Introduction
The programmed death-1 protein (PD-1) pathway is an inhibitory mechanism exploited by tumors to evade destruction by the host immune system. Immunogenic cancers such as lymphoma and melanoma overexpress the PD-1 receptor ligand (PD-L1), which selectively inhibits the host T-cell–mediated antitumor response. Nivolumab, a humanized IgG4 anti–PD-1 antibody, stimulates the native T-cell response by inhibiting PD-1 receptor ligand binding to T cells,1 leading to impressive tumor control. This action is nonspecific, and immune-related adverse events (irAEs) are well described after checkpoint inhibition. Life-threatening immune-related skin toxicity is uncommon.1, 2, 3, 4 We report a case of fatal toxic epidermal necrolysis (TEN) secondary to nivolumab in lymphoma to highlight the challenges of managing this rare complication.
Case report
A 54-year-old man with heavily pretreated relapsed follicular lymphoma enrolled in a phase II clinical trial investigating nivolumab following initial therapy with corticosteroids and allopurinol. On day 10 of the first cycle of nivolumab (3 mg/kg) he developed mild conjunctivitis, visual loss, and a widespread erythematous maculopapular rash. He was admitted for high-dose intravenous methylprednisolone (1 g/d) and intravenous immunoglobulins. Despite this, by day 22 there were bullae and multiple erosions affecting 70% to 80% of body surface area (Figs 1 and 2). A clinical diagnosis of TEN was confirmed on skin biopsy. He stopped prophylactic medications (co-trimoxazole, allopurinol, fluconazole) because of a potential exacerbating effect on TEN. His SCORTEN score was 3, predicting a mortality rate of 32%.5 He continued supportive care, including intravenous methylprednisolone, and regular ophthalmology and dermatology review.
Fig 1.
Toxic epidermal necrolysis. Erosions to the lower back.
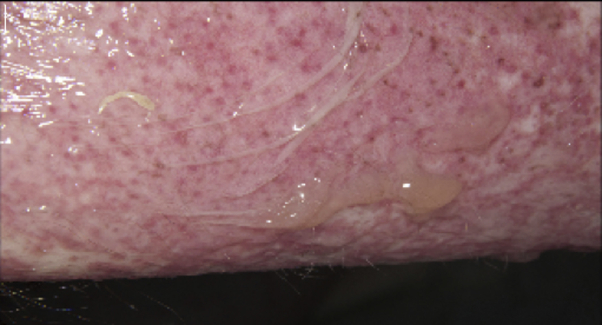
Fig 2.
Toxic epidermal necrolysis. Epidermal detachment and blistering to upper arm.
After initial improvement, he developed a hospital-acquired pneumonia and commenced intravenous tazocin on day 15. Computed tomography pulmonary angiogram confirmed right lower lobe consolidation. Staphylococcus and Candida were cultured from sputum. Inflammatory markers continued to increase on a background of lymphopenia, and oxygen requirements increased. Bronchoscopy showed features of airway ulceration caused by extension of TEN. He was transferred to intensive care. Initially, skin changes were nonprogressive with evidence of re-epithelization. However, he deteriorated with type 2 respiratory failure secondary to mucous plugging and was intubated and ventilated after an episode of carbon dioxide narcosis. TEN extended further, with increasing erythema, desquamation, and erosions involving approximately 90% body surface area. Aspergillus was cultured from bronchial washings and he commenced voriconazole, but multiorgan failure ensued and he died on day 58.
Discussion
TEN is a rare but life-threatening dermatologic emergency, resulting from CD8+ T lymphocytes inducing apoptosis of epithelial keratinocytes.5 An erythematous macular rash evolves with blistering and epidermal desquamation. Mucosal involvement of the gastrointestinal tract, respiratory tract, and genitalia is common. Visual loss caused by ulceration and adhesions can lead to long-term sequelae.5
A multinational European case-control study graded allopurinol as high risk for TEN, with co-trimoxazole and fluconazole implicated to a lesser degree.6 The latent period for TEN is usually less than 5 to 28 days.5 Because our patient had longer treatment (∼49 days of allopurinol and antimicrobial prophylaxis before TEN), in our opinion nivolumab was the most likely causative agent. It is unclear whether concomitant allopurinol and nivolumab increased risk in our patient, considering other reports of TEN mortality using concomitant allopurinol, rituximab, and bendamustine in B-cell lymphoma.7
A favorable safety profile has been reported for nivolumab in clinical trials, although there is a paucity of data for hematologic malignancies. The half-life of 17 to 21 days could lead to lasting toxicity. Low-grade rash was the most common adverse event (n = 5; 22%) reported in a recent phase I trial of nivolumab in relapsed Hodgkin lymphoma (n = 23).4 Similarly, a phase I study in 207 patients with solid tumor reported mostly mild drug-related adverse events including rash, gastrointestinal toxicity, fatigue, and arthralgia.2 No grade 3 to 4 skin toxicity was reported, even at higher doses (10 mg/kg).2
A randomized phase III trial compared nivolumab with chemotherapy in advanced melanoma patients after prior treatment with ipilimumab (an anticytotoxic T-lymphocyte–associated antigen 4 antibody) or a BRAF inhibitor.3 No grade 3 to 4 skin toxicity was observed in nivolumab-treated patients (n = 268).3
Few severe skin toxicity cases have been reported after therapeutic checkpoint inhibition. In the largest international randomized phase III study (n = 945), grade 3 to 4 rash was reported in only 2 cases of advanced-stage melanoma treated with combination nivolumab and ipilimumab.8 Three deaths caused by TEN are reported to date: 1 patient with chemotherapy-naïve advanced-stage non–small cell lung cancer treated with combination nivolumab and ipilimumab in a small phase 1 trial (n = 46),9 1 patient with metastatic melanoma refractory to ipilimumab who died after 2 doses of nivolumab,10 and 1 patient with metastatic melanoma who developed TEN after 3 cycles of nivolumab, the first cycle in combination with ipilimumab.11
It is unknown whether the type of malignancy or previous immunosuppressive treatments could predispose individuals to have severe irAEs. Immunosuppression caused by active lymphoma increases TEN risk, and our patient was also heavily pretreated with rituximab-containing immunochemotherapy and ibritumomab tiuxetan (Zevalin), a radiolabelled anti-CD20 therapy. Depletion of CD20+ B cells following CD20 directed therapy inhibits antigen-specific CD4+ T-cell expansion and response but does not affect CD8+ T-cells. Furthermore, B-cell regeneration over time prevents chronic CD20 depletion. This finding suggests that prior anti-CD20 therapy did not contribute to the pathogenesis of TEN in our patient.
Management of TEN is mainly supportive, and controversy exists regarding systemic treatment, including corticosteroids.5 Tumor necrosis factor is known to be an important inflammatory cytokine in immunotherapy-related reactions. Infliximab, an anti–tumor necrosis factor-α antibody, is used to treat severe IrAEs, such as colitis and pneumonitis but has not been widely used for skin toxicity.3, 8 There is limited evidence that intravenous immunoglobulins improve survival in TEN.5
Life-threatening skin toxicity may become more challenging in the context of new immunotherapies. In our patient, systemic steroids did not reverse or slow the disease process and was a contributing cause of death from opportunistic infection.
Novel checkpoint blockade offers a promising approach for patients with limited standard treatment options, but safety is poorly understood, especially in individuals who are immunocompromised or undergoing concomitant treatment with potentially overlapping toxicities. This case highlights the difficulty of managing life-threatening immune reactions and the competing threat of fatal opportunistic infection complicating high-dose steroid treatment of irAEs in immunosuppressed patients.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Rizvi N.A., Mazieres J., Planchard D. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J.R., Tykodi S.S., Chow L.Q.M. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber J.S., D'Angelo S.P., Minor D. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 4.Ansell S.M., Lesokhin A.M., Borrello I. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creamer D., Walsh S.A., Dziewulski P. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174(6):1194–1227. doi: 10.1111/bjd.14530. [DOI] [PubMed] [Google Scholar]
- 6.Mockenhaupt M., Viboud C., Dunant A. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128(1):35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 7.Fallon M.J., Heck J.N. Fatal Stevens-Johnson syndrome/toxic epidermal necrolysis induced by allopurinol-rituximab-bendamustine therapy. J Oncol Pharm Pract. 2015;21(5):388–392. doi: 10.1177/1078155214533368. [DOI] [PubMed] [Google Scholar]
- 8.Larkin J., Chiarion-Sileni V., Gonzalez R. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonia S.J., Gettinger S.N., Chow L.Q.M. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol. 2014;32(15) [Google Scholar]
- 10.Nayar N., Briscoe K., Penas P.F. Toxic Epidermal necrolysis-like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. 2016;39(3):149–152. doi: 10.1097/CJI.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 11.Vivar K.L., Deschaine M., Messina J. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44(4):381–384. doi: 10.1111/cup.12876. [DOI] [PubMed] [Google Scholar]