Case report
A 69-year-old gentleman was referred to our clinic for a new diffuse erythematous pruritic rash that initially developed on his left chest and rapidly spread to his face, dorsal hands, and lower legs over the course of 2 weeks. His medical history was significant for type II diabetes mellitus, hypertension, and nonmelanoma skin cancer. The initial biopsy showed mildly elongate rete with blunted tips and diffuse parakeratosis with loss of the granular cell layer. There was minimal spongiosis; no basement membrane zone changes or dyskeratotic cells were seen. Only a mild, superficial, dermal, perivascular lymphocytic infiltrate was present. No eosinophils were seen. These findings were consistent with psoriasiform dermatitis. The patient was subsequently started on clobetasol 0.05% ointment, prednisone, and methotrexate, given the extent of the disease and concern for drug eruption.
At his subsequent visit, he was markedly worse, demonstrating an exfoliative erythroderma and severe pruritus (Fig 1). He required admission to the hospital for treatment with topical steroid wet wraps. Repeat biopsies showed changes similar to the first. In addition, a focus with dilated dermal papillae, thinning of the suprapapillary plates, and a few neutrophils in the parakeratotic stratum corneum were found (Fig 2). These findings were diagnostic of psoriasis.
Fig 1.
Presentation of bilateral erythroderma on trunk and upper extremities of patient with paraneoplastic psoriasis treated for psoriasiform dermatitis.
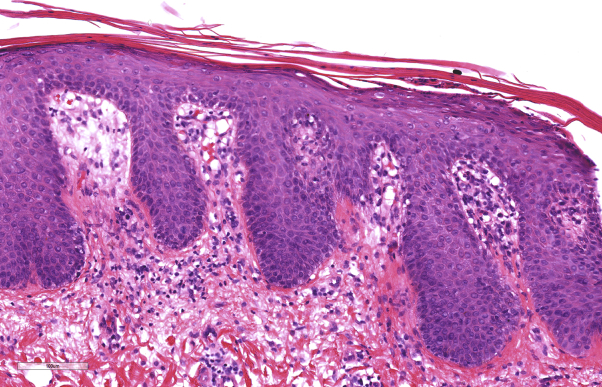
Fig 2.
Skin biopsy showing elongation of rete with thickened tips, dilated and edematous dermal papillae, parakeratosis with loss of the granular cell layer, and parakeratosis with neutrophils. These are diagnostic histopathologic changes of psoriasis.
Despite 5 days of inpatient treatment, he showed minimal improvement. He was subsequently given cyclosporine and acitretin and ultimately transitioned to adalimumab, which resulted in only marginal improvement of his symptoms.
Given the patient's minimal improvement, further investigation was undertaken to search for a possible underlying trigger of his condition. Age-appropriate cancer screening was performed and revealed significantly elevated prostate-specific antigen (8.4 ng/mL), which was increased from prior measurements. Prostate biopsy revealed a high-volume, high-risk prostatic adenocarcinoma with a Gleason score of 9 (4+5). The patient was treated with an 8-week course of external beam radiation therapy (79 Gy) and 2-year androgen-deprivation therapy with leuprolide and bicalutamide.
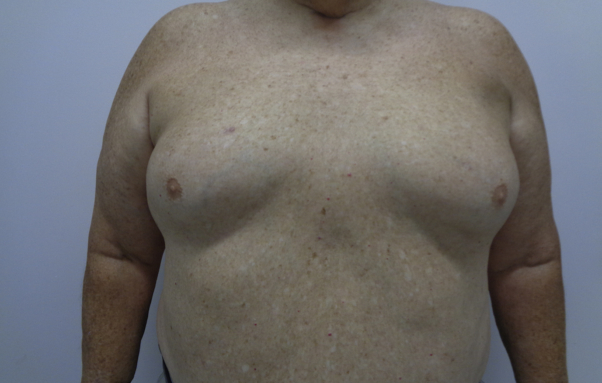
Three weeks after starting radiation therapy, the patient began for the first time since onset of disease to demonstrate appreciable improvement of erythroderma. By the completion of his radiation treatment, his psoriasis had almost completely resolved. As he started to improve, systemic therapies were slowly tapered. Sixteen months after completing radiation therapy, the patient continues to have clear skin (Fig 3) and to be off of all topical and systemic psoriasis medications. In addition, he remains off of androgen-deprivation therapy and has had no recurrence of his prostate cancer.
Fig 3.
Complete clearance without evidence of previous dermatitis on trunk and upper extremities.
Discussion
Paraneoplastic syndromes are diseases associated with malignancy but not attributable to direct tumor invasion or to adverse effects from infection, nutritional deficits, or treatment.1 Prostate cancer is the second most common urologic malignancy to be associated with paraneoplastic syndromes after renal cell carcinoma.2 These syndromes have typically been described in the setting of advanced prostate cancer, and they resolve after treatment of the malignancy.1 Although rare, there have been reported cases of paraneoplastic psoriasis, which has been shown to occur at onset of malignancy, improve after tumor treatment, and recur with tumor relapse or metastasis.3
Skin biopsy findings in patients with erythroderma might not be diagnostic, and distinction between papulosquamous disorders might sometimes only be made after viewing multiple biopsies over time. Such was the case in this patient.4 Two of 3 biopsies had changes overlapping with psoriasis and pityriasis rubra pilaris, including regular elongation of rete, diffuse parakeratosis, loss of the granular cell layer, and follicular hyperkeratosis and parakeratosis. One biopsy, however, showed changes more typical of psoriasis, with elongated rete having thickened tips, widened dermal papillae, papillary capillary ectasia, and neutrophils in the parakeratotic stratum corneum. Acantholysis, a finding of pityriasis rubra pilaris, was not observed in any of the skin biopsies. The clinical and pathologic findings in this case were more in keeping with paraneoplastic psoriasis.5
In our case, the patient developed recalcitrant psoriasis presenting as a severe exfoliative erythroderma not responsive to several therapeutics. The patient's recalcitrant psoriasis suggested that an underlying malignancy could be present which ultimately led to the screening exams that helped diagnose his prostate cancer. To the best of our knowledge, there have been no reported biopsy-proven cases of paraneoplastic psoriasis associated with prostate cancer in a concurrent and parallel fashion. Previous studies have shown that malignant tumors can produce cytokines such as tumor necrosis factor-α, transforming growth factor-α, and epidermal growth factor, which are involved in the pathogenesis of psoriasis and have potent stimulatory effects on epidermal keratinocytes.6, 7 This case demonstrates that an underlying malignancy should be considered in the setting of erythrodermic psoriasis refractory to aggressive treatment. Our patient continues to remain disease free from both a dermatologic and neoplastic standpoint. However, in the event of a psoriatic flare, recurrence of his prostate cancer will need to be investigated.
Footnotes
Dr Pearlstein and Ms Cadmus contributed to this work equally.
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Hong M., Kong J., Namdarian B. Paraneoplastic syndromes in prostate cancer. Nat Rev Urol. 2010;7(12):681–692. doi: 10.1038/nrurol.2010.186. [DOI] [PubMed] [Google Scholar]
- 2.Sacco E., Pinto F., Sasso F. Paraneoplastic syndromes in patients with urological malignancies. Urol Int. 2009;83(1):1–11. doi: 10.1159/000224860. [DOI] [PubMed] [Google Scholar]
- 3.Tsunemi Y., Ihn H., Idezuki T., Okochi H., Tamaki K. Psoriasis guttata in association with hepatocellular carcinoma. Acta Derm Venereol. 2003;83(1):70–71. doi: 10.1080/00015550310002846. [DOI] [PubMed] [Google Scholar]
- 4.Walsh N.M., Prokopetz R., Tron V.A. Histopathology in erythroderma: review of a series of cases by multiple observers. J Cutan Pathol. 1994;21(5):419–423. doi: 10.1111/j.1600-0560.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomasini C., Aloi F., Solaroli C., Pippione M. Psoriatic erythroderma: a histopathologic study of forty-five patients. Dermatology. 1997;194(2):102–106. doi: 10.1159/000246075. [DOI] [PubMed] [Google Scholar]
- 6.Kano Y., Chiba M., Yagita A., Shiohara T. Complete resolution of psoriasis vulgaris after excision of thyroid cancer. Int J Dermatol. 2008;36(4):280–282. doi: 10.1111/j.1365-4362.1997.tb03044.x. [DOI] [PubMed] [Google Scholar]
- 7.Nickoloff B. The cytokine network in psoriasis. Arch Dermatol. 1991;27(6):871–884. [PubMed] [Google Scholar]