Pilomatrix carcinoma is a rare cutaneous malignancy with follicular matrix origin. Since the term pilomatrix carcinoma was first coined in 1980, more than 130 cases have been reported.1 The condition is also known as pilomatrical carcinoma, matrical carcinoma, malignant pilomatricoma, malignant pilomatrixoma, and calcifying epithelial carcinoma of Malherbe. In contrast to pilomatrixomas, pilomatrix carcinomas have locally aggressive behavior with a tendency toward recurrence and metastasis. Management options include surgery or radiation with close long-term follow-up. To our knowledge, Mohs micrographic surgery (MMS) has only been reported in 2 cases off the head and neck.2, 3 We report 2 facial cases treated with MMS.
Case 1
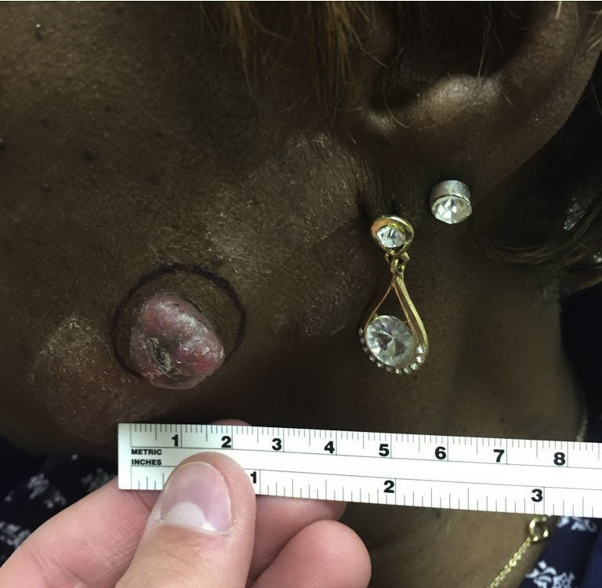
A 68-year-old African-American woman presented with a 5-month history of a 1.8- × 1.9-cm pedunculated nodule on her left cheek (Fig 1) with no palpable lymphadenopathy. She denied a history of skin cancer or radiation or family history of malignancy. She reported an unintended 20-pound weight loss over the last few months with decreased appetite, but otherwise review of systems was negative. Biopsy found a dermal tumor with biphasic nodules of basaloid epithelial cells, aggregates of eosinophilic keratinized cells with matrical differentiation, and broad zones of necrosis (Fig 2). The basaloid cells were markedly atypical with numerous mitoses and pleomorphism (Fig 3). No perineural or perivascular invasion was observed. A histologic diagnosis of pilomatrix carcinoma was made.
Fig 1.
Pilomatrix carcinoma on the left cheek (case 1).
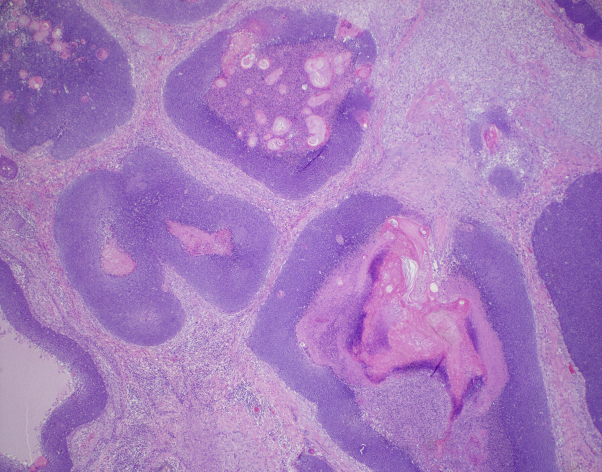
Fig 2.
Asymmetric multinodular dermal proliferation of basaloid cells and eosinophilic cells with zones of necrosis. (Hematoxylin-eosin stain; original magnification: ×20.)
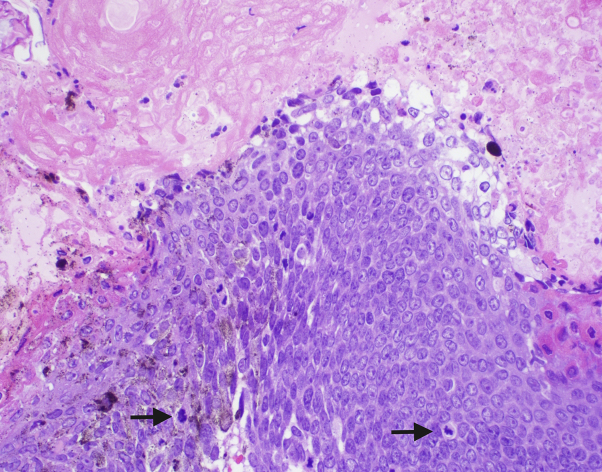
Fig 3.
High-power view of atypical basaloid cells with pleomorphism and frequent mitoses (arrows) with transition to eosinophilic cells showing matrical keratinization. (Hematoxylin-eosin stain; original magnification: ×200.)
A staging computed tomography (CT) scan showed an incidental multinodular thyroid goiter, which was confirmed as papillary thyroid carcinoma on biopsy, and the patient subsequently underwent a total thyroidectomy. Otherwise, the CT scan was unremarkable for metastasis. One stage of MMS with an initial 5-mm margin achieved histologic clearance. The wound was closed primarily, and there was no evidence of recurrence at 7.5-month follow-up.
Case 2
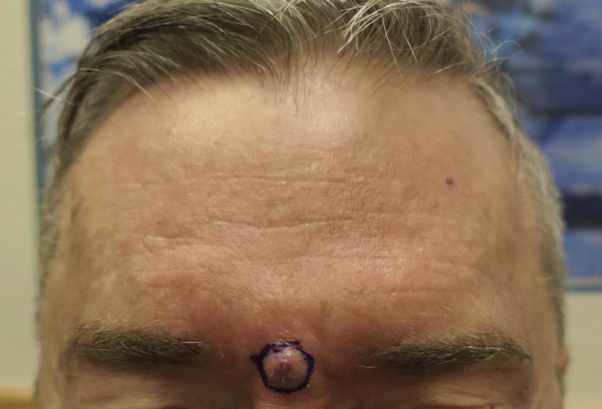
A 68-year-old white man with HIV (CD4 count >600 cells/μL, undetectable viral load) presented with a 4-month history of a 0.9- × 1.1-cm growing nodule on the mid–nasal root (Fig 4). He denied a history of skin cancers or radiation, or family history of malignancy. Concurrently, he was undergoing workup for hematuria, and early high-grade papillary urothelial carcinoma was diagnosed. Review of systems was otherwise negative. Biopsy found a pilomatrix carcinoma.
Fig 4.
Pilomatrix carcinoma on the mid–nasal root (case 2).
After the first stage of MMS with a 5-mm margin, the deep margin showed granulomatous inflammation without tumor, so a second stage of only depth was taken and confirmed clear margins. A linear closure was performed, and at 6-month follow-up there was no evidence of recurrence. CT imaging of head, neck, and chest did not show any evidence of metastatic disease at baseline and at 4 months postoperatively.
Discussion
Pilomatrix carcinoma is a rare tumor originating from the follicular matrix, like the benign pilomatrixoma. Of the more than 130 cases reported, the most common location is the head, followed by neck, back, extremities, chest, buttocks, inguinal region, and axilla. There is a male predominance with an 3:1 ratio, and most cases (81%) are in whites.1 It occurs most frequently in the sixth decade, although an age range from 2 to 93 years has been reported.3
The pathogenesis of pilomatrix carcinoma is unclear, in particular, whether it is a malignant transformation of pilomatrixoma or arises de novo. Mutations in the CTNNB1 gene occur in both, which allude to a possible common pathogenesis, but malignant transformation has not been proven.1, 4 Other contributing factors, such as actinic damage, have been proposed.1
Histologically, both pilomatrix carcinomas and pilomatrixomas show aggregates of basaloid cells and eosinophilic ghost cells, frequently with a transition zone in between them. In contrast to pilomatrixomas, pilomatrix carcinomas show more numerous mitotic figures, cellular atypia, a deeper infiltrating pattern, and areas of necrosis. Proliferating pilomatrixoma is a variant of pilomatrixoma with slight cellular atypia, few mitoses, and an expansive but symmetric growth pattern. The term aggressive or atypical proliferating pilomatrixoma has been used for the entity on the spectrum between proliferating pilomatrixoma and malignant pilomatrix carcinoma, where the growth pattern is more infiltrative and the degree of cellular atypia is more severe. Consequently, the histopathologic distinction between pilomatrix carcinoma and atypical proliferating pilomatrixoma can be unclear. To date, no distinguishing immunohistochemical marker has been found.1, 5
Although no standard management guidelines for pilomatrix carcinoma exist, surgical removal is ideal, but reported margins vary from 5 mm to 3 cm.1 The recurrence rate for wide local excision (WLE) has been reported as 23% compared with 83% for simple excisions, and is not dependent on anatomic location. Local recurrence happened within 2 months after WLE (5-mm margins) with clear margins on standard histologic sectioning, suggesting MMS may be advantageous over standard WLE in ensuring complete margin control.5 Regarding other treatments, radiation has been used as an adjuvant or monotherapy with varying results. Chemotherapy, such as intravenous paclitaxel, has not shown much success.3
The metastatic rate ranges between 13% and 16%, with the most common site being regional lymph nodes, followed by lung, bone, brain, and other viscera.1, 3 Metastatic risk increases with local recurrence but does not appear to depend on excision margins or tumor location.1 After initial diagnosis, a metastatic workup with imaging is commonly performed along with ongoing follow-up, although standard guidelines do not exist on the frequency or type of imaging and follow-up. An association between pilomatrix carcinoma and other malignancies has not been previously described, but, interestingly, our 2 patients were found to have concomitant neoplasms. Although this occurrence is intriguing, there are no other reports linking pilomatrix carcinoma and visceral malignancies.
Because pilomatrix carcinoma is easily identified with hematoxylin-eosin stain, and margin control is crucial given its aggressive nature, MMS may be an optimal treatment. MMS was first reported for a lesion on the back in 2004 with no recurrence at 5-month follow-up.2 In 2011, Melancon et al3 reported another tumor treated with 1 stage of MMS (8.5 mm initial margin) with no recurrence at 6 months.3 We report 2 additional cases of pilomatrix carcinoma treated with MMS and the first facial cases. Although these cases are all limited by short follow-up, given the high recurrence risk and potential for metastasis, we recommend a low threshold for considering MMS to achieve margin control for pilomatrix carcinomas.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Herrmann J.L., Allan A., Trapp K.M., Morgan M.B. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71(1):38–43. doi: 10.1016/j.jaad.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Sable D., Snow S. Pilomatrix carcinoma of the back treated by Mohs micrographic surgery. Dermatol Surg. 2004;30(8):1174–1176. doi: 10.1111/j.1524-4725.2004.30350.x. [DOI] [PubMed] [Google Scholar]
- 3.Melancon J.M., Tom W.L., Lee R.A., Jackson M., Brian Jiang S.I. Management of pilomatrix carcinoma: a case report of successful treatment with mohs micrographic surgery and review of the literature. Dermatol Surg. 2011;37(12):1798–1805. doi: 10.1111/j.1524-4725.2011.02170.x. [DOI] [PubMed] [Google Scholar]
- 4.Sassmannshausen J., Chaffins M. Pilomatrix carcinoma: a report of a case arising from a previously excised pilomatrixoma and a review of the literature. J Am Acad Dermatol. 2001;44:358–361. doi: 10.1067/mjd.2001.105474. [DOI] [PubMed] [Google Scholar]
- 5.Papadakis M., de Bree E., Floros N., Giannikaki E., Xekalou A., Manios A. Pilomatrix carcinoma: more malignant biological behavior than was considered in the past. Mol Clin Oncol. 2017:415–418. doi: 10.3892/mco.2017.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]