Introduction
Acquired idiopathic generalized anhidrosis (AIGA) is a rare disease with approximately 100 reported cases worldwide, many of which occurred in Asia, especially in Japan.1
To diagnose AIGA, other causes of anhidrosis (medications; physical agents; and dermatologic, neurologic, metabolic, and endocrine disorders), must be ruled out.
Three subtypes of AIGA with different pathologic conditions can be distinguished: sweat gland failure, sudomotor neuropathy, and idiopathic pure sudomotoric failure.1, 2 We report a case of AIGA in an Austrian patient. At present, only a few cases have been reported in Europe3, 4, 5 and the United States.6, 7, 8
Case report
A 30-year old, white, athletic man presented with progressive lack of body sweat production, pruritus, and urticaria. During exercise or rest, when surrounding temperatures were >24°C, he felt dizzy and weak, overheated, and required more time to recover after exertion. The frequency of his physical training sessions was reduced because of his symptoms.
The patient's medical and family history were inconspicuous, and previous treatment with any medication was denied. Dermatologic, neurologic, and internal clinical statuses were normal. Cranial and spinal magnetic resonance imaging did not indicate any neurologic disorders. Cardiovascular autonomic reflex screening revealed postural orthostatic tachycardia syndrome and cardioinhibitory reflex syncope.
Laboratory examinations (total and differential blood count, electrolytes, renal and liver function tests, blood glucose level, thyroid stimulating hormone, serum immunoglobulin E level, and carcinoembryonic antigen) were all within the normal ranges. No autoantibodies to SS-A/Ro, SS-B/La, and ganglionic α3 acetylcholine receptors were detected. Biochemical analysis revealed normal enzyme activity of α-galactosidase, ruling out Morbus Fabry. Biopsies from affected areas on the dorsum of the hands and the abdomen showed intact sweat glands with slight CD3+ lymphocytic infiltration.
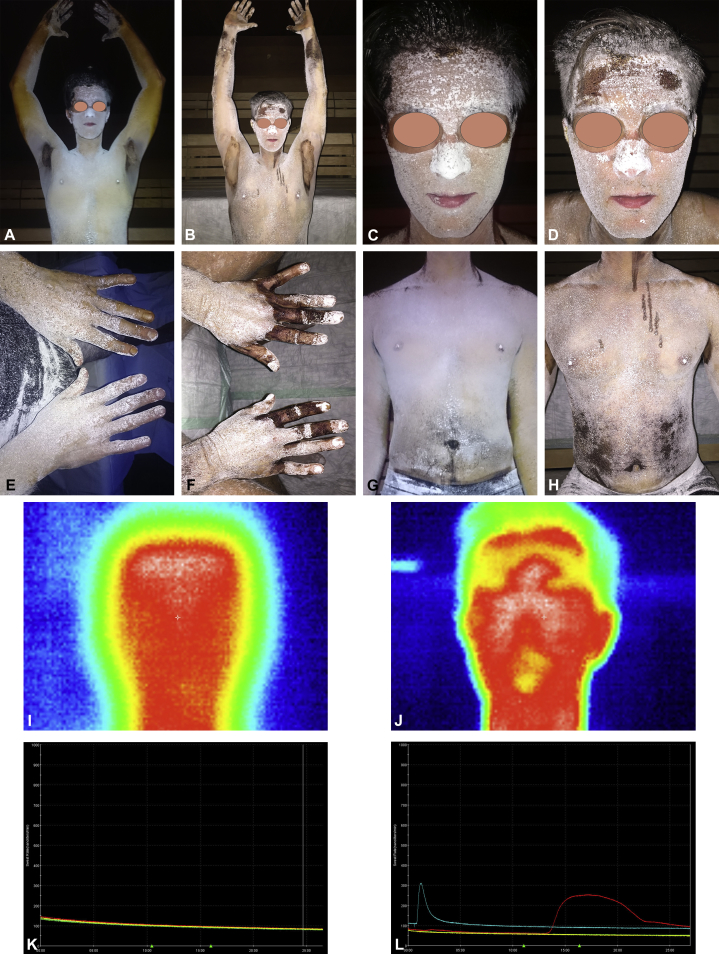
Anhidrosis was confirmed with a qualitative thermoregulatory sweat test (Minor test) combined with thermography and the quantitative sudomotor axon reflex test (QSART). The Minor test confirmed generalized anhidrosis with sweat production only on palms and armpits (Fig 1, A, C, E, and G). QSART showed a severe sudomotoric function deficiency without sweat production (Fig 1, K).
Fig 1.
Minor test, thermography, and quantitative sudomotor axon reflex test (QSART) before and after treatment. A-H, Minor test. After application of potassium iodide solution and starch powder, the patient was exposed to high temperature (40°C) and 50% humidity (conditions in sauna) for 30 minutes. A, C, E, and G, Patient before treatment. Sweat production only on palms and armpits. B, D, F, and H, Patient after treatment. New areas of sweat production on forearms, forehead, dorsum of hands, and abdomen. The linear streaks visible on the chest (H) represent a performance artefact of the Minor test. The potassium iodide solution was not completely dry before the starch powder was applied. I and J, Thermography before (I) and after (J) treatment. K and L, QSART. K, Before treatment, no sweat production on forearm (red line), proximal lower leg (blue line), distal lower leg (green line), and foot (yellow line). L, After treatment, normal sweat production of the forearm. The amplitude of the blue line is attributed to a measurement artefact.
The diagnostic procedures we used ruled out other causes of anhidrosis and confirmed AIGA, subtype idiopathic pure sudomotor failure (IPSF).
After receiving three cycles of high dose methylprednisolone (1 g on each of 3 consecutive days given at 4-week intervals) the patient noticed an increase in body sweat production. The Minor test showed new areas of sweat production on both forearms, forehead, dorsum of hands, and abdomen (Fig 1, B, D, F, and H), and thermography demonstrated cooling down of body surface temperature in areas of new sweat production (Fig 1, I and J). QSART showed normal sweat production on the left forearm (Fig 1, L). The patient's subjective well-being improved, and he started exercising again.
Discussion
Our patient had the IPSF subtype, the seemingly most common form of AIGA diagnosed. Autoimmunologic mechanisms leading to defective cholinergic receptors of the eccrine sweat glands or defective interaction between acetylcholine transmission and cholinergic receptors are thought to play a role in the pathogenesis of IPSF.
The indicators for IPSF seen in our patient were normal histopathology of sweat glands with CD3+ lymphocyte infiltration, early and sudden onset of symptoms with sweating on palms and armpits only (Fig 1, A), episodes of cholinergic urticaria, and marked response to systemic steroids.2
An explanation for continued sweating of armpits in IPSF might be that only eccrine sweat glands with cholinergic innervation are affected. Apocrine sweat glands of the armpits are under adrenergic control and are therefore not involved. Eccrine sweat glands of palms and soles seem to be under both adrenergic and cholinergic control9 and might be only partly affected. According to the literature, 50% of patients with IPSF have episodes of cholinergic urticaria.2 Although the exact pathomechanisms of both disease patterns are not known, acetylcholine seems to play an important role.10
Although intravenous high-dose steroid treatment in our patient proved effective, there is no definite evidence that administration of corticosteroids is effective for AIGA. Currently, treatment of AIGA with steroids is recommended on the basis of findings presented in several case reports, but no randomized controlled trials examining the efficacy of steroids in AIGA have been preformed until recently.1
According to Nakazato et al,2 78% of patients with IPSF respond to treatment with systemic glucocorticoids. However, treatment modalities with systemic steroids reported in the literature vary widely with regard to dose, form of application, and duration of treatment.1
Despite initial efficacy of treatment, relapse rates of 37.5% within 6-12 months after treatment initiation have been reported.3 In case of relapse, another systemic steroid cycle can be administered. Alternatively, there are isolated reports of successful treatment with cyclosporine,11 antihistamines,12 and intravenous immunoglobulins.13
Even though the number of cases of AIGA reported in North America and Europe is low, possibly due to underreporting, clinicians should be aware of it because AIGA can have a severe impact on daily life and sporting activities, especially in young, athletic persons.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Munetsugu T., Fujimoto T., Oshima Y. Revised guideline for the diagnosis and treatment of acquired idiopathic generalized anhidrosis in Japan. J Dermatol. 2017;44:394–400. doi: 10.1111/1346-8138.13649. [DOI] [PubMed] [Google Scholar]
- 2.Nakazato Y., Tamura N., Ohkuma A. Idiopathic pure sudomotor failure: anhidrosis due to deficits in cholinergic transmission. Neurology. 2004;63:1476–1480. doi: 10.1212/01.wnl.0000142036.54112.57. [DOI] [PubMed] [Google Scholar]
- 3.Palm F., Löser C., Gronau W. Successful treatment of acquired idiopathic generalized anhidrosis. Neurology. 2007;68:532–533. doi: 10.1212/01.wnl.0000253221.41124.46. [DOI] [PubMed] [Google Scholar]
- 4.Wolinia U. Acquired idiopathic generalized anhidrosis. J Dermatol Case Rep. 2014;8(4):120–121. doi: 10.3315/jdcr.2014.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Villaverde R., Sánchez Cano D. Acquired idiopathic generalized anhidrosis. Actas Dermasifiliogr. 2006;97:551–552. doi: 10.1016/s0001-7310(06)73464-0. [DOI] [PubMed] [Google Scholar]
- 6.Brantley E., Mutasim D.F., Heaton C. Acquired idiopathic generalized anhidrosis: case report. Cutis. 2011;87(1):21–23. [PubMed] [Google Scholar]
- 7.Kay D., Maibach H. Pruritus and acquired anhidrosis - two unusual cases. Arch Dermatol. 1969;100(3):291–293. [PubMed] [Google Scholar]
- 8.Weigand D., Everett M. Acquired generalized anhidrosis. Arch Dermatol. 1966;93(4):443–445. [PubMed] [Google Scholar]
- 9.Uno H. Sympathetic innervation of the sweat glands and piloarrector muscle of macaques and human being. J Invest Dermatol. 1977;69:112–120. doi: 10.1111/1523-1747.ep12497915. [DOI] [PubMed] [Google Scholar]
- 10.Bito T., Sawada Y., Tokura Y. Pathogenesis of cholinergic urticaria in relation to sweating. Allergol Int. 2012;61:539–544. doi: 10.2332/allergolint.12-RAI-0485. [DOI] [PubMed] [Google Scholar]
- 11.Fujita K., Hatta K. Acquired generalized anhidrosis: review of the literature and report of a case with lymphocytic hidradenitis and sialadenitis successfully treated with cyclosporine. Dermatology. 2013;227:270–277. doi: 10.1159/000355332. [DOI] [PubMed] [Google Scholar]
- 12.Suma A., Murota H., Kitaba S. Idiopathic pure sudomotor failure responding to oral antihistamine with sweating activities. Acta Derm Venereol. 2014;94:723–724. doi: 10.2340/00015555-1820. [DOI] [PubMed] [Google Scholar]
- 13.Masuda T., Obayashi K., Udea M. Therapeutic effects and prevention of recurrence of acquired idiopathic generalized anhidrosis via i.v. immunoglobulin treatment. J Dermatol. 2016;43:336–337. doi: 10.1111/1346-8138.13182. [DOI] [PubMed] [Google Scholar]