Introduction
Cutaneous lymphoid hyperplasia (CLH) is a benign lymphoid infiltrate thought to represent an exaggerated response to antigenic stimulation.1, 2, 3 Treatment includes trigger avoidance and both pharmacologic and surgical modalities.3 We present a case of recalcitrant CLH with durable clinical regression after treatment with thalidomide.
Case report
A 66-year-old white man with a history of ulcerative colitis in remission for 5 years after infliximab treatment presented complaining of a pruritic and painful red rash over his face for greater than a year. Examination found erythematous edematous granulomatous papules and plaques (Fig 1). Histopathology testing found a dense nodular infiltrate of small and large lymphocytes, histiocytes, plasma cells, and eosinophils. Immunohistochemistry findings showed that the small, CD3+ lymphocytes exhibited a normal CD4:CD8 ratio. The large lymphocytes were CD20+. The plasma cells were of polyclonal lineage, expressing both κ or λ light chains. The diagnosis of CLH was made.
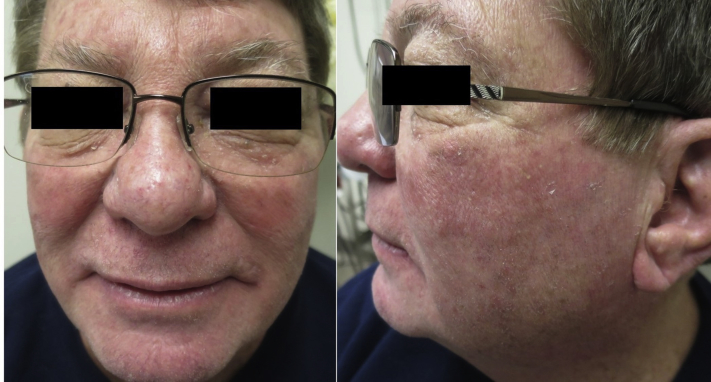
Fig 1.
Before treatment with thalidomide.
Complete blood count and comprehensive metabolic panel were unremarkable. Viral hepatitis and HIV results were negative. Computed tomography of the chest, abdomen, and pelvis and a hematology/oncology workup were normal. The CLH proved refractory to treatment with incomplete responses to clobetasol, intralesional triamcinolone, hydroxychloroquine, mycophenolate mofetil, doxycycline, and methotrexate. The lesions were only responsive to 40 to 60 mg/d of prednisone as a temporizing measure. Finally, thalidomide was started at 50 mg per night and increased to 100 mg per night after 1 month. After a month of 100 mg per night of thalidomide, the patient was substantially improved. After 4 months, the lesions had resolved; however, thalidomide was discontinued because of mild peripheral neuropathy. After discontinuation, the patient has remained in remission during 6 months of follow-up, and his peripheral neuropathy has resolved. Fig 2 shows images after 4 months of treatment.
Fig 2.
After 4 months of treatment with thalidomide.
Discussion
CLH is a benign lymphoid infiltrate of the skin. As a cutaneous pseudolymphoma, CLH often mimics lymphoma both clinically and histologically.1, 3 The pathogenesis of CLH is thought to involve exaggerated responses to antigenic stimulation from a diverse set of exposures. Although often difficult to identify, associated triggers include arthropod bites, scabies, herpes simplex virus infection, Borrelia infection (Europe predominant), tattoos, drugs, and trauma. Less commonly, CLH is seen after herpes zoster in a dermatomal distribution or after vaccination (hepatitis A, B).2
The approach to diagnosis includes a composite assessment of clinical presentation and behavior, routine histology, immunophenotyping, and molecular studies. Clinically, CLH classically presents insidiously as erythematous-to-violaceous papules, plaques, or nodules located mainly on the face, trunk, and extremities.2 On histopathology, CLH often follows a nodular or bandlike pattern. CLH with a nodular pattern mimics cutaneous B-cell lymphomas, whereas CLH with a bandlike pattern mimics mycosis fungoides and is commonly seen in drug-induced cases.2, 3 Regarding immunophenotyping and molecular studies, the lymphocytes may be of B-cell or T-cell predominance.2, 4 Generally, polyclonal polymerase chain reaction bands should be expected with immunoglobulin heavy chain rearrangement and T-cell receptor γ gene rearrangement studies in CLH. However, monoclonality can be observed in about 10% of CLH cases. Light-chain restriction in plasma cells is not seen in CLH.4
Treatment modalities include pharmacologic and surgical treatments. If identified, triggers can be avoided or treated.3 Initial pharmacologic options include systemic corticosteroids and intralesional triamcinolone. Other therapies include methotrexate, hydroxychloroquine, and antibiotics (doxycycline, minocycline). Procedural options include cryotherapy, laser ablation, excision, or radiotherapy of localized disease.1, 3 In our patient's case, standard pharmacologic treatments failed, and the involved areas were too large for surgical treatment. Thalidomide represented another option for his treatment of refractory disease.
Thalidomide and the newer analogues lenalidomide and pomalidomide possess immunomodulatory effects. The mechanism of action is still being elucidated but includes antiangiogenic, anti-inflammatory, and oxidative stress-inducing effects.5, 6 Thalidomide is currently approved by the US Food and Drug Administration for multiple myeloma and erythema nodosum leprosum. However, it has been successfully used off label in many refractory inflammatory skin conditions.7 A literature search yielded 4 case reports documenting successful treatment of CLH with thalidomide.7, 8, 9 Side effects include drowsiness, dizziness, nausea, peripheral neuropathy, venous thromboembolism, teratogenicity, and bicytopenia (leukocytes and platelets). As in our patient, peripheral neuropathy is a limiting factor and warrants close observation.5, 7
Thalidomide use in multiple refractory inflammatory skin conditions has been reported in the dermatology literature. We presented a case of treatment-refractory CLH that showed durable remission with thalidomide. Our patient had been quite troubled by his extensive and symptomatic lesions and was pleased with his response. This case report demonstrates the use of thalidomide in lymphoid aggregate conditions and hopefully assists dermatologists in caring for patients with treatment-refractory CLH.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Gilliam A.C., Wood G.S. Cutaneous lymphoid hyperplasias. Semin Cutan Med Surg. 2000;19(2):133–141. doi: 10.1016/s1085-5629(00)80011-5. [DOI] [PubMed] [Google Scholar]
- 2.Winfield H.L., Smoller B.R. Other Lymphoproliferative and Myeloproliferative Diseases. In: Bolognia J., Jorizzo J., Schaffer J., editors. Dermatology. 3rd ed. Elsevier; 2012. pp. 2037–2047. [Google Scholar]
- 3.Albrecht J., Fine L.A., Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25(2):233–244. doi: 10.1016/j.det.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Charli-Joseph Y., Gatica-Torres M., Pincus L. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61(4):351–374. doi: 10.4103/0019-5154.185698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng S., Pak G., Washenik K., Pomeranz M.K., Shupack J.L. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol. 1996;35(6):969–979. doi: 10.1016/s0190-9622(96)90122-x. [DOI] [PubMed] [Google Scholar]
- 6.Millrine D., Kishimoto T. A brighter side to thalidomide: its potential use in immunological disorders. Trends Mol Med. 2017;23(4):348–361. doi: 10.1016/j.molmed.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Nahmias Z., Nambudiri V., Vleugels R.A. Thalidomide and lenalidomide for the treatment of refractory dermatologic conditions. J Am Acad Dermatol. 2016;75(1):210–212. doi: 10.1016/j.jaad.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Benchikhi H., Bodemer C., Fraitag S. Treatment of cutaneous lymphoid hyperplasia with thalidomide: report of two cases. J Am Acad Dermatol. 1999;40(6):1005–1007. doi: 10.1016/s0190-9622(99)70094-0. [DOI] [PubMed] [Google Scholar]
- 9.Pham-Ledard A., Vergier B., Doutre M.-S., Beylot-Barry M. Disseminated cutaneous lymphoid hyperplasia of 12 years' duration triggered by vaccination. Dermatology. 2010;220(2):176–179. doi: 10.1159/000269845. [DOI] [PubMed] [Google Scholar]