Abstract
Background
Baicalin is a flavonoid derived from Scutellaria baicalensis, used in Chinese herbal medicine. Activation of the sirtuin 1 gene (SIRT1) and adenosine monophosphate (AMP)-activated protein kinase gene (AMPK), the SIRT1/AMPK signaling pathway, is associated with human malignant tumors. The aim of this study was to investigate the effects of baicalin on the cell viability, apoptosis, proliferation, and migration of human non-small cell lung cancer (NSCLC) cells, A549 and H1299, in vitro.
Material/Methods
Human NSCLC cells, A549 and H1299, were treated with serial doses of baicalin. Small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes was performed using cell transfection. The MTT assay was used to determine cell viability, flow cytometry was used to measure cell apoptosis, wound healing and transwell assays were used to assess cell migration of A549 and H1299 cells. Western blotting was used to measure protein expression and phosphorylation levels in untreated A549 and H1299 cells, and cells treated with increasing doses of baicalin.
Results
Baicalin inhibited the viability, migration, and invasion of A549 and H1299 cells, and increased cell apoptosis in a dose-dependent manner. Baicalin activated the SIRT1/AMPK and mechanistic target of rapamycin (mTOR), and SIRT1/AMPK and matrix metalloproteinase (MMP) signaling in A549 and H1299 cells in a dose-dependent manner. siRNA silencing of SIRT1 and AMPK reduced the effects of baicalin on cell proliferation and migration.
Conclusions
Baicalin, a flavonoid used in Chinese herbal medicine, inhibited the proliferation and migration of human NSCLC cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway.
MeSH Keywords: Apoptosis; Lung Neoplasms; Medicine, Chinese Traditional; Neoplasm Invasiveness
Background
Worldwide, non-small cell lung cancer (NSCLC) is one of the most common malignant tumors of the respiratory system and is currently recognized as one of the leading causes of cancer-related death worldwide [1]. Surgical resection can be a curative treatment for early-stage NSCLC, but most patients are diagnosed at an advanced stage when patients present with clinical symptoms. The prognosis for patients with advanced-stage NSCLC, which has invaded the lung locally, invaded beyond the lung, invaded blood and lymphatic vessels, and metastasized, is poor [2]. The molecular mechanisms that underly the development and progression of NSCLC are complicated and remain poorly understood. Therefore, further understanding of the mechanisms underlining this malignancy, from continuing studies, would be helpful in identifying the potential molecular therapeutic targets for NSCLC.
Sirtuins are a protein family responsible for various cellular biological events including cell proliferation, apoptosis, and cell migration [3]. Sirtuin 1, is also known as NAD-dependent deacetylase sirtuin-1, and is encoded by the SIRT1 gene, and is the most studied protein of sirtuin family. The down-regulation of the SIRT1 gene has been described in previous studies, indicating SIRT1 as a tumor suppressor gene [4]. The adenosine monophosphate (AMP)-activated protein kinase gene (AMPK) was recognized as a downstream effector of SIRT1, playing a role as a cellular metabolic stress sensor [5]. It has also been suggested that the AMPK gene can act as a tumor suppressor [6]. Previous studies have shown that cancer cell proliferation could be inhibited via activation of the AMPK gene, whereas inactivation of AMPK was associated with tumor progression [7,8].
Recently, components of natural Chinese herbal medicines have attracted increasing numbers of research studies, as novel anti-cancer agents were extracted from medicinal herbs. Baicalin (5,6-dihydroxy-7-O-glucuronide flavone) is a flavonoid derived from Scutellaria baicalensis Georgi (or Chinese skullcap), and has been used and studied in Chinese herbal medicine for the treatment of several types of cancer [9,10]. However, there have been few previous studies on the effects of baicalin in NSCLC.
However, baicalin and its metabolites have been shown to upregulate the activation of the SIRT1 and AMPK genes [11,12]. For this reason, the aim of this study was to investigate the effects of baicalin on the cell viability, apoptosis, proliferation, and migration of human NSCLC cells, A549 and H1299, in vitro.
Material and Methods
Cell culture
The human non-small cell lung cancer (NSCLC) cell lines, A549 and H1299 used in this study were purchased from the Cell Bank of Typical Preservation Committee, Chinese Academy of Science, Shanghai, China. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Invitrogen) and a cell culture antibiotic mixture (Sigma-Aldrich) in a humidified cell incubator providing an atmosphere providing 5% CO2 and 95% air at 37°C.
The cultured A549 and H1299 cells were treated with baicalin (Sigma-Aldrich) at increasing doses, from 0, 20, 40, and 80 μmol/l, respectively for 24 hours. These doses of baicalin were chosen according to the findings from previous preliminary experiments.
Small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes
The A549 and H1299 cells were transfected with small interfering RNA (siRNA), silencing the expression of the SIRT1 and AMPK genes. Commercially available siRNA kits used included SignalSilence SIRT1 siRNA 1 kit (Catalog No. 12241) (Cell Signaling Technology) and the SignalSilence AMPKα2 siRNA II kit (Catalog No. 6620) (Cell Signaling Technology) were used to knockdown the expression of the SIRT1 and the AMPK gene expression, respectively. Cultured A549 and H1299 cells were transfected with the siRNAs with the TransIT-TKO reagent (Mirus Bio LLC) in accordance with the protocols provided by the manufacturer.
MTT cell viability assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl terazolium bromide (MTT) assay was used to assess the cell viability of cultured human NSCLC cells. Briefly, cultured A549 and H1299 cells were seeded into 96-well culture plates at a cell density of 5×103 cells per well. The cells were treated with baicalin and/or siRNAs. Then, 20 μl of MTT solution (5 mg/ml) (Sigma–Aldrich) was added to each well and the cells were incubated for 4 hours at 37°C, followed by the addition of 100 μl of dimethylsulfoxide (DMSO) to dissolve the resultant formazan crystals. A plate reader was used to detect the optical density (OD) absorbance at 490 nm. The cell viability was calculated as: OD of treatment/OD of control ×100%.
Flow cytometry to measure cell apoptosis
The apoptosis of the cultured NSCLC cells, A549 and H1299, was determined by flow cytometry in this study. Briefly, treated A549 and H1299 cells were harvested by centrifugation and then washed with PBS. After resuspension, cells were incubated with 100 μl of binding buffer containing 5 μl Annexin V- fluorescein isothiocyanate (FITC) and 1 μl of propidium iodide (PI) for 30 minutes in a humidified cell incubator. Cell apoptosis was then analyzed with a BD FACSCalibur flow cytometer (BD Biosciences).
Cell migration and invasion evaluated by a wound healing assay
The migration ability of cultured human NSCLC cells, A549 and H1299, was evaluated by a wound healing assay. Briefly, A549 and H1299 cells were seeded and cultured into 60 mm culture dishes. A 2 mm razor blade was used to form the wound, and the edges were marked. The cells were treated with baicalin and/or siRNAs. Acetone was used to fix the cells, which were then stained with 4′,6-diamidino-2-phenylindole (DAPI), a blue cell nuclear fluorescence stain. Cells were observed with an inverted fluorescence microscope. The invasion capacity of human A549 and H1299 cells was evaluated by a transwell assay using Matrigel-coated transwells (BD Biosciences). The assay was carried according to the protocol provided by the manufacturer. The number of cells that invaded through the wells to the opposite side of the membranes were counted.
Western blotting
Cells were lysed by a radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime) supplemented with phenylmethylsulfonyl fluoride, (PMSF) (Santa Cruz). The protein was extracted using a Total Protein Extraction kit (Beyotime) according to the manufacturer’s instruction. The doses of protein were examined with the bicinchoninic acid (BCA) protein assay kit (Beyotime). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate the proteins, which was then transferred to polyvinylidene difluoride (PVDF) membranes.
Primary antibodies used included antibodies to the proteins, SIRT1 (1: 4000)(Abcam)), AMPK (1: 2000) (Cell Signaling Tech), phosphorylated (p-AMPK) (1: 2000) (Cell Signaling Technology), mechanistic target of rapamycin (mTOR) (1: 4000) (Abcam), phosphorylated mTOR (p-mTOR) (1: 4000) (Abcam), cleaved caspase-3 (c-caspase-3) (1: 2500) (AbCam), active matrix metalloproteinase-2 (MMP-2) (1: 4000) (Abcam), active MMP-9 (1: 2000) (Abcam) and GAPDH (1: 5000) (Abcam) for 8 hours at 4°C. After washing, the membranes were further incubated with horseradish-peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology). Luminol enhancer reagent (Millipore) was used to develop the membranes, which were visualized on X-ray films. The intensity of the Western blots was analyzed by ImageJ software.
Statistical analysis
The data collected in this study was presented as the mean ± standard deviation (SD). Differences between groups were analyzed by one-way analysis of variance (ANOVA) and using the Student’s t-test. Tests of least significant difference (LSD) were performed as post hoc tests. A P-value <0.05 was considered to be statistically significant.
Results
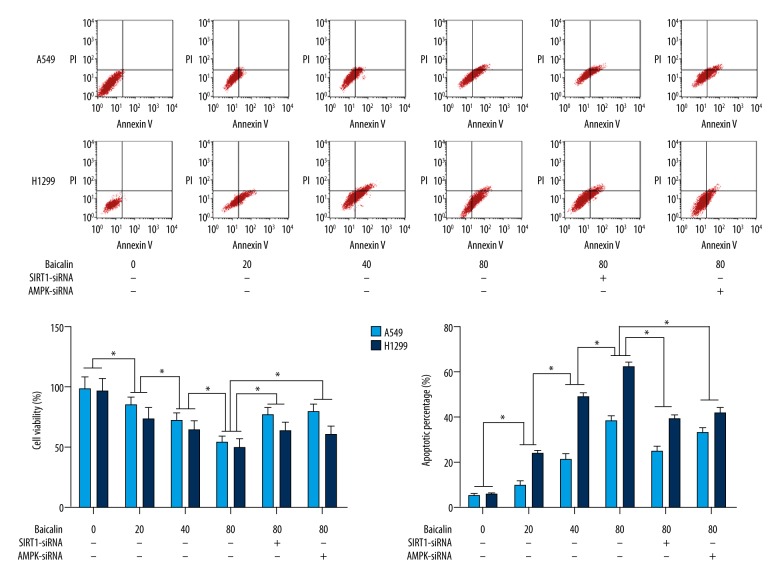
Baicalin treatment of cultured non-small cell lung cancer (NSCLC) cells, A549 and H1299, increased cell viability by inducing apoptosis in a dose-dependent manner
The results of cell viability findings are shown in Figure 1. The cell viability of A549 and H1299 were assessed by the MTT assay and cell apoptosis was detected by flow cytometry. The cell viability of both A549 and H1299 cells were significantly inhibited by baicalin in a dose-dependent manner. The baicalin incubation significantly also elevated the apoptotic rate in both A549 and H1299 cells in a dose-dependent manner.
Figure 1.
Effects of baicalin treatment and small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes on the cell viability and rates of apoptosis of the non-small cell lung cancer (NSCLC) cells, A549 and H1299. The columns on the left of this figure indicate the cell viabilities of cultured A549 cells (white columns) and H1299 cells (black columns) treated with baicalin and/or small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes, respectively. The columns on the right of this figure include the upper panel that shows the plotted charts of the flow cytometry detection of cell apoptosis; the lower panel show the apoptotic cell rates of cultured A549 cells (white columns) and H1299 cells (black columns) treated with baicalin and/or siRNAs silencing of the SIRT1 and AMPK genes, respectively. * Indicates differences that were statistically significant.
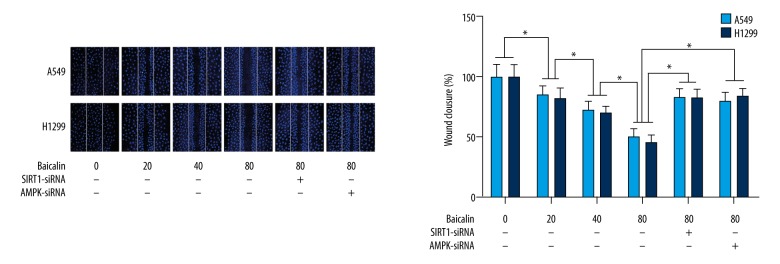
Baicalin treatment of cultured NSCLC cells, A549 and H1299, decreased cell migration and invasion in a dose-dependent manner
The capacity of migration and invasion were elevated by wound healing assay and transwell assay respectively, which were shown in Figures 2 and 3. Following cell incubation with baicalin at various doses, the migration and invasion abilities were significantly inhibited dramatically in a dose-dependent manner in both A549 and H1299 cells.
Figure 2.
Effects of baicalin treatment on the wound healing assay results of the non-small cell lung cancer (NSCLC) cells, A549 and H1299. The upper panel of the figure shows the fluorescence images of cultured A549 cells and H1299 cells during the wound healing assay. The nuclei of the cells are positively-stained blue with 4′,6-diamidino-2-phenylindole (DAPI). The columns on the lower panel show the percentage of wound closure involving cultured A549 cells (white columns) and H1299 cells (black columns) treated with baicalin and/or small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes. * Indicates differences that were statistically significant.
Figure 3.
Effects of baicalin treatment on cell migration in the transwell assay on the non-small cell lung cancer (NSCLC) cells, A549 and H1299. The upper panel of this figure shows the images of the transwell assay of the cultured cells. Columns on the lower panel indicate the average cell number count per field (total=5 fields) and cells that have penetrated through the membranes of the transwells of cultured A549 cells (white columns) and H1299 cells (black columns) treated with baicalin and/or small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes. * Indicates differences that were statistically significant.
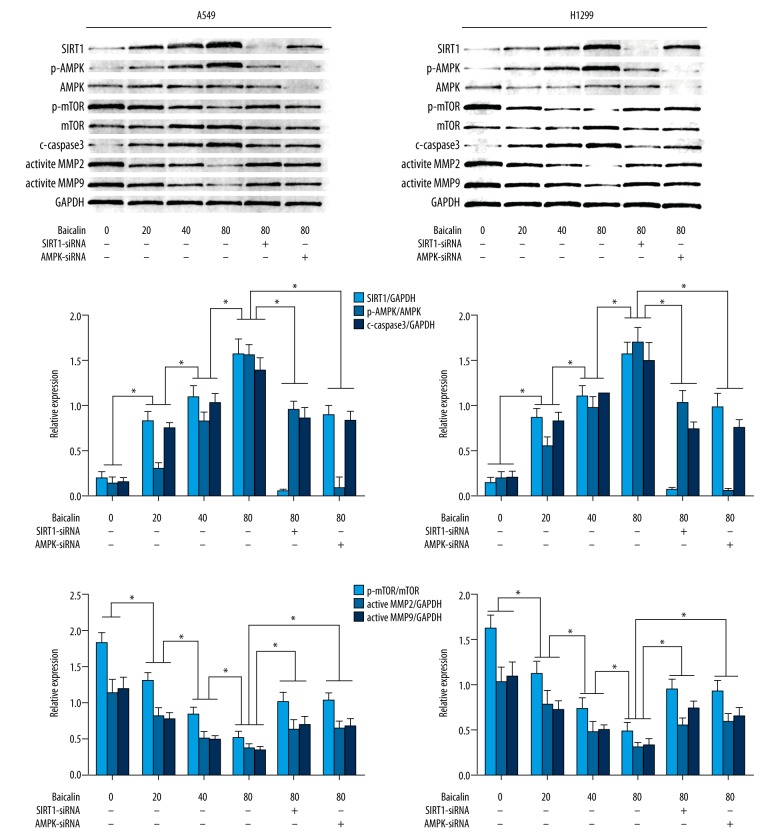
Baicalin treatment of cultured NSCLC cells, A549 and H1299, regulated the activation of the SIRT1/AMPK signaling pathway in a dose-dependent manner
The immunoblots indicating the activation of SIRT1/AMPK signaling in A549 and H1299 cells are shown in Figure 4. The baicalin incubation dramatically increased the expression levels of SIRT1 and cleaved caspase-3, as well as the phosphorylation levels of AMPK in NSCLC cells in a dose-dependent manner. Also, the baicalin incubation also suppressed the expression levels of active matrix metalloproteinase (MMP)-2 and MMP-9, as well as the phosphorylation levels of m-TOR in NSCLC cells in a dose-dependent manner.
Figure 4.
Effects of baicalin treatment and the Western blotting results on the non-small cell lung cancer (NSCLC) cells, A549 and H1299. The upper part of this figure demonstrates the immunoblots for SIRT1, p-AMPK, AMPK, p-mTOR, mTOR, cleaved caspase-3 (c-caspase-3) encoded by the CASP3 gene, active matrix metalloproteinase (MMP)-2, active MMP-9 and GAPDH in cultured human NSCLC cells, A549 and H1299. The central part (columns) indicates the ratios of SIRT1/GAPDH (white columns), p-AMPK/AMPK (grey columns) and c-caspase-3/GAPDH (black columns) in cultured A549 cells and H1299 cells treated with baicalin and/or small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes. The columns on the bottom of this figure indicate the ratios of p-mTOR/mTOR (white columns), active MMP-2/GAPDH (grey columns), active MMP-9/GAPDH (black columns) in cultured A549 cells and H1299 cells treated with baicalin and/or small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes. * Indicates differences that were statistically significant.
Small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes reduced the inhibitory effects of baicalin on cultured NSCLC cells, A549 and H1299
As shown in Figure 1, the specific siRNAs silencing SIRT1 and AMPK reduced the anti-proliferative effect of baicalin on both A549 and H1299 cells. The results shown in Figures 2 and 3 demonstrate that both SIRT1 and AMPK silencing impaired the anti-migration and anti-invasion effects of baicalin on both A549 and H1299 cells.
As shown in Figure 4, small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes effectively down-regulated the expression of SIRT1 and AMPK in the NSCLC cells, A549 and H1299 in vitro. As a result, both SIRT1 and AMPK silencing increased phosphorylation levels of mechanistic target of rapamycin (mTOR) as well as the expression levels of active MMP-2 and MMP-9 in baicalin- treated NSCLC cancer cells, A549 and H1299 in vitro. Silencing of the SIRT1 and AMPK genes also decreased the expression levels of cleaved caspase-3 in A549 and H1299 cells treated with baicalin.
Discussion
Baicalin is a flavonoid extracted from the root of the medicinal herb, Scutellaria baicalensis Georgi, which is also known as the traditional Chinese herb, Huangqin. Baicalin exerts a wide spectrum of biological activities including anti-oxidant, anti-proliferation, anti-bacterial and immune-regulating activities [13–15]. Previously published studies have evaluated the safety, toxicity, and tolerability properties of baicalin in both in vivo and in vitro experiments [16,17]. Previously, baicalin has been shown to have anti-cancer activities in several human cancer cell types including breast cancer, ovarian cancer, prostate cancer, and colonic cancer [18–20].
There have been few reports on the anti-cancer activity of baicalin on non-small cell lung cancer (NSCLC) cells. In the current study, two human NSCLC cell lines were used, A549 cells and H1299 cells. Baicalin treatment of NSCLC in vitro significantly reduced the cell viability and induced cell apoptosis of both A549 and H1299 cells in a dose-dependent manner. Also, baicalin incubation reduced the migration and invasion capacities of cells in a dose-dependent manner. These results suggested that baicalin had inhibitory effects on human NSCLC cells in vitro.
Although previously published studies have proposed molecular mechanisms for targeted therapy in NSCLC, this malignancy is characterized by cell proliferation, invasion, and metastasis capacities, and so a pathway mediating these properties might represent an effective therapeutic target. SIRT1 is a class III histone deacetylase that participates in many critical biological processes including cell death, cell proliferation, and cell migration [4]. As one of the substrates of SIRT1, AMPK is activated by phosphorylation. The significant down-regulated expression of SIRT1 and phosphorylation of AMPK have been previously identified in several human cancers [21,22]. Therefore, SIRT1 and AMPK have been recognized as tumor suppressor genes. From the findings of the present study, it is possible to propose that SIRT1/AMPK signaling might be involved in the anti-cancer effects of baicalin on NSCLC cells in vitro.
In human NSCLC cells, incubated with increasing doses of baicalin, the expression level of SIRT1 as well as the phosphorylation level of AMPK increased significantly. This result indicated that baicalin activated the tumor suppressor pathway SIRT1/AMPK signaling in human NSCLC cells in vitro. The serine/threonine kinase mechanistic target of rapamycin (mTOR) functions as a mediator of cell survival, which is believed to be oncogenic [23]. The activation of AMPK might have a negative regulatory effect on mTOR expression, which is known to be resistant to apoptosis by regulating the activation of the caspase cascade and autophagy [24,25]. The results of this study showed that baicalin activated the SIRT1/AMPK signaling, which further inhibited the phosphorylation of mTOR, resulting in an increase in apoptosis of human NSCLC cells in vitro. The matrix metalloproteinases (MMPs) have been reported to be associated with the invasion and metastasis of malignant cells due to their ability to degrade extracellular matrix and promote cell mobility [26,27]. It has previously been reported that reduced AMPK phosphorylation resulted in MMP expression [28]. In the current study, baicalin treatment was found to reduce the migration and invasion abilities of human NSCLC cells in vitro by reducing the expression of MMP-2 and MMP-9.
To further validate these preliminary findings, small interfering RNA (siRNA) silencing of the SIRT1 and AMPK genes were undertaken. The siRNA silencing of SIRT1 and AMPK significantly reduced the anti-proliferative and anti-migration effects of baicalin on human NSCLC cells in vitro. The results of this study support the possibility that baicalin might inhibit tumor cell viability, invasion, and metastasis of human NSCLC cells by activating the SIRT1/AMPK signaling pathway.
Conclusions
The findings of this study showed that baicalin, a flavonoid used in Chinese herbal medicine, inhibited the proliferation and migration of human non-small cell lung cancer (NSCLC) cells, A549 and H1299, by activating the SIRT1/AMPK signaling pathway. These preliminary in vitro findings warrant further studies that add to the understanding of the molecular mechanisms of baicalin on tumor cell activity. The findings of this study also indicate that the SIRT1/AMPK signaling pathway might be a potential pathway for further studies as a potential diagnostic or therapeutic target in NSCLC.
Footnotes
Source of support: Departmental sources
References
- 1.Song F, Wang H, Wang Y. Myeloid ecotropic viral integration site 1 inhibits cell proliferation, invasion or migration in human gastric cancer. Oncotarget. 2017;8:90050–60. doi: 10.18632/oncotarget.21376. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Qian L, Ji AH, Zhang WJ, Zhao N. HuR, TTP, and miR-133b expression in NSCLC and their association with prognosis. Eur Rev Med Pharmacol Sci. 2018;22(2):430–42. doi: 10.26355/eurrev_201801_14192. [DOI] [PubMed] [Google Scholar]
- 3.Jin MS, Hyun CL, Park IA, et al. SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer. Tumor Biol. 2016;37:4743–53. doi: 10.1007/s13277-015-4231-3. [DOI] [PubMed] [Google Scholar]
- 4.Kang YY, Sun FL, Zhang Y, Wang Z. SIRT1 acts as a potential tumor suppressor in oral squamous cell carcinoma. J Chinese Med Ass. 2017;78:1–7. doi: 10.1016/j.jcma.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Salomone F, Barbagallo I, Godos J, et al. Silibinin restores NAD(+) levels and induces the SIRT1/AMPK pathway in non-alcoholic fatty liver. Nutrients. 2017;9:10. doi: 10.3390/nu9101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Fang Q, Ji S, Han Z, et al. Resveratrol-mediated apoptosis in renal cell carcinoma via the p53/AMPactivated protein kinase/mammalian target of rapamycin autophagy signaling pathway. Mol Med Rep. 2018;17:502–8. doi: 10.3892/mmr.2017.7868. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh FS, Hung MH, Wang CY, et al. Inhibition of protein phosphatase 5 suppresses non-small cell lung cancer through AMP-activated kinase activation. Lung Cancer. 2017;112:81–89. doi: 10.1016/j.lungcan.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Zhu MJ. AMP-activated protein kinase: A therapeutic target in intestinal diseases. Open Biol. 2017;7(8) doi: 10.1098/rsob.170104. pii: 170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou T, Zhang A, Kuang G, et al. Baicalin inhibits the metastasis of highly aggressive breast cancer cells by reversing epithelial-to-mesenchymal transition by targeting beta-catenin signaling. Oncol Rep. 2017;38:3599–607. doi: 10.3892/or.2017.6011. [DOI] [PubMed] [Google Scholar]
- 10.Li K, Wang J, Shi M, et al. Prescription consisting of Vitamin C and Baicalin inhibits tumor growth by enhancing the antioxidant capacity in vivo. J BUON. 2015;20:1368–72. [PubMed] [Google Scholar]
- 11.Wang HZ, Wang HH, Huang SS, et al. Inhibitory effect of baicalin on collagen-induced arthritis in rats through the nuclear factor-kappaB pathway. J Pharmacol Exp Ther. 2014;350:435–43. doi: 10.1124/jpet.114.215145. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Jiang H, Cao S, et al. Baicalin and its metabolites suppresses gluconeogenesis through activation of AMPK or AKT in insulin resistant HepG-2 cells. Eur J Medicinal Chem. 2017;141:92–100. doi: 10.1016/j.ejmech.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Jiang WB, Zhao W, Chen H, et al. Baicalin protects H9c2 cardiomyocytes against hypoxia/reoxygenation-induced apoptosis and oxidative stress through activation of mitochondrial aldehyde dehydrogenase 2. Clin Exp Pharmacol Physiol. 2018;45:303–11. doi: 10.1111/1440-1681.12876. [DOI] [PubMed] [Google Scholar]
- 14.Cheng P, Wang T, Li W, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-kappaB pathway. Front Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen M, Wang L, Yang G, et al. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: Inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One. 2014;9:e88389. doi: 10.1371/journal.pone.0088389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S, Xu L, Li S, et al. Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-kappaB) signaling during Haemophilus parasuis infection. Vet Res. 2016;47:80. doi: 10.1186/s13567-016-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinda B, Dinda S, DasSharma S, et al. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Gao C, Zhou Y, Li H, et al. Antitumor effects of baicalin on ovarian cancer cells through induction of cell apoptosis and inhibition of cell migration in vitro. Mol Med Rep. 2017;16:8729–34. doi: 10.3892/mmr.2017.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miocinovic R, McCabe NP, Keck RW, et al. In vivo and in vitro effect of baicalein on human prostate cancer cells. Int J Oncol. 2005;26:241–46. [PubMed] [Google Scholar]
- 20.Wang XF, Zhou QM, Du J, et al. Baicalin suppresses migration, invasion and metastasis of breast cancer via p38MAPK signaling pathway. Anticancer Agents Med Chem. 2013;13:923–31. doi: 10.2174/18715206113139990143. [DOI] [PubMed] [Google Scholar]
- 21.Huang B, Cheng X, Wang D, et al. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1alpha signaling. Oncotarget. 2014;5:4732–45. doi: 10.18632/oncotarget.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gollavilli PN, Kanugula AK, Koyyada R, et al. AMPK inhibits MTDH expression via GSK3beta and SIRT1 activation: potential role in triple negative breast cancer cell proliferation. FEBS Journal. 2015;282:3971–85. doi: 10.1111/febs.13391. [DOI] [PubMed] [Google Scholar]
- 23.Yan G, Ru Y, Wu K, et al. GOLM1 promotes prostate cancer progression through activating PI3K-AKT-mTOR signaling. Prostate. 2017;78:166–77. doi: 10.1002/pros.23461. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Peng F, Shu M, et al. Isogambogenic acid inhibits the growth of glioma through activation of the AMPK-mTOR pathway. Cell Physiol Biochem. 2017;44:1381–95. doi: 10.1159/000485535. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharjee A, Hasanain M, Kathuria M, et al. Ormeloxifene-induced unfolded protein response contributes to autophagy-associated apoptosis via disruption of Akt/mTOR and activation of JNK. Sci Rep. 2018;8(1):2303. doi: 10.1038/s41598-018-20541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ukaji T, Lin Y, Okada S, Umezawa K. Inhibition of MMP-2-mediated cellular invasion by NF-kappaB inhibitor DHMEQ in 3D culture of breast carcinoma MDA-MB-231 cells: A model for early phase of metastasis. Biochem Biophys Res Commun. 2017;485:76–81. doi: 10.1016/j.bbrc.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Shin SY, Kim CG, Jung YJ, et al. Euphorbia humifusa Willd exerts inhibition of breast cancer cell invasion and metastasis through inhibition of TNFalpha-induced MMP-9 expression. BMC Complement Altern Med. 2016;16:413. doi: 10.1186/s12906-016-1404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YH, Kee JY, Kim DS, et al. Arctii fructus inhibits colorectal cancer cell proliferation and MMPs mediated invasion via AMPK. Am J Chinese Med. 2017;45:1309–25. doi: 10.1142/S0192415X17500720. [DOI] [PubMed] [Google Scholar]