Introduction
Primary mucinous carcinoma of the skin (PMCS) is a rare malignant adnexal tumor with a high rate of local recurrence.1 Given its rarity, no standard of care for PMCS has been established. In general, surgical excision with a wide margin with or without regional lymph node dissection is the treatment of choice. Although distant metastases are infrequent, recurrent and metastatic PMCS are highly resistant to radiotherapy and chemotherapy,2, 3 requiring alternative treatment. In this report, we describe a case of repeated recurrences of PMCS with lymph node and lung metastases, which was successfully controlled by endocrine therapy with an aromatase inhibitor.
Case
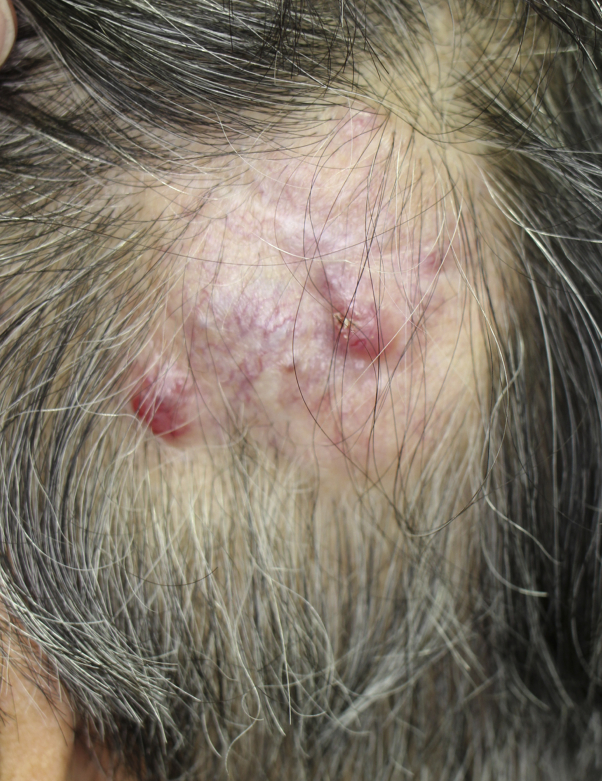
A 66-year-old postmenopausal Japanese woman with no history of skin or breast cancer visited a dermatology clinic for an asymptomatic red nodule of the left temporal region that had gradually increased in size over the past 5 years. Histopathologic examination of the completely excised nodule revealed a well-circumscribed mucinous lesion separated into compartments by strands of fibrous tissue extending from the dermis to the subcutaneous fat. In each compartment, basophilic neoplastic cells with nuclear atypia and mitosis, some of which were forming tubular lumens, were floating in the mucinous pool. Using postoperative evaluations, including [18-F]-fluorodeoxy-D-glucose (FDG) positron emission tomography–computed tomography (PET-CT), we failed to detect a primary tumor at a site other than the skin. Therefore, she was diagnosed with PMCS. Two years later, she was referred to our dermatology clinic for multiple red nodules in the left temporal region and cervical lymphadenopathy (Fig 1). Excision of the nodule, with a 2-cm horizontal margin and vertical margin including part of the skull, and cervical lymph node dissection (level V) revealed a recurrent PMCS with cervical lymph node metastasis.
Fig 1.
Clinical features of the first recurrence in patient with primary mucinous carcinoma of the skin. Multiple red nodules appeared in the left temporal region 2 years after the initial excision.
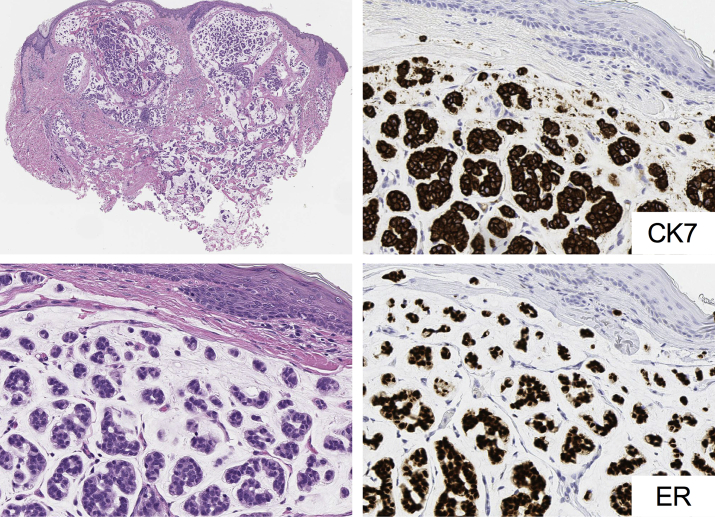
Sixteen months after the second operation, a left preauricular red nodule resembling previous cutaneous lesions was noted at follow-up. FDG PET-CT revealed cutaneous and subcutaneous masses, with FDG uptake in a cervical lymph node and the right lower lung lobe (lung segment S6) (Fig 2, A). Skin biopsy results of one of the cutaneous red nodules and results of the transbronchial biopsy of the lung nodule were histopathologically consistent with mucinous carcinoma (Fig 3). Immunohistochemical examination findings of the tumor cells were positive for cytokeratin 7 but negative for cytokeratin 20. The high Ki-67 proliferation index (∼60%) indicated aggressive clinical features. A coincidental onset of primary lung cancer was ruled out on the basis of negative staining results for thyroid transcription factor 1, anaplastic lymphoma kinase, and napsin A. Both skin and lung biopsy specimens showed estrogen receptor (ER) and progesterone receptor (PgR) positivity (Fig 3). Needle biopsy results of the cervical lymphadenopathy were consistent with metastatic adenocarcinoma. To rule out mucinous breast carcinoma, mammography and ultrasound were performed, and the findings were negative. Thus, she was diagnosed with repeated recurrences of PMCS with lymph node and distant lung metastases.
Fig 2.
Imaging of patient with primary mucinous carcinoma of the skin with [18F]-fluorodeoxy-D-glucose (FDG) positron emission tomography–computed tomography (PET-CT). A, Four months before the treatment with letrozole, FDG PET-CT demonstrated cutaneous (not shown) and subcutaneous (left red circular outline) masses, with FDG uptake in the left cervical lymph node and right lower lung lobe (S6) (middle and right red circular outline). B, Eight months after starting letrozole treatment. FDG uptake had decreased in the cutaneous and subcutaneous masses (left red circular outline) and the lung metastasis (right red circular outline). Moreover, FDG uptake of the cervical lymph node metastasis had disappeared (middle red circular outline).
Fig 3.
Histopathologic features of cutaneous recurrence of primary mucinous carcinoma of the skin. Well-circumscribed mucinous lesion separated into compartments by strands of fibrous tissue. In each compartment, basophilic neoplastic cells with nuclear atypia and mitosis, some forming tubular lumens, are seen floating in the mucinous lesion. Neoplastic cells are positive for cytokeratin 7 and strongly positive for estrogen receptor.
We initially proposed a total resection of the local recurrence, metastatic cervical lymph node, and lung metastasis, but she refused surgical treatments. Thus, the PMCS was deemed unresectable, and the treatment was discussed with the multidisciplinary tumor board and the clinical ethics committee of Chiba University Hospital. Because the cutaneous recurrence and lung metastasis were positive for ER and PgR, we decided to treat her with letrozole, an aromatase inhibitor, because endocrine therapy is a treatment for hormone receptor–positive breast cancer. No clinical symptoms related to the metastases were noted at this point, and the patient requested a treatment with the least adverse effects. Thus, we decided to omit radiotherapy and treat her with letrozole alone.
At baseline (4 months after the FDG PET-CT scan shown in Fig 2, A), the longest diameter of the lung metastasis had rapidly increased from 15 mm to 21 mm. After daily oral letrozole (2.5 mg) administration for 8 months, the lung metastasis diameter decreased to 17 mm. Eight months after starting letrozole, FDG uptake decreased in the lung metastasis and disappeared from the cervical lymph node metastasis (Fig 2, B). The patient continued letrozole treatment and has maintained at stable disease status for 11 months without any adverse events.
Discussion
Once the patient is diagnosed with PMCS, imaging studies, such as FDG PET-CT, are useful for detecting lymph node and distant organ metastases.4 While ∼30% of PMCS cases have local recurrences, metastasis to a distant organ is extremely rare, and only a few cases have been reported.3, 5, 6 However, distant metastases of PMCS are highly resistant to radiotherapy and chemotherapy.2, 3 Therefore, alternative treatments for advanced PMCS are needed.
Endocrine therapy with an aromatase inhibitor is an established treatment for hormone receptor–positive breast cancer.7 However, metastatic sites could have discordant expression of hormone receptors compared with the primary lesion. Therefore, performing biopsy and comparing the expression of hormone receptors, particularly ER, with the primary lesion is important before starting antiestrogenic therapy. Endocrine therapy generally has fewer adverse effects and toxicities than chemotherapy. The main adverse effect to be monitored during treatment with aromatase inhibitors is osteoporosis with fractures.8
On immunohistopathologic examination, PMCS lesions frequently express ER and PgR.9, 10 Therefore, PMCS might respond to endocrine therapies. Two previous case reports have described the benefits of aromatase inhibitors in combination with radiotherapy for advanced PMCS.5, 6 In line with these 2 case reports, we treated the distant metastasis of PMCS using an aromatase inhibitor and observed a positive response without any adverse events.
In conclusion, this case report, together with 2 previous ones,5, 6 demonstrates the benefits of the endocrine therapy with an aromatase inhibitor for hormone receptor–positive advanced PMCS. Thus, endocrine therapy can be considered for hormone receptor–positive PMCS as an alternative treatment to radiotherapy and chemotherapy owing to the efficacy of treatment and low incidence of adverse events. Furthermore, findings from breast cancer treatments suggest that endocrine therapy might be beneficial as a neoadjuvant therapy for PMCS, although further investigation is required. Given the rarity of the PMCS, further case reports and series of cases involving endocrine therapy are important factors in the development of a standard of care for PMCS.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Breiting L., Christensen L., Dahlstrom K. Primary mucinous carcinoma of the skin: a population-based study. Int J Dermatol. 2008;47:242–245. doi: 10.1111/j.1365-4632.2008.03558.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamalpour L., Brindise R.T., Nodzenski M. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380–384. doi: 10.1001/jamadermatol.2013.6006. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka M., Tanaka R., Hirabayashi K. Primary mucinous carcinoma of the skin: a case of metastasis after 10 years of disease-free interval. Eur J Plast Surg. 2009;32:189–193. doi: 10.1007/s00238-008-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitamura S., Hata H., Inamura Y. Positron emission tomography-computed tomography can be useful in the early detection of metastases in primary mucinous carcinoma of the skin on the head and neck. Br J Dermatol. 2015;173:1263–1265. doi: 10.1111/bjd.14011. [DOI] [PubMed] [Google Scholar]
- 5.Shockman S., Krug L., Lountzis N. Remission of metastatic primary mucinous carcinoma of the skin with anastrozole. J Am Acad Dermatol. 2014;71:e27–e28. doi: 10.1016/j.jaad.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 6.Tanemura A., Deguchi A., Tanaka A. A rare case of mucinous carcinoma of the skin with multiple organ metastases. Eur J Dermatol. 2015;25:483–484. doi: 10.1684/ejd.2015.2545. [DOI] [PubMed] [Google Scholar]
- 7.Mauri D., Pavlidis N., Polyzos N.P. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–1291. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 8.Chien A.J., Goss P.E. Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol. 2006;24:5305–5312. doi: 10.1200/JCO.2006.07.5382. [DOI] [PubMed] [Google Scholar]
- 9.Hanby A.M., McKee P., Jeffery M. Primary mucinous carcinomas of the skin express TFF1, TFF3, estrogen receptor, and progesterone receptors. Am J Surg Pathol. 1998;22:1125–1131. doi: 10.1097/00000478-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Kwatra K.S., Prabhakar B.R., Jain S. Oestrogen and progesterone receptors in primary mucinous carcinoma of skin. Australas J Dermatol. 2005;46:246–249. doi: 10.1111/j.1440-0960.2005.00193.x. [DOI] [PubMed] [Google Scholar]