Abstract
Background
Protein kinase C (PKC), interleukin (IL)-13, prostaglandin E2 (PGE2), and prostacyclin 2 (PGI2) can all play crucial roles in pulmonary fibrosis. However, their functions remain unclear in hepatic fibrosis mediated by hepatic stellate cells (HSCs), which has been demonstrated to be related to transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF).
Material/Methods
All the experiments were based on LX-2 Hepatic stellate cells. The expression of TGF-β1 and PDGF were assessed by ELISA, RT-PCR, and Western blotting in human HSCs treated by IL-13, PGE2, and PGI2, respectively. At the same time, bridge assay and CCK8 assay were used to detect the cell proliferation and activity, PKC activity assay was used to test the activity of PKC, and PKC agonist and antagonist were used to verify the results obtained previously.
Results
We found that IL-13, PGE2, and PGI2 significantly enhanced the expression of TGF-β1 and PDGF in human HSCs, which also clearly improved the proliferation and cell activity of HSCs. Moreover, PKC activity was significantly increased following IL-13, PGE2, and PGI2 treatments. We also found that the expression of TGF-β1 and PDGF, as well as the proliferation and cell activity of HSCs, were significantly enhanced by the PKC agonist phorbol 12-myristate 13-acetate (PMA), but suppressed by the PKC antagonist calphostin C.
Conclusions
We found that IL-13, PGE2, and PGI2 stimulated HSCs proliferation and secretion of TGF-β1 and PDGF by activating PKC, which predicted their potential roles in hepatic fibrosis.
MeSH Keywords: 16,16-Dimethylprostaglandin E2; Hepatic Stellate Cells; Receptors, Epoprostenol; Receptors, Interleukin-13; Transforming Growth Factor beta1
Background
Hepatic fibrosis, a wound-healing response following chronic liver injury, is a dynamic process that involves over-deposition of extracellular matrix (ECM) components [1–3]. Hepatic stellate cells (HSCs) are important in hepatic fibrosis because they produce an abnormal ECM [1,3,4], although normal HSCs function in the metabolism and storage of vitamin A. As activation is initiated, HSCs exhibit a proliferative “myofibroblast-like” phenotype and express α-smooth muscle actin (SMA) [4–6], which is absent in other resident liver cells except smooth muscle cells in large vessels [3]. However, the activation process of HSCs is complex, including the paracrine and autocrine involvement of many types of cytokines, such as transforming growth factor-β (TGF-β) [7] and platelet-derived growth factor (PDGF) [1,3,8,9]. TGF-β promotes the synthesis of the ECM by decreasing the expression of degradation enzyme matrix metalloproteinases and increasing expression of a specific inhibitor of matrix metalloproteinases [10]. TGF-β also promotes the synthesis of PDGF and inhibits the proliferation of most immune cells [9,11–13]. Thus, the degree of hepatic fibrosis is associated with the presence of TGF-β [13–16]. PDGF plays a role in hepatic fibrosis by promoting HSC proliferation [9,17]. Previous studies reported that direct disruption of the TGF-β gene in mice induced inflammatory reactions and even tumors [18]. Thus, it is important to identify upstream regulators of TGF-β and PDGF.
Protein kinase C (PKC), a protein kinase that participates in the expression and regulation of multiple cytokines, likely regulates TGF-β1 and PDGF in HSCs with similar effects as in mesangial cells [3,19–21]. Interestingly, other studies revealed that interleukin (IL)-13, prostaglandin E2 (PGE2), and prostacyclin 2 (PGI2) play crucial roles in pulmonary fibrosis [22]. Therefore, these regulators may also be involved in the process of hepatic fibrosis. In the present study, we investigated the effects and fundamental mechanisms of IL-13, PGE2, and PGI2 in the secretion of TGF-β1 and PDGF in HSCs.
Material and Methods
Materials
Phorbol 12-myristate 13-acetate (PMA), calphostin C, IL-13, PGE2, and PGI2 were purchased from Sigma Aldrich (St. Louis, MO, USA). The PKC assay kit was from Promega (Madison, WI, USA). The BrdU assay kit was obtained from Sigma Aldrich. The CCK8 assay kit was obtained from Beyotime Biotechnology (Shanghai, China). TGF-β1 and PDGF ELISA kits were from Boyao Biotechnology (Shanghai, China).
Cell culture
The LX-2 human HSC line was purchased from Millipore (Billerica, MA, USA). HSCs were cultured in RMPI-1640 medium supplemented with 10% fetal bovine serum and maintained under standard conditions (5% CO2, 37°C, and humidified atmosphere).
ELISA
The supernatant of HSCs was collected 12 h after stimulation and assayed in an enzyme-linked immunosorbent assay (ELISA) for TGF-β1 and PDGF proteins. We used the double antibody sandwich method to assess the quantity of TGF-β1 and PDGF using their respective kits according to the manufacturer’s instruction. Equal protein amounts were loaded into all wells for optical density comparison. The experiment was tested with 6 parallel wells.
RT-PCR
RT-PCR analysis was conducted to determine the differences in the expression of TGF-β1 and PDGF. The total RNA was extracted from HSCs in groups treated with PMA, calphostin C, IL-13, PGE2, PGI2, and drug solvents, and 6 parallel wells were tested in each group. The primers sequences were as follows [23–25]: TGF-β1, 5′-CAC TGA TAC GCC TGA GTG-3′ and 5′-CTC CCG TGG CTT CTA GTG C-3′; PDGF, 5′-AAG AAG TCC AGG TGA GGT TAG AG-3′ and 5′-GGC TGC TTT AGG TGG GTT T-3′; β-actin: 5′-TGT TAC CAA CTG GGA CGA CA-3′ and 5′-CTG GGT CAT CTT TTC ACG GT-3′. cDNA synthesis was performed to analyze gene expression. Reverse transcription was performed in accordance with the following protocol: RNA was incubated with the primer at 42°C for 60 min followed by enzyme inactivation for 10 min at 95°C, after which the samples were immediately placed on ice. PCR was performed as follows: 30 s of denaturation at 94°C, 30 s of annealing at 45°C, and 30 s of extension at 72°C. The results were normalized to the level of actin cDNA as an internal control.
Western blotting
Western blotting was performed as previously described [26]. Briefly, the proteins were concentrated from the culture supernatant of HSCs. Thirty micrograms of proteins were loaded into each well of a 10% SDS polyacrylamide gel and then transferred to a polyvinylidene fluoride membrane. Membranes were incubated with Tris-buffered saline containing Tween 20 and 5% non-fat dried milk for 1 h at room temperature to block non-specific binding sites. Next, proteins were immunoblotted with specific primary antibodies against TGF-β1 or PDGF (1: 200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C followed by incubation with horseradish peroxidase-conjugated secondary antibody to detect the protein-antibody complex. Finally, the membranes were incubated with ECL followed by exposure in a ChemiDoc XRS imaging system. The intensity of protein bands was normalized to that of β-actin. The experiments were done in triplicate.
PKC activity assay
PKC activity was analyzed with a PKC Activity Assay Kit in accordance with the manufacturer’s protocol. PKC was extracted from lysed HSCs. Equal protein amounts were loaded into each well. The experiment was performed with 5 parallel wells.
Cell proliferation and activity
Cell proliferation was evaluated by conducting a bridge assay. Twenty-four hours before drug treatment, cells were seeded in 6-well plates at a density of 5×103 cells per well followed by incubation with Bride-containing medium for another 2 h. Next, we counted bridge-positive (deep red) cells under a microscope. Cell activity was evaluated using the CCK8 assay according to the manufacturer’s instructions. Briefly, cells were seeded into a 96-well plate at a density of 1×104 cells per well followed by the addition of PMA, calphostin C, IL-13, PGE2, or PGI2. The cells were incubated with CCK8 reagents for 2 h at 37°C. Finally, absorbance was measured at a wavelength of 490 nm with an automated plate reader. All the experiments were repeated with 6 parallel wells.
Statistical analysis
Statistical analyses were performed using Prism software (GraphPad, Inc., Chicago, IL, USA). Analyses were conducted using the unpaired t test. Values were expressed as the means ±SD. P<0.05 was considered statistically significant.
Result
IL-13, PGE2, and PGI2 enhanced HSC proliferation and the synthesis of TGF-β1 and PDGF
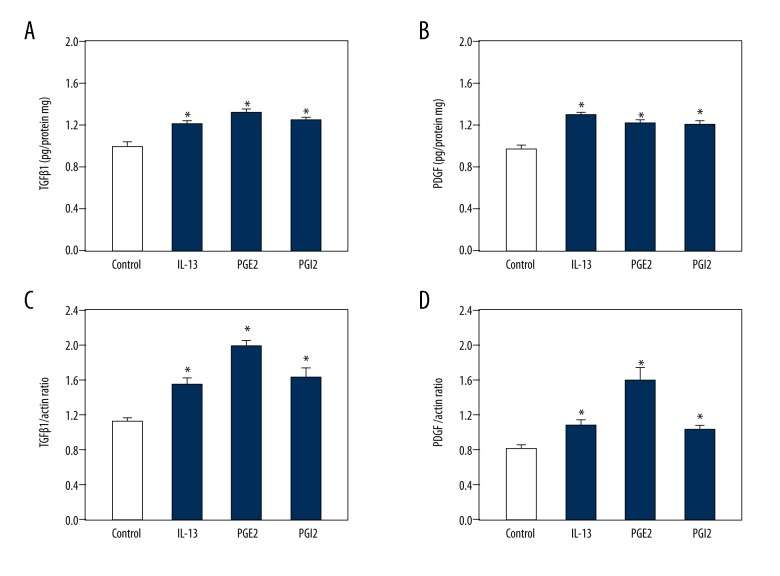
Because IL-13, PGE2, and PGI2 play a role in pulmonary fibrosis, they may also participate in hepatic fibrosis and affect the synthesis and secretion of TGF-β1 and PDGF in HSCs. HSCs were incubated with the 3 drugs for 24 h. ELISA showed that the synthesis of TGF-β1 and PDGF proteins was increased in response to 5 μM IL-13, 2 μM PGE2, and 2 μM PGI2 compared to in controls (Figure 1A, 1B). To confirm this result, RT-PCR was conducted to detect changes in the mRNA levels of TGF-β1 and PDGF. The data showed that both TGF-β1 and PDGF mRNA were increased in response to IL-13, PGE2, and PGI2 compared to in controls in accordance with the ELISA results (Figure 1C, 1D). Therefore, IL-13, PGE2, and PGI2 can up-regulate the synthesis and secretion of TGF-β1 and PDGF in HSCs.
Figure 1.
IL-13, PGE2, and PGI2 induced the expression and secretion of TGF-β1 and PDGF in HSCs. HSCs were treated with the solvent of drugs (Control), 5 μM IL-13, 2 μM PGE2, and 2 μM PGI2. A summary of data from the ELISA showing increased secretion of TGF-β1 (A) and PDGF (B) in response to IL-13, PGE2, and PGI2 compared to the control. Reverse transcription PCR results showing the augmented synthesis of TGF-β1 (C) and PDGF (D) mRNA in response to all IL-13, PGE2, and PGI2 compared to the control. Data are shown as the means ±SD. All differences were considered significant when * P<0.05 (n=6) compared with the solvent of drugs (Control).
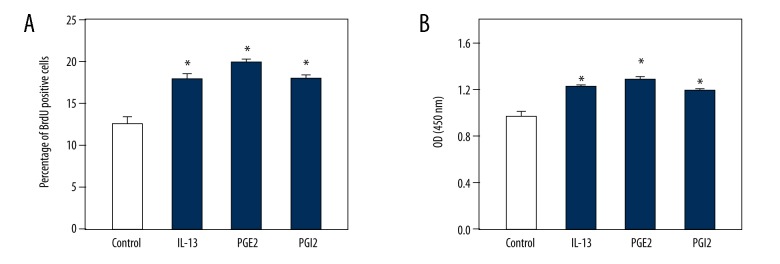
Because changes in the expression and secretion of TGF-β1 and PDGF may influence the function of HSCs, particularly their proliferation [27], we next conducted BrdU and CCK8 assays to evaluate the proliferation and cell activity changes of HSCs, respectively. Counting of BrdU-positive cells revealed that the proliferation of HSCs was significantly increased following treatment with IL-13, PGE2, and PGI2 compared to control cells (Figure 2A). Furthermore, cell activity was markedly enhanced following treatment with IL-13, PGE2, and PGI2 (Figure 2B). Thus, our data indicate that IL-13, PGE2, and PGI2 enhance the proliferation and cell activity of HSCs.
Figure 2.
IL-13, PGE2, and PGI2 enhanced the proliferation and cell activity of HSCs. HSCs were treated with the solvent of drugs (Control), 5 μM IL-13, 2 μM PGE2, and 2 μM PGI2. BrdU assay results showing the enhanced proliferation of HSCs in response to IL-13, PGE2, and PGI2 compared to the control (A). CCK8 assay results showing the increased optical density (OD) of cell activity in response to IL-13, PGE2, and PGI2 compared to the control (B). Data are shown as the means ±SD. All differences were considered significant when * P<0.05 (n=6) compared with the solvent of drugs (Control).
IL-13, PGE2, and PGI2 enhanced PKC activity in HSCs
PKC has been found to play a role in increasing the expression of TGF-β in mesangial cells [20]. Thus, we hypothesized that it may also be involved in inducing TGF-β1 and PDGF secretion by IL-13, PGE2, or PGI2. We used IL-13, PGE2, and PGI2 to stimulate HSCs directly. HSCs were incubated with IL-13, PGE2, and PGI2 for 24 h before functional analysis. The PKC activity assay showed that PKC activity was significantly enhanced in response to IL-13, PGE2, and PGI2 (Figure 3).
Figure 3.

IL-13, PGE2, and PGI2 increased the activity of PKC in HSCs. HSCs were treated with the solvent of drugs (control), 5 μM IL-13, 2 μM PGE2, and 2 μM PGI2. PKC activity assay results showing the enhanced optical density (OD) of PKC activity in response to IL-13, PGE2, and PGI2 compared to the control. Data are shown as the means ±SD. All differences were considered significant when * P<0.05 (n=5) compared with the solvent of drugs (Control).
PKC affects secretion and synthesis of TGF-β1 and PDGF in HSCs
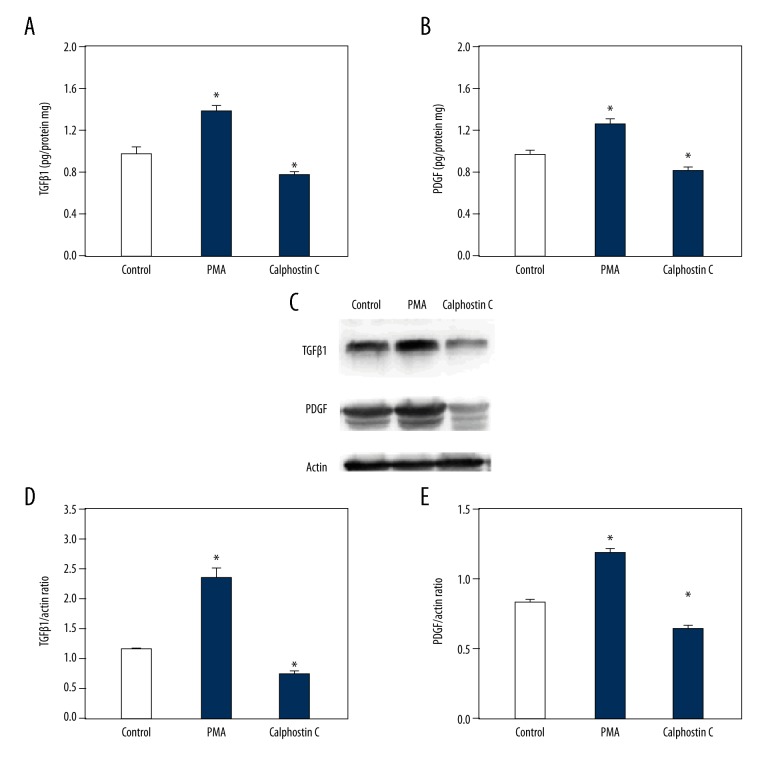
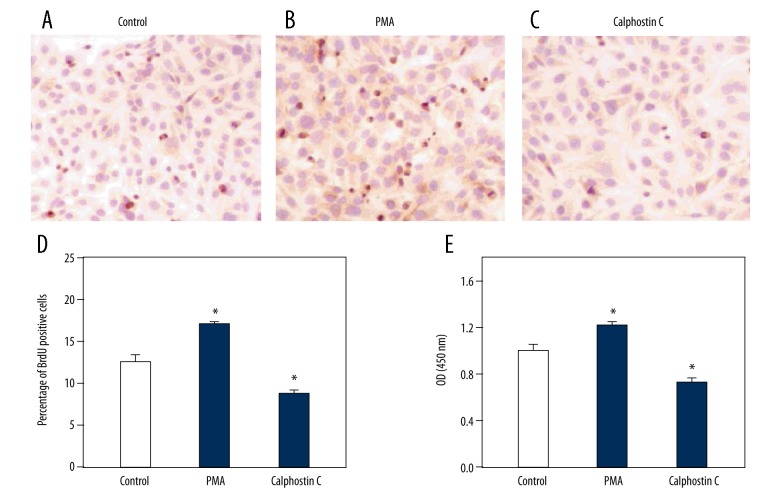
The above data indicate that PKC was increased in HSCs along with TGF-β1 and PDGF in response to IL-13, PGE2, and PGI2. To investigate the potential effects of PKC on the synthesis of TGF-β1 and PDGF in HSCs, we treated the cells with 3 μM PMA (a PKC agonist) and 1 μM calphostin C (a PKC antagonist). HSCs were incubated with PMA and calphostin C for 24 h before functional analysis. ELISA showed that the secretion of TGF-β1 and PDGF proteins were increased in the PMA group and decreased in the calphostin C group compared to in the control group (Figure 4A, 4B). Similar results were obtained by Western blotting (Figure 4C). In addition, we tested TGF-β1 and PDGF mRNA expression. The results showed that both TGF-β1 and PDGF mRNA were increased in response to PMA, but decreased in response to calphostin C (Figure 4D, 4E). Therefore, PKC up-regulated the synthesis and secretion of TGF-β1 and PDGF in HSCs. Furthermore, we conducted BrdU and CCK8 assays. The representative images shown indicate that the proliferation and cell activity of HSCs were both significantly enhanced by PMA, but suppressed by calphostin C (Figure 5).
Figure 4.
Effect of PKC on expression and secretion of TGF-β1 and PDGF in HSCs. HSCs were treated with the solvent of drugs (Control), 3 μM phorbol 12-myristate 13-acetate (PMA, PKC agonist), and 1 μM calphostin C (PKC antagonist). ELISA results showing the secretion changes of TGF-β1 (A) and PDGF (B) in response to the solvent control (Control), PMA, and calphostin C. Representative blots showing TGF-β1 and PDGF expression levels in response to the solvent control (Control), PMA, and calphostin C (C). Reverse transcription PCR results showing mRNA changes in TGF-β1 (D) and PDGF (E) in response to the solvent control (Control), PMA, and calphostin C. Data are shown as the means ±SD. All differences were considered significant when * P<0.05 (n=7) compared with the solvent of drugs (Control).
Figure 5.
Effect of PKC on proliferation and cell activity of HSCs. HSCs were treated with the solvent of drugs (Control), 3 μM phorbol 12-myristate 13-acetate (PMA, PKC agonist), and 1 μM calphostin C (PKC antagonist). BrdU assay representative images showing the number of BrdU-positive HSCs in response to the solvent control (Control, A), PMA (B), and calphostin C (C). Proliferation of HSCs in response to the solvent control (Control), PMA, and calphostin C (D). CCK8 assay results showing the optical density (OD) in response to the solvent control (Control), PMA, and calphostin C (E). Data are shown as the means ±SD. All differences were considered significant when * P<0.05 (n=6) compared with the solvent of drugs (Control).
Discussion
In the present study, we investigated potent upstream regulators of TGF-β1 and PDGF in HSCs, focusing on IL-13, PGE2, and PGI2. We demonstrated that IL-13, PGE2, and PGI2 regulate TGF-β1 and PDGF via the PKC pathway (Figure 6). The major findings of this study are as follows: (1) ELISA and RT-PCR demonstrated that IL-13, PGE2, and PGI2 enhanced the expression of TGF-β1 and PDGF. (2) BrdU and CCK8 assays revealed that IL-13, PGE2, and PGI2 dramatically enhanced the proliferation and cell activity of HSCs. (3) The PKC activity assay showed that the activity of PKC was increased by IL-13, PGE2, and PGI2. (4) ELISA, Western blotting, and RT-PCR demonstrated that PKC enhanced the expression of TGF-β1 and PDGF at both the protein and mRNA levels. (5) BrdU and CCK8 assays revealed that PKC significantly enhanced the proliferation and cell activity of HSCs. Overall, these results suggest that IL-13, PGE2, and PGI2 significantly enhance the synthesis and secretion of TGF-β1 and PDGF in HSCs via PKC activation. All of these proteins notably increased the proliferation and cell activity of HSCs.
Figure 6.

Graphical abstract of this study, showing HSCs effected by IL-13, PGE2, and PGI2 through PKC pathway.
Liver fibrosis is a dynamic wound-healing response that occurs following liver injury, hepatotropic virus infection (mainly hepatitis virus B and virus C), and long-term alcohol consumption [28]. The key source of liver fibrosis is HSCs [3,9], which can synthesize TGF-β1 and PDGF in a paracrine and autocrine manner. Previous studies showed that both TGF-β1 and PDGF exert their functions on HSCs and accelerate the fibrosis process, even when initial hepatic injury factors are removed [1,9]. Recently, liver fibrosis was suggested to be reversible before its final stage [3]. If the synthesis of TGF-β1 and PDGF by HSCs can be inhibited, liver fibrosis can be relieved or reversed [3,9]. However, the upstream treatment targets remain unclear. Direct disruption of the TGF-β gene induces serious inflammatory reactions and tumors [18]. PKC is thought to participate in regulating TGF-β in glomerulus mesangial cells [20]. IL-13, PGE2, and PGI2 are involved in pulmonary fibrosis, which originates from similar regions during embryonic development. The present study evaluated the functions of IL-13, PGE2, and PGI2 in HSC activation.
In vitro, to elucidate whether IL-13, PGE2, and PGI2 are involved in the synthesis of TGF-β1 and PDGF, we treated HSCs with these drugs and then evaluated the expression of TGF-β1 and PDGF. Our results showed that IL-13, PGE2, and PGI2 significantly increased the secretion of TGF-β1 and PDGF compared to the control. These results were further examined by ELISA, Western blotting, and RT-PCR. Hence, IL-13, PGE2, and PGI2 regulate the synthesis and secretion of TGF-β1 and PDGF in HSCs. We also found that the proliferation and cell activity of HSCs was greatly enhanced by IL-13, PGE2, and PGI2 according to BrdU and CCK8 assay results. We also found that the activity of PKC was enhanced in response to IL-13, PGE2, and PGI2. To confirm PKC function in the IL-13, PGE2, and PGI2 pathways and determine whether PKC is involved in the synthesis of TGF-β1 and PDGF, we treated HSCs with PMA (a PKC agonist) and calphostin C (a PKC antagonist) to assess the expression of TGF-β1 and PDGF. PKC is a protein kinase involved in multiple cellular activities. Our results showed that PMA significantly increased the secretion of TGF-β1 and PDGF compared to in control cells, whereas calphostin C inhibited TGF-β1 and PDGF secretion according to ELISA, Western blotting, and RT-PCR. These results were supported by the observations that proliferation and cell activity of HSCs were greatly enhanced by PMA but suppressed by calphostin C according to the BrdU and CCK8 assays, which agree with the results of a previous study [3]. Hence, PKC, which was increased by treatment with IL-13, PGE2, and PGI2, up-regulates the synthesis and secretion of TGF-β1 and PDGF in HSCs. Therefore, IL-13, PGE2, and PGI2 stimulate the synthesis and secretion of TGF-β1 and PDGF via the PKC pathway.
However, the present study did not assess the direct relationship between these cytokines (IL-13, PGE2, and PGI2) and some significant markers of hepatic fibrosis in vivo, such as over-deposition of the ECM, α-SMA, and procollagen. The latter 2 markers indicate HSC activation and the occurrence and degree of hepatic fibrosis [2,3,13]. H3 histone is a significant and clear indicator of cell proliferation [13,29]. If IL-13, PGE2, and PGI2 are considered as potential treatment targets, their siRNAs must be developed to down-regulate the cytokines and elucidate how hepatic fibrosis is inhibited. IL-13, PGE2, and PGI2 gene knockout mice will be valuable for investigating their effects on the susceptibility to hepatic fibrosis in normal mice. A recent study suggested that hepatocytes, rather than bystander cells, are involved in hepatic fibrosis and exerts effects on the ECM, matrix metalloproteinases, and proliferation of HSCs directly by secreting various cytokines and hormones, such as TGF-β, connective tissue growth factor, PDGF, and angiotensin II [2]. Therefore, we hypothesized that hepatocytes are regulated by IL-13, PGE2, and PGI2. Other studies suggested that the classical intracellular effectors of TGF-β, Smad proteins, particularly Smad 1, 2, and 3, are involved in hepatic fibrosis [30–32]. Smad7, an antagonist related to Smad 2 and 3, is a potent target for the inhibition or reversion of hepatic fibrosis [32–34]. Hence, the relationship between these cytokines (IL-13, PGE2, and PGI2) and Smad proteins should be evaluated to determine the signaling pathway involving TGF-β1.
Conclusions
In conclusion, we found that IL-13, PGE2, and PGI2 stimulated the activation of HSCs by increasing the synthesis and secretion of TGF-β1 and PDGF in HSCs. In addition, PKC stimulated the secretion of TGF-β1 and PDGF in HSCs. These results indicate that IL-13, PGE2, and PGI2 are therapeutic targets for hepatic fibrosis via the PKC pathway.
Abbreviations
- α-SMA
α-smooth muscle actin
- BrdU
bromodeoxyuridine
- CCK8
cell counting kit-8
- ECL
electrochemiluminescence
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- HSC
hepatic stellate cell
- IL-13
interleukin-13
- PDGF
platelet-derived growth factor
- PGE2
phenyl glycidyl ether 2
- PGI2
prostacyclin 2
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- RT-PCR
reverse transcription-polymerase chain reaction
- Smad
Sma- and Mad-related protein
- TGF-β
transforming growth factor-β
Footnotes
Source of support: This work was supported by the Natural Science Foundation of Shandong Province (Y2005C45)
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Friedman SL. Liver fibrosis – from bench to bedside. J Hhepatol. 2003;38:38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Tu T, Calabro SR, Lee A, et al. Hepatocytes in liver injury: Victim, bystander, or accomplice in progressive fibrosis? J Gastroenterol Hepatol. 2015;30(12):1696–704. doi: 10.1111/jgh.13065. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinzani M, Gentilini P. Biology of hepatic stellate cells and their possible relevance in the pathogenesis of portal hypertension in cirrhosis. Semin Liver Dis. 1999;19(4):397–410. doi: 10.1055/s-2007-1007128. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt-Gräff A, Krüger S, Bochard F, et al. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991;138:1233–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL, Rockey DC, McGuire RF, et al. Isolated hepatic lipocytes and Kupffer cells from normal human liver: Morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–43. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 7.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 8.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–93. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hautekeete ML, Geerts A. The hepatic stellate (Ito) cell: Its role in human liver disease. Virchows Arch. 1997;430:195–207. doi: 10.1007/BF01324802. [DOI] [PubMed] [Google Scholar]
- 10.Knittel T, Mehde M, Kobold D, et al. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: Regulation by TNF-α and TGF-β1. J Hepatol. 1999;30:48–60. doi: 10.1016/s0168-8278(99)80007-5. [DOI] [PubMed] [Google Scholar]
- 11.Friedman SL, Yamasaki GY, Wong L. Modulation of transforming growth factor p receptors of rat lipocytes during the hepatic wound-healing response. J Biol Chem. 1994;269(14):10551–58. [PubMed] [Google Scholar]
- 12.Pinzani M, Gentilini A, Caligiuri A, et al. Transforming growth factor-β1 regulates platelet-derived growth factor receptor β subunit in human liver fat-storing cells. Hepatology (Baltimore, Md) 1995;21:232–39. [PubMed] [Google Scholar]
- 13.Castilla A, Prieto J, Fausto N. Transforming growth factors β1 and α in chronic liver disease effects of interferon alfa therapy. N Engl J Med. 1991;324:933–40. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- 14.Nagy P, Schaff Z, Lapis K. Immunohistochemical detection of transforming growth factor-beta 1 in fibrotic liver diseases. Hepatology. 1991;14:269–73. [PubMed] [Google Scholar]
- 15.Gressner AM. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22:28–36. [PubMed] [Google Scholar]
- 16.Annoni G, Weiner FR, Zern MA. Increased transforming growth factor-beta 1 gene expression in human liver disease. J Hepatol. 1992;14:259–64. doi: 10.1016/0168-8278(92)90168-o. [DOI] [PubMed] [Google Scholar]
- 17.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S79–84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 18.Shull MM, Ormsby I, Kier AB, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–99. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Studer RK, DeRubertis FR, Craven PA. Nitric oxide suppresses increases in mesangial cell protein kinase C, transforming growth factor-beta, and fibronectin synthesis induced by thromboxane. J Am Soc Nephrol. 1996;7:999–1005. doi: 10.1681/ASN.V77999. [DOI] [PubMed] [Google Scholar]
- 20.Studer RK, Craven PA, DeRubertis FR. Antioxidant inhibition of protein kinase C – signaled increases in transforming growth factor-beta in mesangial cells. Metabolism. 1997;46:918–25. doi: 10.1016/s0026-0495(97)90080-9. [DOI] [PubMed] [Google Scholar]
- 21.Di Sario A, Bendia E, Baroni GS, et al. Intracellular pathways mediating Na+/H+ exchange activation by platelet-derived growth factor in rat hepatic stellate cells. Gastroenterology. 1999;116:1155–66. doi: 10.1016/s0016-5085(99)70019-3. [DOI] [PubMed] [Google Scholar]
- 22.Laurent GJ, McAnulty RJ, Hill M, Chambers R. Escape from the matrix: Multiple mechanisms for fibroblast activation in pulmonary fibrosis. Proc Am Thorac Soc. 2008;5:311–15. doi: 10.1513/pats.200710-159DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu X, Xiao J, Wei Y, et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 2015;25(6):655–73. doi: 10.1038/cr.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Liu Y, Liu Z, et al. Transcriptome profiling of a multiple recurrent muscle-invasive urothelial carcinoma of the bladder by deep sequencing. PLoS One. 2014;9(3):e91466. doi: 10.1371/journal.pone.0091466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Lu R, Xia Y, Sun J. Global analysis of the eukaryotic pathways and networks regulated by Salmonella typhimurium in mouse intestinal infection in vivo. BMC Genomics. 2010;11:722. doi: 10.1186/1471-2164-11-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao R, Zhou M, Li J, et al. Increased TRPP2 expression in vascular smooth muscle cells from high-salt intake hypertensive rats: The crucial role in vascular dysfunction. Mol Nutr Food Res. 2015;59:365–72. doi: 10.1002/mnfr.201400465. [DOI] [PubMed] [Google Scholar]
- 27.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 28.Povero D1, Busletta C, Novo E, et al. Liver fibrosis: A dynamic and potentially reversible process. Histol Histopathol. 2010;25(8):1075–91. doi: 10.14670/HH-25.1075. [DOI] [PubMed] [Google Scholar]
- 29.Rickles R, Marashi F, Sierra F, et al. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci USA. 1982;79(3):749–53. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitkopf K, Godoy P, Ciuclan L, et al. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44(1):57–66. doi: 10.1055/s-2005-858989. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki Y, Okazaki I. Emerging insights into transforming growth factor β Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–92. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heldin C-H, Miyazono K, Ten Dijke P. TGF-β signalling from cell membrane to nucleus through Smad proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 33.Dooley S, Hamzavi J, Breitkopf K, et al. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–91. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- 34.Kopp J, Preis E, Said H, et al. Abrogation of transforming growth factor-β signaling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem. 2005;280:21570–76. doi: 10.1074/jbc.M502071200. [DOI] [PubMed] [Google Scholar]