and mammillary bodies in recall versus recognition memory. Nat. Neurosci. 2008;11(7):834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorder controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Shannon B.J., Kahn I., Buckner R.L. Parietal lobe contributions to episodic memory retrieval. Trends Cognit. Neurosci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wellman B.J., Rockland K.S. Divergent cortical connections to entorhinal cortex from area TF in the macaque. J. Comp. Neurol. 1997;389(3):361–376. [PubMed] [Google Scholar]
- Wendelken C., Lee J.K., Pospisil J., Sastre M., Ross J.M., Bunge S.A., Ghetti S. White matter tracts connected to the medial temporal lobe support the development of mnemonic control. Cereb. Cortex. 2015;18(9):2116–2208. doi: 10.1093/cercor/bhu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjornerud A., Due-Tonnessen P., Engvig A., Grydeland H., Tamnes C.L., Ostby Y., Fjell A.M. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Wheeler M.E., Buckner R.L. Functional anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Willford J.A., Richardson G.A., Leech S.L., Day N.L. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin. Exp. Res. 2004;28(3):497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Yim H., Dennis S.J., Sloutsky V.M. The development of episodic memory: items, contexts and relations. Psychol. Sci. 2013;24:2153–2172. doi: 10.1177/0956797613487385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhuang L., Sachdev P.S., Trollor J.N., Reppermund S., Kochan N.A., Brodaty H. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One. 2013;8(3):e58887. doi: 10.1371/journal.pone.0058887. [DOI] [PMC free article] [PubMed] [Google Scholar]