Abstract
Transcription termination by Rho is essential for viability in various bacteria, including some major pathogens. Since Rho acts by targeting nascent RNAs that are not simultaneously translated, it also regulates antisense transcription. Here we show that RNase H-deficient mutants of Escherichia coli exhibit heightened sensitivity to the Rho inhibitor bicyclomycin, and that Rho deficiency provokes increased formation of RNA–DNA hybrids (R-loops) which is ameliorated by expression of the phage T4-derived R-loop helicase UvsW. We also provide evidence that in Rho-deficient cells, R-loop formation blocks subsequent rounds of antisense transcription at more than 500 chromosomal loci. Hence these antisense transcripts, which can extend beyond 10 kb in their length, are only detected when Rho function is absent or compromised and the UvsW helicase is concurrently expressed. Thus the potential for antisense transcription in bacteria is much greater than hitherto recognized; and the cells are able to retain viability even when nearly one-quarter of their total non-rRNA abundance is accounted for by antisense transcripts, provided that R-loop formation from them is curtailed.
INTRODUCTION
Transcription termination at the ends of genes and operons in bacteria occurs by two processes, factor independent (or intrinsic) and factor dependent, whose respective contributions are believed to be approximately equal in Escherichia coli (1,2). The latter process is also known as Rho-dependent transcription termination (RDTT).
The molecular mechanism of RDTT is reasonably well characterized (reviewed in 1,2,3,4,5,6). Briefly stated, it is mediated by the binding to a nascent transcript of Rho protein, whose subsequent interaction with RNA polymerase (RNAP) in the transcription elongation complex leads to dissociation of the enzyme from the DNA template. The coupling of transcription with translation (which is the norm in bacteria) protects against RDTT within the open-reading frame (ORF) regions, since translating ribosomes sterically prevent Rho’s access to the nascent transcript. A second protein NusG is also required for RDTT at some termination sites, and both Rho and NusG are essential for viability in several bacteria including E. coli (1–6).
RDTT has been suggested to participate, directly or indirectly, in several functions (that may not be mutually exclusive). These include: the silencing of horizontally transferred genes (7); maintenance of chromosomal integrity (8); prevention of gratuitous excision of prophages (9); regulation of gene expression by attenuation, small RNAs or riboswitches (10–15); suppression of pervasive antisense transcription (16–20); and avoidance of formation of excessive RNA–DNA hybrids or R-loops (21–24).
Antisense transcripts are those that are templated from the ‘wrong’ strand of ORFs in the genome. Although early studies had identified and characterized a limited number of such RNAs as regulators of gene product abundance, more recent data from next-generation-sequencing experiments have revealed an unexpected and substantial level of antisense transcription in both prokaryotes (25–31) and eukaryotes (32–34), which may be designated as the ‘constitutive antisense transcriptome’.
Peters et al. (16) have subsequently shown that the potential for antisense transcription in E. coli is much higher than that suggested by the constitutive antisense data (35,36), and that this potential is indeed kept in check by RDTT; in their study, following growth in the presence of sublethal concentrations of the Rho inhibitor bicyclomycin (BCM), there was a substantial increase in extent of antisense transcription in the cells. Their findings are consistent with the discovery of numerous intragenic promoters in E. coli (37–42) as well as with the concept that nascent untranslated transcripts are the target of RDTT (1,22), since antisense transcripts are by definition not translated. RDTT has also been shown to inhibit antisense transcription in other bacteria (17,19).
With respect to RDTT and R-loops, Leela et al. (23) have shown that the lethality conferred by deletion of rho or nusG in wild-type (WT) E. coli can be rescued by ectopic expression of UvsW, an R-loop helicase of T4 phage (43,44). The model is that, in the absence of RDTT, nascent transcripts that are not being translated are prone to re-annealing with upstream template DNA to generate R-loops which are toxic (22,24). By exploiting the property that C residues in the displaced single-stranded DNA of an R-loop suffer modification upon treatment with bisulfite, Leela et al. (23) were also able to infer the genome-wide locations of R-loops purportedly from both sense and antisense transcripts in E. coli, and to demonstrate their increased prevalence in a mutant deficient for RDTT.
In this work, we identify more than 500 sites on the E. coli chromosome from which antisense transcription is elevated in RDTT-deficient derivatives only when they are also expressing the R-loop helicase UvsW. These loci are well correlated with the antisense regions that were shown to be high-bisulfite-reactive by Leela et al. (23), suggesting that, for this subset, it is R-loop formation that precludes their detection following Rho inhibition alone.
MATERIALS AND METHODS
Growth media, bacterial strains, plasmids and primers
Unless otherwise indicated, LB and minimal A (with 0.2% glucose) were used as rich and defined media (45), respectively, and the growth temperature was 37°C. Supplementation with ampicillin (Amp), kanamycin (Kan) and trimethoprim (Tp) were at concentrations described previously (23). Xgal was added at 25 μg/ml, and isopropyl β-D-thiogalactoside (IPTG) at the indicated concentrations in different experiments.
Escherichia coli strain SA1751 (λ int+ xis439 cI857 [cro-chlA]ΔH1) used for preparation of supercoiled minicircle templates has been described earlier (46). All other strains are derivatives of the reference E. coli K-12 strain MG1655, and are listed in Supplementary Table S1.
The following plasmids have been described previously (salient features in parentheses): pSA508 (AmpR, for generation of supercoiled minicircle templates with multiple-cloning-site and Rho-independent terminator) (46); and pHYD2411 (TpR, single-copy-number rho+ lacZ+) and pHYD2412 (TpR, single-copy-number nusG+ lacZ+) (23). Other plasmids constructed in this study, and the primers used therefor, are described in Supplementary Table S2.
Immunoblotting with S9.6 monoclonal antibody
Immunoblotting with S9.6 monoclonal antibody (Kerafast, USA) for detection of RNA–DNA hybrids was performed essentially as described (47–49). Total nucleic acids were prepared by spooling following chloroform isoamyl alcohol extraction as described in Ausubel et al. (50) for preparation of chromosomal DNA, with the modification that the step of RNase treatment was omitted. Cultures of the WT strain GJ13519 grown to mid-exponential phase without or with 25 μg/ml (sublethal) BCM were used, and the yield was ∼10 μg per ml of culture. For each culture, a pair of 10–20 μg aliquots of the preparations was immobilized, with the aid of vacuum suction through a Bio-Dot microfiltration apparatus (Bio-Rad, USA) followed by UV-crosslinking at 1200 J/m2, on Hybond-N+ nylon membrane (Amersham Biosciences); one aliquot for each pair was treated with two units of RNase H for 1 h at 37°C before the immobilization. A 1:5000 dilution of the antibody preparation was used for immunoblotting overnight at 4°C, followed by reaction with enzyme-conjugated anti-mouse secondary antibody and detection with a chemiluminesence kit.
To determine the equivalence of immobilization of the different nucleic acid samples, the membrane was washed in water, soaked in 5% acetic acid with shaking for 15 min, and then stained with 0.05% methylene blue in 0.5 M sodium acetate buffer (pH5.2) (51).
For strain GJ13531 (Δrho Ptac-UvsW), an overnight-grown culture in glucose-minimal A supplemented with 150 μM IPTG was washed twice in minimal A and then inoculated 1:50 in fresh glucose-minimal A medium (without IPTG). This IPTG-depleted cell suspension was incubated for 12 h before it was processed for S9.6 immunoblotting as above; an aliquot of the culture was also plated on appropriate media to confirm the absence of accumulation in it of suppressors or contaminants. Cells from an IPTG-supplemented log-phase culture of GJ13531 were used as the control for this experiment.
Protocols for RNA-Seq experiments
Viable clones of the UvsW-expressing strains GJ13531 (Δrho) and GJ13507 (ΔnusG) were obtained as white colonies from their respective shelter plasmid-carrying derivatives GJ13531/pHYD2411 and GJ13507/pHYD2412 on glucose-minimal A plates supplemented with Xgal and IPTG at 200 μM (for Δrho) or 3 μM (for ΔnusG), as previously described (23).
Starting from single colonies, the following cultures were set up in triplicate for overnight incubation: GJ13507, GJ13519, GJ13531 and GJ13533 in glucose-minimal A; and GJ13519 also in 0.2% glycerol-minimal A. All the cultures were supplemented with 200 μM IPTG, with the exception of the cultures of GJ13507 whose supplementation with IPTG was at 3 μM. The overnight-grown cultures were each subcultured into 20 ml of fresh medium of the same composition, with an inoculum of 1:50 for GJ13507 and GJ13531 and of 1:100 for the remainder, and grown to an A600 of 0.4–0.45, before the cells were harvested for making the RNA preparations as described below. Growth rates for the cultures were determined, and are given in Supplementary Table S3. Aliquots of the cultures were also plated on appropriate media to confirm the absence of accumulation of suppressors or contaminants in any of them. At the appropriate optical density, culture growth was instantaneously arrested by addition of an equal volume of chilled 100% ethanol, and the cells were stored at −80°C until they were processed for RNA extraction. The cells were lysed and total RNA was prepared by the hot phenol method essentially as described (52,53) after chromosomal DNA has been digested with RNase-free DNase. The quality of RNA preparations was validated by microchannel electrophoresis (Agilent, USA). Strand-specific RNA-Seq data were generated, following rRNA depletion with a Ribo-Zero kit, with the aid of the di-tagged cDNA strategy (ScriptSeq) on an Illumina NextSeq platform.
The sequence data from these experiments, as also from the other publicly available datasets [Peters et al. (16); Larson et al. (54); and Sedlyarova et al. (55)], were then analyzed as described in the Supplementary Data.
In vitro transcription with supercoiled minicircle templates
Techniques for DNA manipulations and polymerase chain reaction were as described (50,56). Derivatives of plasmid pSA508 (46) each carrying the phage T7 A1 promoter and a specified E. coli genomic fragment were constructed as described in Supplementary Table S2, and all the insert regions were sequence verified. Transformants of strain SA1751 carrying these plasmids were temperature-induced in one litre-culture volumes as described (46), to enable the generation of supercoiled minicircles that were then purified from 1.5% agarose gels following electrophoresis.
In vitro transcription reactions were each performed essentially as described (57), with 0.45 pmol of supercoiled minicircle DNA as template, 3 pmol of RNAP holoenzyme and 32P-α-CTP as radiolabel, in a total volume of 150 μl, with the modification that heparin was omitted from the mixture and the transcription reaction was stopped by heating to 65°C for 20 min. A total of 10 μl of the mix was retained as ‘no-treatment’ control; of the remainder, one-half was treated with two units of RNase H for 5 min and 0.5 μg/ml RNase A for 3 min (‘A+H’ sample) (58), while the other half was treated only with 0.5 μg/ml of RNase A for 3 min. (‘A’ sample). All three fractions were then treated with a stop-solution mix containing 70 μl of ice-cold precipitation buffer (40 mM ethylenediaminetetraacetic acid, 300 μg/ml salmon sperm DNA, 0.6 M sodium acetate), ethanol precipitated, and subjected to electrophoresis on denaturing 6% polyacrylamide— 8 M urea gels followed by autoradiography, as described (57).
RESULTS AND DISCUSSION
R-loop abundance is increased under RDTT-deficient conditions
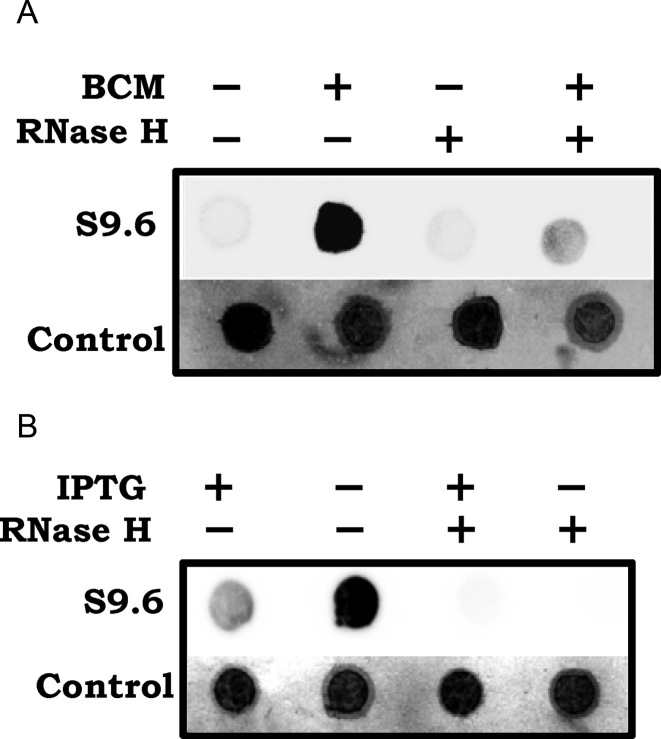
To examine whether R-loops are more prevalent following Rho inhibition, we used S9.6 monoclonal antibody, which recognizes RNA–DNA hybrids (59) to perform immunoblotting experiments with total nucleic acid preparations from cultures grown without or with sublethal concentrations of BCM. The BCM-grown culture preparation displayed significantly more reactivity to S9.6 antibody than did the control; this reactivity was also abolished upon RNase H treatment, confirming that the antibody is specific to RNA–DNA hybrids (Figure 1A).
Figure 1.
Immunoblot with S9.6 monoclonal antibody of total nucleic acid preparations from cultures (A) of WT strain (GJ13519) grown without or with sublethal BCM at 25 μg/ml, and (B) of Δrho-UvsW strain (GJ13531) grown under IPTG-replete or -depleted conditions. Where indicated, the nucleic acid preparations were treated in vitro with RNase H. To serve as loading controls, methylene blue-stained images of the blotted membranes are also shown.
We have earlier shown (23) that lethality conferred by Δrho in WT E. coli can be overcome by expression of UvsW, the R-loop helicase from phage T4. Reactivity to S9.6 antibody was low in nucleic acid preparations from cells of the Δrho-UvsW strain that had been grown in presence of the inducer IPTG needed for expression of UvsW, but it was strongly elevated in equivalent preparations from the culture that was growth-inhibited following IPTG withdrawal; once again, this signal was abolished upon treatment with RNase H (Figure 1B). We conclude that R-loops are increased in cells deficient for RDTT, and furthermore that UvsW expression under these conditions is correlated with both reduced R-loop prevalence and restoration of viability.
Heightened BCM sensitivity of RNase H-deficient mutants
In E. coli, RNA–DNA hybrids can be disrupted by the pair of endogenous RNase H enzymes I and II that are encoded, respectively, by rnhA and rnhB. BCM tolerance phenotypes for rnhA and rnhB mutants have been reported previously (60,61), but only in the context of studies on whole-genome single-gene knockout collections. In a more detailed and systematic characterization of these phenotypes in the rnhA and rnhB single and double mutants, we found that the rnhA derivative is more sensitive than is the WT strain to inhibition by BCM at both 37 and 42°C (Supplementary Figure S1A and B, respectively), and that the rnhB mutation confers no defect. The rnhA rnhB double mutant [which is known to be growth-sensitive at temperatures of 37°C and above (23,62)] was even more BCM-sensitive than rnhA alone at 30°C (Supplementary Figure S1C). These results indicate that the compromised ability in rnhA and rnhA rnhB mutants to remove R-loops is associated also with increased sensitivity to BCM.
Inverse correlation between Rho-inhibited antisense transcription and reactivity to bisulfite
Given both that Rho acts to terminate synthesis of nascent transcripts that are not simultaneously translated, and that such transcripts are also the perceived substrates for R-loop generation, we examined whether a correlation exists between those ORFs where antisense RNA synthesis had been induced following Rho inhibition by sublethal BCM [in the data of Peters et al. (16)], and the sites of presumed high-antisense R-loop prevalence [as had previously been determined in the bisulfite reactivity experiments (23)]; for the former, we applied a log2 induction ratio threshold of 3.
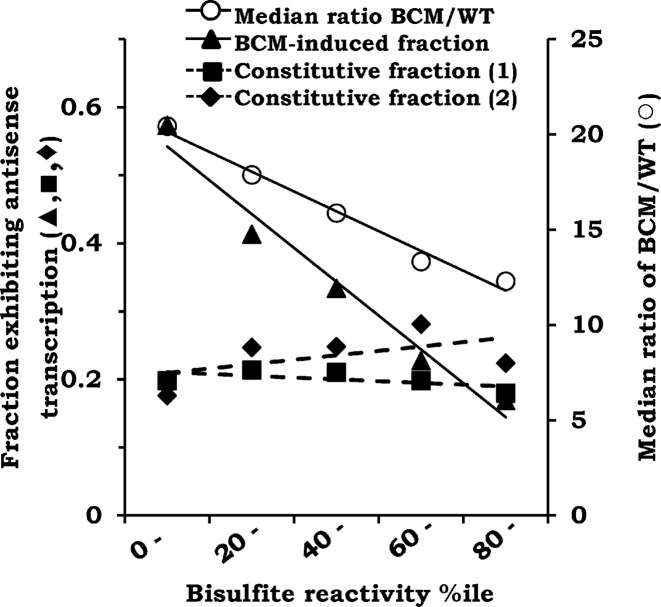
Against our initial expectation of a possible direct correlation between the two datasets, however, we in fact observed the opposite, that they were strongly inversely correlated across the entire range of bisulfite reactivity rankings (Figure 2; R2 = 0.97, P < 0.002). Thus, whereas more than one-half of regions in the lowest-ranking interval of bisulfite reactivity exhibited BCM-induced antisense transcription, only one-sixth of those in the highest-ranking interval did so. This same counterintuitive pattern was observed even when the log2 BCM-induction ratio threshold was reduced to 2 (Supplementary Figure S2A; R2 = 0.99, P < 0.001). The median value for the BCM induction ratio also exhibited a progressive decline with increasing bisulfite reactivity (Figure 2 and Supplementary Figure S2A; R2 = 0.98, P < 0.001 and R2 = 0.92, P < 0.01, respectively). On the other hand, there was little or no correlation between the constitutive antisense transcriptome [from each of two different datasets, namely those of Dornenburg et al. (35) and Peters et al. (16)], and the regions of varying bisulfite reactivity (Figure 2; R2 = 0.24, P = 0.39 and R2 = 0.38, P = 0.26, respectively, for the two datasets).
Figure 2.
Inverse correlation between BCM-induced antisense transcription [Peters et al. (16)] and bisulfite reactivity. Across the different intervals of bisulfite reactivity percentiles are plotted both the fraction of BCM-induced antisense loci (filled triangles), and the median induction ratio of antisense transcripts for these loci (open circles), within each interval. Also shown (interrupted lines) are the fractions of the constitutively transcribed antisense loci (i.e. >80th percentile in the WT strain) for each of the intervals, the data for which have been taken from Peters et al. (16) (filled squares, constitutive fraction 1) and Dornenburg et al. (35) (filled diamonds, constitutive fraction 2).
To account for this apparent paradox of inverse correlation between two phenomena that are individually both ostensibly upregulated upon Rho inhibition, we postulated that if a nascent antisense transcript synthesized following Rho inhibition were to form an R-loop, it would inhibit movement of succeeding RNAP molecules and hence lead to reduced transcript abundance for that locus (63–65). Thus, only those RNAs would be identified as BCM-induced that do not form R-loops when they are synthesized following Rho inhibition. Such a model could explain the progressive under-representation of BCM-induced antisense transcription loci with increasing bisulfite reactivity.
Antisense transcriptomes in RDTT-deficient strains expressing an R-loop helicase
One prediction of our model is that the antisense transcription sites above which are under-represented because of R-loop formation will be revealed upon concomitant expression of an R-loop helicase in the RDTT-deficient strain. (Of course, the transcripts previously identified as BCM-induced [that is, which do not form R-loops] would also be expected to be present in this strain).
Accordingly, we designed strand-specific RNA-Seq experiments to determine the transcription profiles in triplicate cultures of Δrho or ΔnusG derivatives expressing UvsW (designated hereafter as Δrho-UvsW and ΔnusG-UvsW, respectively). We also generated RNA-Seq data for cultures of the WT (rho+nusG+) strain, and of the WT strain expressing UvsW (WT-UvsW), as controls.
After the sequence reads were aligned to the reference E. coli genome, base read counts (normalized to both ORF length and aggregate read counts) were determined for sense and antisense strands of each of 4091 ORFs that together comprise 84% of the chromosome length in WT E. coli (Supplementary Table S4, sheet 1). (In the process, we confirmed both the Δrho and ΔnusG status of the test strains and that the ΔnusG derivative exhibited a 5-fold increase in transcription of the autoregulated rho gene, which is consistent with western blot data from an earlier study (66)). Normalized base read counts were similarly computed for these ORFs from the data of Peters et al. (16) for cultures that had been grown without or with BCM (Supplementary Table S4, sheet 1).
Antisense transcripts induced by BCM are also expressed in Δrho-UvsW strain
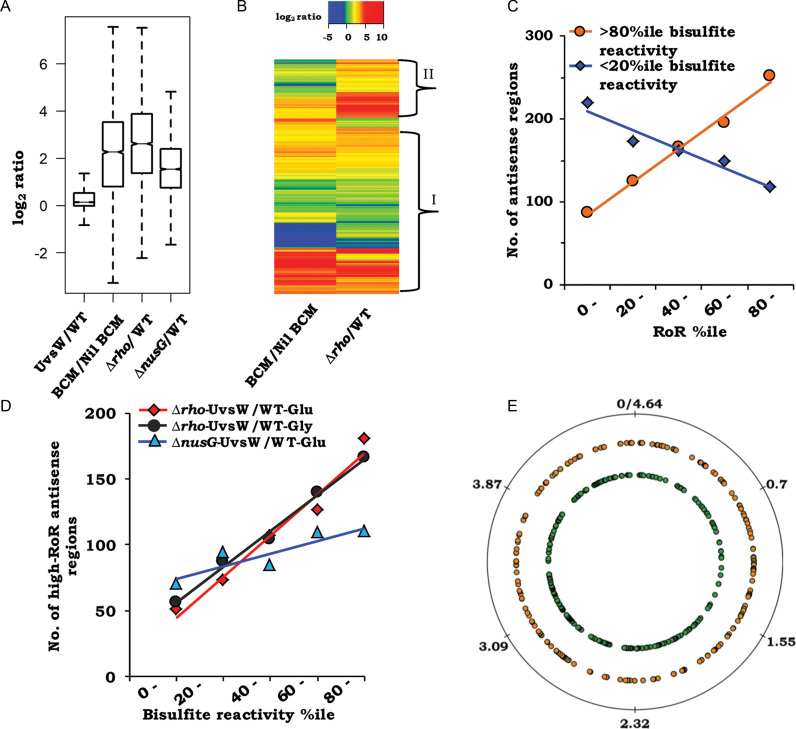
We initially established that UvsW expression alone had negligible effect on global antisense transcription (Figure 3A, log2 median ratio of antisense expression for WT-UvsW relative to WT = 0.14, 95% confidence interval [CI] = 0.13–0.15), whereas there was substantial induction of antisense expression with either sublethal BCM exposure [from data of Peters et al. (16)] or in the Δrho-UvsW derivative (log2 median ratios relative to WT, respectively, 2.26 [95% CI = 2.19–2.33] and 2.61 [95% CI = 2.55–2.67]) (Figure 3A).
Figure 3.
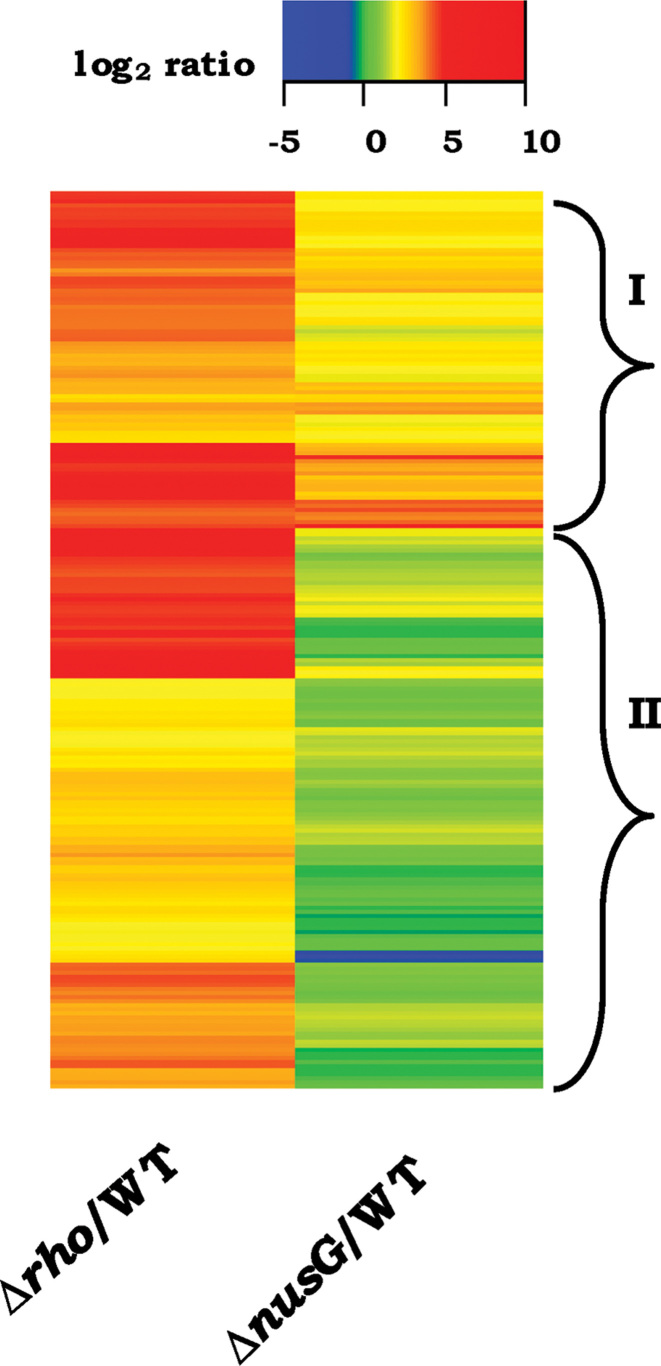
Analysis of antisense RNA-Seq data from RDTT-deficient strains expressing UvsW. (A) Box plot representations of the log2 antisense expression ratios (relative to glucose-grown WT) for the strains WT-UvsW, Δrho-UvsW and ΔnusG-UvsW (labeled, respectively, as UvsW/WT, Δrho/WT and ΔnusG/WT), as well as for the culture grown in presence of BCM to that in its absence (BCM/Nil BCM) [taken from the data of Peters et al. (16)]. (B) Heat map representation of the ratio data from panel A for the pair: BCM/Nil BCM and Δrho/WT; hierarchical clusters were ordered by complete linkage method. Regions marked ‘I’ and ‘II’ are explained in the text. (C) Frequency distribution curves, across the range of Δrho-UvsW RoR percentiles, of antisense loci that are (i) above the 80th percentile (orange circles) and (ii) below the 20th percentile (blue diamonds) of bisulfite reactivity. (D) Frequency distribution curves, across the range of bisulfite reactivity percentiles, of high-RoR antisense loci determined from the following comparisons: Δrho-UvsW to WT grown in glucose (red diamonds); ΔnusG-UvsW to WT grown in glucose (blue triangles); and Δrho-UvsW to WT grown in glycerol (black circles). (E) Representation, on map of circular Escherichia coli chromosome, of 535 high-RoR antisense regions in the Δrho-UvsW strain, corresponding to ORFs oriented clockwise (orange, middle circle) and counterclockwise (green, inner circle). Genome coordinates (in Mb) are marked on the outer circle.
As mentioned above, the expectation from our model was that antisense transcripts that do not form R-loops [and thus had been detected earlier as BCM-induced (16)] would also be present in the Δrho-UvsW strain, and that the latter would additionally contain the R-loop forming antisense RNAs as well. These expectations were validated upon inspection of the heat map representation of the log2 induction ratios for all 4091 antisense regions in the two sets of data (Figure 3B). For the subset of regions marked ‘I’ in Figure 3B, there was for the major part either no induction or approximately equivalent induction of antisense expression both upon BCM addition and in Δrho-UvsW; in addition, there was also a subset (marked ‘II’ in Figure 3B) that was more prominently induced in Δrho-UvsW than in the BCM-exposed cultures. These data also serve to confirm our previous finding (23), this time on a genome-wide scale, that in rescuing Δrho lethality, UvsW expression does not reverse the transcription termination defect in the strain.
Identification of novel antisense transcripts that are abundant only with combined RDTT deficiency and UvsW expression
To follow-up on the heat map data and to identify antisense regions that were differentially induced upon the combined influence of RDTT deficiency and R-loop helicase expression compared to that with RDTT deficiency alone, we determined the ratio of ratios (RoR) for the antisense strand of each of the ORFs. In the RoR calculation, the numerator and denominator ratios were the magnitudes of induction, respectively, (i) in Δrho-UvsW (relative to WT) from our data, and (ii) by sublethal BCM (relative to the cultures without BCM) in the data of Peters et al. (16). The expectation was that high-RoR regions would represent those in which R-loop formation had precluded their induction by BCM addition alone.
For antisense regions which are high-ranking (>80th percentile) for bisulfite reactivity (that is, with presumed maximal R-loop prevalence), the frequency distribution plot showed a strong direct correlation with RoR for the Δrho-UvsW derivative (Figure 3C; R2 = 0.99, P < 0.001). The converse was also true, in that antisense regions that were the least ranking (<20th percentile) for bisulfite reactivity (minimal R-loop prevalence) exhibited a strong inverse correlation and were under-represented at high RoRs (Figure 3C; R2 = 0.93, P < 0.01).
From the data, we then identified 535 sites of antisense transcription that fulfilled the following criteria to indicate that they are transcribed preferentially only under the combined conditions of Rho deficiency and UvsW expression (compared to that with Rho inhibition alone): log2 ratio of induction by BCM in data of Peters et al. (16) <3; log2 ratio of expression in Δrho-UvsW strain relative to WT >2; and RoR >80th percentile (corresponding to log2 RoR of around two and above). Their distribution showed a strong positive correlation across the range of ranks of bisulfite reactivity, with a 3.5-fold difference in numbers between the highest and lowest quintile rank intervals (Figure 3D; R2 = 0.97, P < 0.003). When the criterion relating to BCM induction was modified to log2 ratio <2, the identified antisense region numbers were decreased to 412 but the strong positive correlation across the range of ranks of bisulfite reactivity remained, this time with a 5-fold enrichment for the highest rank interval compared to the lowest (Supplementary Figure S2B; R2 = 0.91, P < 0.01).
Taken together, these findings are strongly supportive of the model that a subset of antisense loci which are transcribed following Rho inhibition suffer R-loop formation and therefore need expression of an R-loop helicase as well for their detection. The 535 regions identified, which we refer to below as high-RoR antisense regions, are organized in 370 clusters and show a fairly uniform distribution across the genome on both strands (Supplementary Table S4, sheet 2; see also Figure 3E). Given that UvsW expression alone had no significant effect on antisense expression (Figure 3A), these results suggest that the majority of R-loops upon which the helicase acts are formed only after inhibition of RDTT.
Analysis of antisense transcription in representative high-RoR clusters
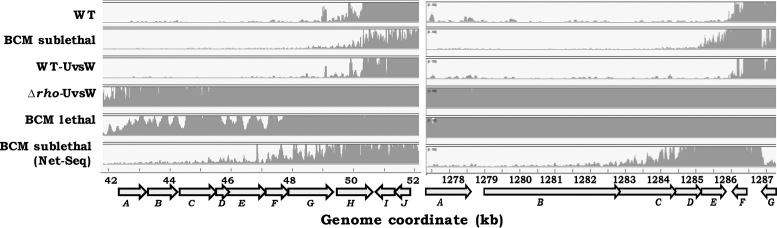
For two representative high-RoR clusters, a contig analysis of the RNA-Seq read data clearly established the occurrence of novel antisense transcripts that could span multiple ORF boundaries and extend even beyond 10 kb in their length (Figure 4). These RNAs were present only in the Δrho-UvsW strain, but not in the WT strain nor in cultures exposed to just one of the two perturbations in isolation (BCM addition, or UvsW expression).
Figure 4.
Contig analysis of sequence data from two representative genomic regions. For each panel, genome coordinates (in kb) and ORF annotations are given at the bottom. Plotted are the base-specific read numbers, with an upper cut-off limit at 100, in the data for RNA preparations from one replicate each of the following cultures: WT, glucose-grown GJ13519; (+) BCM sublethal, culture grown with sublethal BCM in study of Peters et al. (16); WT-UvsW, GJ13533; Δrho-UvsW, GJ13531; (+) BCM lethal, culture harvested after lethal BCM exposure in study of Sedlyarova et al. (55); and (+) BCM sublethal (Net-Seq), culture grown with sublethal BCM in study of Larson et al. (54). In both the panels, data for only one strand (bottom) is shown which is antisense for the ORFs in clockwise orientation. ORFs have been alphabetically listed in the following order (from left to right): left panel, fixA, -B, -C, -X, yaaU, kefF, -C, folA, apaH, -C; and right panel, narK, -G, -H, -J, -I, tpr, purU.
To obtain a more fine-grained insight from the RNA-Seq data, we determined the read counts for successive 100-base regions across the genome for both strands, and calculated log2 ratios (relative to WT) after normalization and thresholding, as described in the Supplementary Data (Supplementary Table S4, sheet 3). Plots for the representative examples of antisense regions that display unchanged transcription (nil or constitutive) under all conditions, transcription with RDTT deficiency, and transcription for multi-ORF clusters only upon combined RDTT deficiency and UvsW expression, are shown in Supplementary Figure S3. Our data indicate once again that at multiple loci, antisense transcripts are detected only with the last combination, and that the induction is quite marked (>100-fold) under these conditions. These high-RoR regions were also the ones that exhibited high bisulfite reactivity.
Sense transcription is not inhibited in Δrho-UvsW strain
In cells of the Δrho-UvsW strain, antisense RNAs comprised 22% of the total non-rRNA abundance (determined as the average of data for the three replicate cultures). Nevertheless, sense transcription in the Δrho-UvsW strain was not inhibited (and in fact was moderately elevated), as determined both for the full set of 4091 ORFs (log2 median expression for WT and Δrho-UvsW, respectively, 6.01 [95% CI = 5.90–6.11] and 6.45 [95% CI = 6.40–6.51]) and for the subset of 535 ‘high-RoR’ ORFs (corresponding values, respectively, 5.71 [95% CI = 5.44–5.98] and 6.02 [95% CI = 5.87–6.18]) (see Supplementary Figure S4). Our findings are in agreement with the conclusions of Peters et al. (16) that increased antisense transcription following Rho inhibition is not associated with concomitant decrease in sense transcription.
Antisense transcription is similarly affected by UvsW expression in ΔnusG as in Δrho, and is unrelated to growth rate changes
From the box plot representation (Figure 3A), it was evident that antisense expression in ΔnusG-UvsW was increased relative to that in WT (log2 median induction ratio = 1.54, 95% CI = 1.49–1.58), but to a less extent than in Δrho-UvsW. It is known that RDTT is compromised more severely in absence of Rho than in absence of NusG (7,16,67,68).
From the ΔnusG-UvsW data, high-RoR antisense regions were determined by the approach similar to that described above for the Δrho-UvsW strain, with the modification that a lower threshold of log2 induction ratio >1 (relative to WT) was employed. The frequency distribution of the high-RoR regions in ΔnusG-UvsW was moderately positively correlated across the range of ranks of bisulfite reactivity (Figure 3D; R2 = 0.78, P < 0.05).
Peters et al. (16) had shown that antisense transcripts induced upon BCM addition can be divided into two categories depending upon whether they are, or are not, also induced in NusG-deficient cells; they had designated the cognate sites of Rho termination as, respectively, NusG-dependent and NusG-independent, whose relative proportion was ∼1:4 across the genome. The latter also exhibited stronger ‘rho utilization’ sites, with higher C/G ratios in the transcript sequences, than the former.
A comparative analysis of antisense transcription data for the Δrho-UvsW and ΔnusG-UvsW derivatives was reasonably consistent with the findings above of Peters et al. (16). Thus, in the heat map representation of the 535 high-RoR antisense regions [which are completely non-overlapping with the BCM-induced regions of Peters et al. (16)], both NusG-dependent (that is, also induced in ΔnusG-UvsW albeit to less extent than in Δrho-UvsW) and NusG-independent subsets could be identified, which are demarcated as ‘I’ and ‘II’, respectively, in Figure 5. The median C/G ratio of the deduced antisense transcript sequences for the NusG-independent subset of 353 regions (log2 induction ratio for ΔnusG-UvsW relative to WT <1) was 1.14, which was significantly higher than that for the NusG-dependent subset of 182 regions, at 1.07 (P < 10–5, Mann–Whitney U test).
Figure 5.
Heat map representation of log2 antisense expression ratios (relative to glucose-grown WT) for the strains Δrho-UvsW and ΔnusG-UvsW (labeled, respectively, as Δrho/WT, and ΔnusG/WT) with respect to the 535 high-RoR regions; hierarchical clusters were ordered by wardD2 linkage method. Regions marked ‘I’ and ‘II’ are explained in the text.
To exclude the possibility that slow growth of Δrho-UvsW and ΔnusG-UvsW derivatives (relative to WT) may account for the differences in their transcriptomes, we also performed RNA-Seq analyses for triplicate cultures of the WT strain grown with glycerol, wherein the growth rate was about 60% of that for glucose-grown cultures [Supplementary Table S3, see also (69)]. A strong correlation was observed when the log2 antisense RNA induction ratios for the Δrho-UvsW strain, calculated relative to the WT strain grown with glucose on the one hand or glycerol on the other, were compared (Supplementary Figure S5; R2 = 0.84, P < 10–15). The distribution of high-RoR regions determined from the data for the glycerol-grown cultures (using the same criteria as that for the glucose-grown cultures) was also positively correlated across the range of ranks of bisulfite reactivity (Figure 3D; R2 = 0.99, P < 0.0003).
R-loops are generated in a high-RoR antisense region during in vitro transcription
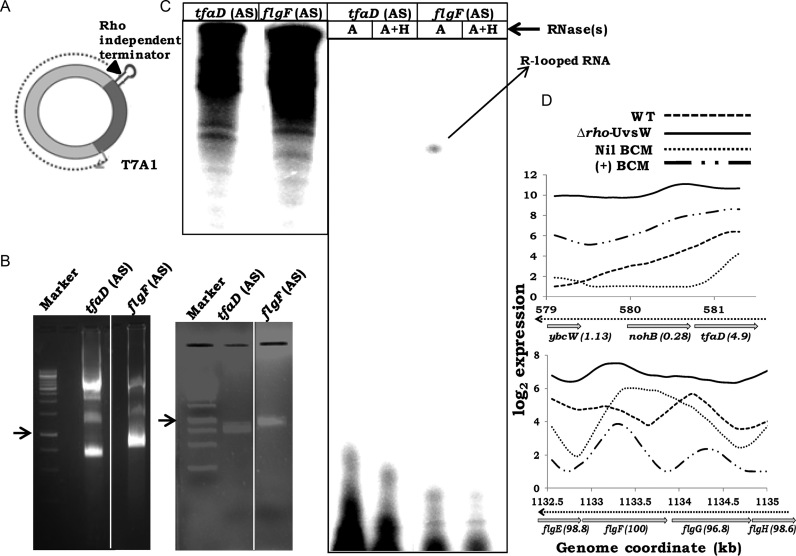
R-loops generated during in vitro transcription can be identified by the property that the radiolabeled transcripts would be resistant to RNase A and sensitive to RNase H (58). We generated templates (Supplementary Table S2) in antisense orientation from both a region (flgF) exhibiting high-antisense RoR, and a second control region (tfaD) whose antisense transcription was induced to equivalent extent either by BCM alone or in the Δrho-UvsW derivative (bisulfite reactivity percentiles 100 and 4.9, respectively; see Figure 6D). The DNA fragments were cloned such as to reside downstream of the phage T7 A1 promoter and upstream of a Rho-independent terminator. Since R-loop formation is facilitated by negative supercoiling (70), the templates were prepared as supercoiled minicircles by a protocol of in vivo site-specific recombination from plasmids, as previously described (46) (Figure 6A and B).
Figure 6.
Detection of R-loops during in vitro transcription. (A) Schematic depiction of supercoiled minicircle template following excision from pSA508 derivative, with cloned genomic fragment between T7 A1 promoter and Rho-independent terminator. (B) Agarose gel electrophoregrams of pSA508 derivatives [bearing antisense (AS) orientations of fragments from tfaD and flgF] following temperature induction in SA1751 cultures (left), and after purification of the supercoiled minicircles (right). Arrows denote 1-kb band in each of the two marker lanes. (C) Autoradiographs following denaturing gel electrophoresis of in vitro transcription products from the supercoiled minicircles, before (left panel) and after (right panel) treatment with RNase(s) A or A with H (A+H), as indicated. Lanes for samples without RNase treatment were loaded with one-seventh of the amounts loaded on each of the lanes for RNase-treated samples. (D) Plots of the log2 antisense expression values for the tfaD and flgF loci for cultures of WT strain without (Nil BCM) and with sublethal BCM (+ BCM) [data from Peters et al. (16)], and from this study for WT and Δrho-UvsW cultures. Genome coordinates (in kb) and ORF annotations are marked, with the antisense bisulfite reactivity percentile indicated in parentheses beside each ORF. Direction of antisense transcription is shown by the interrupted arrow.
Following in vitro transcription from the pair of supercoiled minicircle templates, RNase A-resistant transcripts were observed only for the flgF ORF region in its antisense orientation (Figure 6C); these transcripts were sensitive to digestion by RNase H. Transcripts from the control tfaD antisense template were fully RNase A-sensitive (Figure 6C). We conclude that transcripts from a high-RoR antisense region do form R-loops in vitro on a supercoiled template.
Antisense expression analyses in other transcriptome datasets of Rho inhibition
We analyzed two other public sets of E. coli transcriptome data related to RDTT (Supplementary Table S4, sheet 1): one was of nascent transcript sequencing (Net-Seq) (54) from cultures without or with sublethal BCM, that is, conditions that were similar to those employed earlier for RNA-Seq by Peters et al. (16); and the other was from RNA-Seq experiments of cultures without or with exposure for 20 min to lethal concentrations of BCM (55). As described below, the former set [Net-Seq with sublethal BCM (54)] resembled that of Peters et al. (16), whereas the latter [RNA-Seq with lethal BCM (54)] was similar to that from the Δrho-UvsW derivative.
Thus, the BCM-induced antisense regions from the Net-Seq data exhibited an inverse correlation in their distribution across the range of ranks of bisulfite reactivity, whereas those from the lethal-BCM exposure experiment showed no significant correlation (Supplementary Figure S6A; R2 = 0.98, P < 0.001 and R2 = 0.57, P = 0.13, respectively). Furthermore as depicted in Supplementary Figure S6B for the 535 high-RoR antisense regions identified above, there was little induction following sublethal BCM exposure of synthesis of both free transcripts (data of Peters et al. (16)) and nascent transcripts (Net-Seq data) (log2 median induction ratios, respectively, 1.05 [95% CI = 0.92–1.18] and 1.32 [95% CI = 1.23–1.41]); on the other hand, these regions were induced by lethal BCM exposure (log2 median induction ratio = 4.89 [95% CI = 4.76–5.02]) to the same extent as in the Δrho-UvsW derivative (log2 median induction ratio = 3.85 [95% CI = 3.72–3.97]). The same pattern was noted in the contig analysis of the sequence read data as well, wherein lethal BCM exposure was associated with strong antisense transcription of those regions that were not induced by sublethal BCM (Figure 4).
The finding that relative antisense transcript abundance in the high-RoR regions (in absence of UvsW expression) is low when the Rho deficiency is chronic (after sublethal BCM exposure for several hours), and high when it is acute (after lethal BCM exposure for 20 min), suggests that R-loops at these sites may accumulate with time, and furthermore that these R-loops then act to block progressive rounds of transcription elongation (63–65).
CONCLUSIONS
We have shown here that transcripts from more than 500 antisense loci are synthesized in RDTT-deficient strains predominantly only when UvsW is also expressed in them. These high-RoR regions were also correlated with the loci that had previously been inferred, based on their reactivity to bisulfite, to be R-looped in vivo. The simplest interpretations for these observations is that at a subset of regions where antisense transcription occurs following Rho inhibition, R-loops are generated that serve as road-blocks for further transcription (63–65); and that UvsW’s action as an R-loop helicase relieves the road-blocks at these regions. The direct demonstration (i) in Rho-deficient cells of elevated R-loop prevalence that is alleviated upon UvsW expression, and (ii) of R-loop occurrence during in vitro transcription at a high-RoR antisense locus, supports this model. In an earlier study (23), we had also shown that UvsW expression rescues the inviability of an rnhA rnhB double mutant at 42°C.
Apart from its R-loop helicase activity, UvsW has also been shown to possess activity in vitro as a junction DNA helicase that can catalyze branch migration and fork regression reactions (71–74). It has also been suggested that the mechanism of UvsW’s action in rescuing Δrho or ΔnusG lethality may perhaps be related to its ability to resolve problems related to DNA replication in the mutants (75). In the context of the present study, however, it is quite unlikely that UvsW’s DNA junction helicase activity would explain the detection of novel antisense RNAs in RDTT-deficient cells from the loci of high bisulfite reactivity. Indeed, the strong and direct correlation established in this study, between bisulfite-reactive loci and the sites of novel antisense transcription, offers mutual reinforcement to the twin notions (i) that the former represent R-looped regions, and (ii) that the latter reflect UvsW’s ability to unwind them.
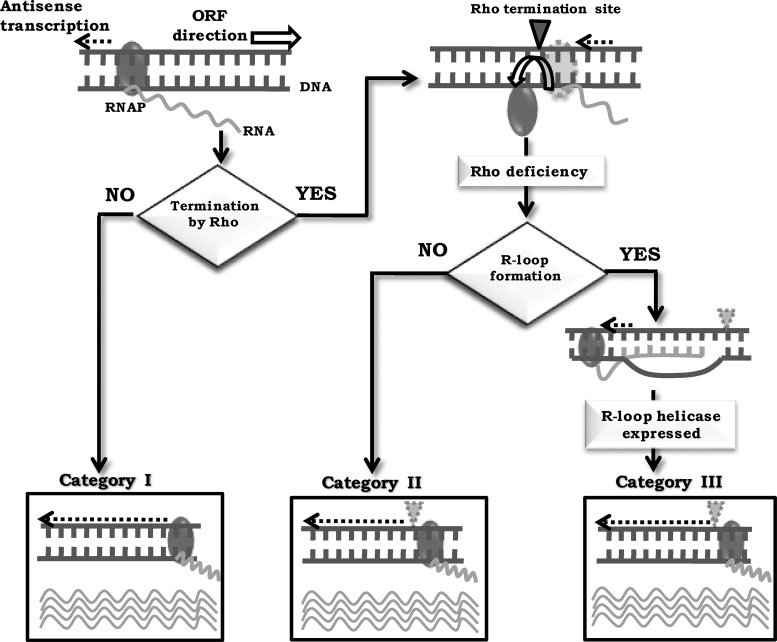
Thus, three categories of bacterial antisense transcription may be envisaged (Figure 7). The first is constitutive antisense transcription whereas in the second and the third, transcripts are only synthesized following Rho inhibition. The second category are antisense RNAs that do not form R-loops whereas the third category (represented by the transcripts from high-RoR regions) form R-loops. The features that distinguish between the latter two categories remain to be determined; previous genome-scale studies in other organisms have variously implicated high levels of transcript expression, as well as different characteristics of the RNA sequences such as G-richness and G/C skew or poly-A tracts and A/T skew, for R-loop formation (76–80).
Figure 7.
Model to explain three categories of bacterial antisense transcription; see text for details.
The fact that the Δrho-UvsW derivative, containing high levels of antisense RNAs, is viable (while Δrho is lethal) suggests that the antisense transcripts themselves are not toxic and that they become so only upon associating with DNA to form R-loops. Rho’s essential role therefore is in curtailing antisense transcription and consequential R-loop formation. Rho is essential for viability in many other bacteria including major pathogens such as Mycobacterium tuberculosis (20), and here too it is possible that R-loops from untranslated antisense transcripts are the cause of lethality under the Rho-deficient conditions.
R-loop toxicity may be caused not only by transcriptional road-blocking as mentioned above (63–65), but also by arrest and titration of RNAP molecules (41), impedance of replication fork progression (81,82), and aberrant initiation of DNA replication (24,83,84). Washburn and Gottesman (8,75) have earlier suggested that Rho inhibition can lead to transcription-replication conflicts and thus to loss of genome integrity, which is consistent with the model for R-loop toxicity in bacterial cells. In eukaryotes as well, R-loops are now similarly recognized to be a major contributor to compromise of genomic integrity and thus to cancer pathogenesis (85–89), and action of an R-loop helicase has recently been shown to ameliorate R-loop toxicity in mammalian cells (90).
DATA AVAILABILITY
The RNA-Seq data generated in this study are available at GEO https://www.ncbi.nlm.nih.gov/geo/ under accession number GSE103937.
Supplementary Material
ACKNOWLEDGEMENTS
The RNA-Seq data for this study were generated at the Plateforme de Séquençage à Haut Débit facility of I2BC. BCM was a kind gift from Max Gottesman. We thank Saswat Mohapatra, Audrey Vingadassalon and especially Rohan Misra for assistance with experiments and data analysis; and COE team members for advice and discussions.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Centre of Excellence (COE) Project for Microbial Biology—Phase 2; Indo-French DST-ANR Project 2013–06; DST-INSPIRE Fellowships (to N.R., J.M.); ICMR Research Fellowship (to R.M.K.); J C Bose Fellowship (to J.G.); INSA Senior Scientist Award (to J.G.).
Conflicting interest statement. None declared.
REFERENCES
- 1. Adhya S., Gottesman M.E.. Control of transcription termination. Annu. Rev. Biochem. 1978; 47:967–996. [DOI] [PubMed] [Google Scholar]
- 2. Ray-Soni A., Bellecourt M.J., Landick R.. Mechanisms of bacterial transcription termination: all good things must end. Annu. Rev. Biochem. 2016; 85:319–347. [DOI] [PubMed] [Google Scholar]
- 3. Boudvillain M., Figueroa-Bossi N., Bossi L.. Terminator still moving forward: expanding roles for Rho factor. Curr. Opin. Microbiol. 2013; 16:118–124. [DOI] [PubMed] [Google Scholar]
- 4. Grylak-Mielnicka A., Bidnenko V., Bardowski J., Bidnenko E.. Transcription termination factor Rho: a hub linking diverse physiological processes in bacteria. Microbiology. 2016; 162:433–447. [DOI] [PubMed] [Google Scholar]
- 5. Kriner M.A., Sevostyanova A., Groisman E.A.. Learning from the leaders: gene regulation by the transcription termination factor Rho. Trends Biochem. Sci. 2017; 41:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitra P., Ghosh G., Hafeezunnisa M., Sen R.. Rho protein: roles and mechanisms. Annu. Rev. Microbiol. 2017; 71:687–709. [DOI] [PubMed] [Google Scholar]
- 7. Cardinale C.J., Washburn R.S., Tadigotla V.R., Brown L.M., Gottesman M.E., Nudler E.. Termination factor Rho and its cofactors NusA and NusG silence foreign DNA in E. coli. Science. 2008; 320:935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Washburn R.S., Gottesman M.E.. Transcription termination maintains chromosome integrity. Proc. Natl. Acad. Sci. U.S.A. 2011; 108:792–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menouni R., Champ S., Espinosa L., Boudvillain M., Ansaldi M.. Transcription termination controls prophage maintenance in Escherichia coli genomes. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:14414–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bossi L., Schwartz A., Guillemardet B., Boudvillain M., Figueroa-Bossi N.. A role for Rho-dependent polarity in gene regulation by a noncoding small RNA. Genes Dev. 2012; 26:1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Proshkin S., Mironov A., Nudler E.. Riboswitches in regulation of Rho-dependent transcription termination. Biochim. Biophys. Acta Gene Regul. Mech. 2014; 1839:974–977. [DOI] [PubMed] [Google Scholar]
- 12. Hollands K., Sevostiyanova A., Groisman E.A.. Unusually long-lived pause required for regulation of a Rho-dependent transcription terminator. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:E1999–E2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kriner M.A., Groisman E.A.. The bacterial transcription termination factor Rho coordinates Mg2+ homeostasis with translational signals. J. Mol. Biol. 2015; 427:3834–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gall A.R., Datsenko K.A., Figueroa-bossi N., Bossi L., Masuda I., Hou Y., Csonka L.. Mg2+ regulates transcription of mgtA in Salmonella typhimurium via translation of proline codons during synthesis of the MgtL peptide. Proc. Natl. Acad. Sci. U.S.A. 2016; 113:15096–15101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bastet L., Chauvier A., Singh N., Lussier A., Wade J.T., Lamontagne A., Prevost K., Mass E., Lafontaine A.. Translational control and Rho-dependent transcription termination are intimately linked in riboswitch regulation. Nucleic Acids Res. 2017; 45:7474–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters J.M., Mooney R.A., Grass J.A., Jessen E.D., Tran F., Landick R.. Rho and NusG suppress pervasive antisense transcription in Escherichia coli. Genes Dev. 2012; 26:2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicolas P., Mäder U., Dervyn E., Rochat T., Leduc A., Pigeonneau N., Bidnenko E., Marchadier E., Hoebeke M., Aymerich S. et al. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science. 2012; 335:1103–1106. [DOI] [PubMed] [Google Scholar]
- 18. Mäder U., Nicolas P., Depke M., Pané-Farré J., Debarbouille M., van der Kooi-Pol M.M., Guérin C., Dérozier S., Hiron A., Jarmer H. et al. Staphylococcus aureus transcriptome architecture: from laboratory to infection-mimicking conditions. PLOS Genet. 2016; 12:e1005962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bidnenko V., Nicolas P., Grylak-Mielnicka A., Delumeau O., Auger S., Aucouturier A., Guerin C., Francis R., Bardowski J., Aymerich S. et al. Termination factor Rho: from the control of pervasive transcription to cell fate determination in Bacillus subtilis. PLoS Genet. 2017; 13:e1006909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Botella L., Vaubourgeix J., Livny J., Schnappinger D.. Depleting Mycobacterium tuberculosis of the transcription termination factor Rho causes pervasive transcription and rapid death. Nat. Commun. 2017; 8:14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harinarayanan R., Gowrishankar J.. Host factor titration by chromosomal R-loops as a mechanism for runaway plasmid replication in transcription termination-defective mutants of Escherichia coli. J. Mol. Biol. 2003; 332:31–46. [DOI] [PubMed] [Google Scholar]
- 22. Gowrishankar J., Harinarayanan R.. Why is transcription coupled to translation in bacteria. Mol. Microbiol. 2004; 54:598–603. [DOI] [PubMed] [Google Scholar]
- 23. Leela J.K., Syeda A.H., Anupama K., Gowrishankar J.. Rho-dependent transcription termination is essential to prevent excessive genome-wide R-loops in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gowrishankar J., Leela J.K., Anupama K.. R-loops in bacterial transcription: their causes and consequences. Transcription. 2013; 4:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomason M.K., Storz G.. Bacterial antisense RNAs: how many are there and what are they doing. Annu. Rev. Genet. 2011; 44:167–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sesto N., Wurtzel O., Archambaud C., Sorek R., Cossart P.. The excludon: a new concept in bacterial antisense RNA-mediated gene regulation. Nat. Rev. Microbiol. 2013; 11:75–82. [DOI] [PubMed] [Google Scholar]
- 27. Lybecker M., Bilusic I., Raghavan R.. Pervasive transcription: detecting functional RNAs in bacteria. Transcription. 2014; 5:e944039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wade J.T., Grainger D.C.. Pervasive transcription: illuminating the dark matter of bacterial transcriptomes. Nat. Rev. Microbiol. 2014; 12:647–653. [DOI] [PubMed] [Google Scholar]
- 29. Grainger D.C. The unexpected complexity of bacterial genomes. Microbiology. 2016; 162:1167–1172. [DOI] [PubMed] [Google Scholar]
- 30. Mars R.A.T., Nicolas P., Denham E.L., van Dijl J.M.. Regulatory RNAs in Bacillus subtilis: a Gram-positive perspective on bacterial RNA-mediated regulation of gene expression. Microbiol. Mol. Biol. Rev. 2016; 80:1029–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lloréns-Rico V., Cano J., Kamminga T., Gil R., Latorre A., Chen W.-H., Bork P., Glass J.I., Serrano L., Lluch-Senar M.. Bacterial antisense RNAs are mainly the product of transcriptional noise. Sci. Adv. 2016; 2:e1501363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelechano V., Steinmetz L.M.. Gene regulation by antisense transcription. Nat. Genet. 2013; 14:880–893. [DOI] [PubMed] [Google Scholar]
- 33. Khorkova O., Myers A.J., Hsiao J., Wahlestedt C.. Natural antisense transcripts. Hum. Mol. Genet. 2014; 23:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun Y., Li D., Zhang R., Peng S., Zhang G., Yang T., Qian A.. Strategies to identify natural antisense transcripts. Biochimie. 2017; 132:131–151. [DOI] [PubMed] [Google Scholar]
- 35. Dornenburg J.E., DeVita A.M., Palumbo M.J., Wade J.T.. Widespread antisense transcription in Escherichia coli. Mbio. 2010; 1:e00024-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raghavan R., Sloan D.B., Ochman H.. Antisense transcription is pervasive but rarely conserved in enteric bacteria. Mbio. 2012; 3:e00156-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawano M., Storz G., Rao B.S., Rosner J.L., Martin R.G.. Detection of low-level promoter activity within open reading frame sequences of Escherichia coli. Nucleic Acids Res. 2005; 33:6268–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimada T., Yamazaki Y., Tanaka K., Ishihama A.. The whole set of constitutive promoters recognized by RNA polymerase RpoD holoenzyme of Escherichia coli. PLoS One. 2014; 9:e90447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wade J.T. Where to begin? Mapping transcription start sites genome-wide in Escherichia coli. J. Bacteriol. 2015; 197:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomason M.K., Bischler T., Eisenbart S.K., Förstner K.U., Zhang A., Herbig A., Nieselt K., Sharma C.M., Storz G.. Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J. Bacteriol. 2015; 197:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamberte L.E., Baniulyte G., Singh S.S., Stringer A.M., Bonocora R.P., Stracy M., Kapanidis A.N., Wade J.T., Grainger D.C.. Horizontally acquired AT-rich genes in Escherichia coli cause toxicity by sequestering RNA polymerase. Nat. Microbiol. 2017; 2:16249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wade J.T., Grainger D.C.. Spurious transcription and its impact on cell function. Transcription. 2018; doi:10.1080/ 21541264. 2017.1381794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carles-Kinch K., George J.W., Kreuzer K.N.. Bacteriophage T4 UvsW protein is a helicase involved in recombination, repair and the regulation of DNA replication origins. EMBO J. 1997; 16:4142–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dudas K.C., Kreuzer K.N.. UvsW protein regulates bacteriophage T4 origin-dependent replication by unwinding R-loops. Mol. Cell. Biol. 2001; 21:2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller JH. A Short Course in Bacterial Genetics: a Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. 1992; NY: Cold Spring Harbor Lab Press. [Google Scholar]
- 46. Choy H.E., Adhya S.. RNA polymerase idling and clearance in gal promoters: use of supercoiled minicircle DNA template made in vivo. Proc. Natl. Acad. Sci. U.S.A. 1993; 90:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wahba L., Gore S.K., Koshland D.. The homologous recombination machinery modulates the formation of RNA–DNA hybrids and associated chromosome instability. Elife. 2013; 2:e00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwab R.A., Nieminuszczy J., Shah F., Langton J., Martinez D.L., Liang C., Cohn M.A., Gibbons R.J., Deans A.J., Niedzwiedz W.. The Fanconi anemia pathway maintains genome stability by coordinating replication and transcription. Mol. Cell. 2015; 60:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stork C.T., Bocek M., Crossley M.P., Sollier J.. Co-transcriptional R-loops are the main cause of estrogen-induced DNA damage. Elife. 2016; 5:e17548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K.. Short Protocols in Molecular Biology. 1995; 3rd ednNY: Wiley. [Google Scholar]
- 51. Ko M., Huang Y., Jankowska A.M., Pape U.J., Tahiliani M., Bandukwala H.S., An J., Lamperti E.D., Koh K.P., Ganetzky R. et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010; 468:839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aiba H., Adhya S., de Crombrugghe B.. Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem. 1981; 256:11905–11910. [PubMed] [Google Scholar]
- 53. Bury-Moné S., Nomane Y., Reymond N., Barbet R., Jacquet E., Imbeaud S., Jacq A., Bouloc P.. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 2009; 5:e1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Larson M.H., Mooney R. a, Peters J.M., Windgassen T., Nayak D., Gross C.A., Block S.M., Greenleaf W.J., Landick R., Weissman J.S.. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science. 2014; 344:1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sedlyarova N., Shamovsky I., Bharati B.K., Epshtein V., Chen J., Gottesman S., Schroeder R., Nudler E.. sRNA-mediated control of transcription termination in E. coli. Cell. 2016; 167:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sambrook J., Russell D.. Molecular Cloning: a Laboratory Manual. 2001; 3rd ednNY: Cold Spring Harbor Lab Press. [Google Scholar]
- 57. Rajkumari K., Kusano S., Ishihama A., Mizuno T., Gowrishankar J.. Effects of H-NS and potassium glutamate on σS - and σ70 -directed transcription in vitro from osmotically regulated P1 and P2 promoters of proU in Escherichia coli. J. Bacteriol. 1996; 178:4176–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li X., Manley J.L.. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell. 2005; 122:365–378. [DOI] [PubMed] [Google Scholar]
- 59. Boguslawski S., Smith D., Michalak M., Mickelson K., Yehle C., Patterson W., Carrico R.. Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J. Immunol. Methods. 1986; 89:123–130. [DOI] [PubMed] [Google Scholar]
- 60. Tran L., van Baarsel J. A., Washburn R.S., Gottesman M.E., Miller J.H.. Single-gene deletion mutants of Escherichia coli with altered sensitivity to bicyclomycin, an inhibitor of transcription termination factor Rho. J. Bacteriol. 2011; 193:2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nichols R.J., Sen S., Choo Y.J., Beltrao P., Zietek M., Chaba R., Lee S., Kazmierczak K.M., Lee K.J., Wong A. et al. Phenotypic landscape of a bacterial cell. Cell. 2011; 144:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Itaya M., Omori A., Kanaya S., Crouch R.J., Tanaka T., Kondo K.. Isolation of RNase H genes that are essential for growth of Bacillus subtilis. J. Bacteriol. 1999; 181:2118–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tous C., Aguilera A.. Impairment of transcription elongation by R-loops in vitro. Biochem. Biophys. Res. Commun. 2007; 360:428–432. [DOI] [PubMed] [Google Scholar]
- 64. Belotserkovskii B.P., Hanawalt P.C.. Anchoring nascent RNA to the DNA template could interfere with transcription. Biophys. J. 2011; 100:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Belotserkovskii B., Soo Shin J., Hanawalt P.C.. Strong transcription blockage mediated by R-loop formation within a G-rich homopurine – homopyrimidine sequence localized in the vicinity of the promoter. Nucleic Acids Res. 2017; 45:6589–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saxena S., Gowrishankar J.. Modulation of Rho-dependent transcription termination in Escherichia coli by the H-NS family of proteins. J. Bacteriol. 2011; 193:3832–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shashni R., Qayyum M.Z., Vishalini V., Dey D., Sen R.. Redundancy of primary RNA-binding functions of the bacterial transcription terminator Rho. Nucleic Acids Res. 2014; 42:9677–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Valabhoju V., Agrawal S., Sen R.. Molecular basis of NusG-mediated regulation of Rho-dependent transcription termination in bacteria. J. Biol. Chem. 2016; 291:22386–22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hermsen R., Okano H., You C., Werner N., Hwa T.. A growth-rate composition formula for the growth of E. coli on co-utilized carbon substrates. Mol. Syst. Biol. 2015; 11:801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drolet M. Growth inhibition mediated by excess negative supercoiling: the interplay between transcription elongation, R-loop formation and DNA topology. Mol. Microbiol. 2006; 59:723–730. [DOI] [PubMed] [Google Scholar]
- 71. Webb M.R., Plank J.L., Long D.T., Hsieh T.S., Kreuzer K.N.. The phage T4 protein UvsW drives Holliday junction branch migration. J. Biol. Chem. 2007; 282:34401–34411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nelson S.W., Benkovic S.J.. The T4 phage UvsW protein contains both DNA unwinding and strand annealing activities. J. Biol. Chem. 2007; 282:407–416. [DOI] [PubMed] [Google Scholar]
- 73. Nelson S.W., Benkovic S.J.. Response of the bacteriophage T4 replisome to non-coding lesions and regression of a stalled replication fork. J. Mol. Biol. 2011; 401:743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Manosas M., Perumal S.K., Bianco P., Ritort F., Benkovic S.J., Croquette V.. RecG and UvsW catalyse robust DNA rewinding critical for stalled DNA replication fork rescue. Nat. Commun. 2013; 4:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Washburn R., Gottesman M.. Regulation of transcription elongation and termination. Biomolecules. 2015; 5:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ginno P.A., Lim Y.W., Lott P.L., Korf I., Chédin F.. GC skew at the 5′ and 3′ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 2013; 23:1590–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chan Y.A., Aristizabal M.J., Lu P.Y.T., Luo Z., Hamza A., Kobor M.S., Stirling P.C., Hieter P.. Genome-wide profiling of yeast DNA:RNA hybrid prone sites with DRIP-Chip. PLoS Genet. 2014; 10:e1004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. El Hage A., Webb S., Kerr A., Tollervey D.. Genome-wide distribution of RNA-DNA hybrids identifies RNase H targets in tRNA genes, retrotransposons and mitochondria. PLoS Genet. 2014; 10:e1004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wahba L., Costantino L., Tan F.J., Zimmer A., Koshland D.. S1-DRIP-seq identifies high expression and polyA tracts as major contributors to R-loop formation. Genes Dev. 2016; 30:1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sanz L.A., Hartono S.R., Lim Y.W., Steyaert S., Rajpurkar A., Ginno P.A., Xu X., Chédin F.. Prevalent, dynamic, and conserved R-loop structures associate with specific epigenomic signatures in mammals. Mol. Cell. 2017; 63:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gan W., Guan Z., Liu J., Gui T., Shen K., Manley J.L., Li X.. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev. 2011; 25:2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kuzminov A. When DNA topology turns deadly—RNA polymerases dig in their R-loops to stand their ground: new positive and negative (super)twists in the replication-transcription conflict. Trends Genet. 2018; 34:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wimberly H., Shee C., Thornton P.C., Sivaramakrishnan P., Rosenberg S.M., Hastings P.J.. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nat. Commun. 2013; 4:2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gowrishankar J. End of the beginning: elongation and termination features of alternative modes of chromosomal replication initiation in bacteria. PLoS Genet. 2015; 11:e1004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Groh M., Gromak N.. Out of balance: R-loops in human disease. PLoS Genet. 2014; 10:e1004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sollier J., Cimprich K.A.. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 2015; 25:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Santos-Pereira J.M., Aguilera A.. R loops: new modulators of genome dynamics and function. Nat. Rev. Genet. 2015; 16:583–597. [DOI] [PubMed] [Google Scholar]
- 88. Richard P., Manley J.L.. R loops and links to human disease. J. Mol. Biol. 2017; 429:3168–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stirling P.C., Hieter P.. Canonical DNA repair pathways influence R-loop driven genome instability. J. Mol. Biol. 2017; 429:3132–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Song C., Hotz-Wagenblatt A., Voit R., Grummt I.. SIRT7 and the DEAD-box helicase DDX21 cooperate to resolve genomic R loops and safeguard genome stability. Genes Dev. 2017; 31:1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-Seq data generated in this study are available at GEO https://www.ncbi.nlm.nih.gov/geo/ under accession number GSE103937.