Abstract
COX-2 and YAP are shown to be highly associated with hepatocellular carcinoma (HCC) and frequently upregulated during tumor formation. However, despite their importance, whether there is a mutual interaction between COX-2 and YAP and how they regulate each other are not clear. In this paper, we showed that COX-2 overexpression in HCC cell lines resulted in increased levels of YAP mRNA, protein, and its target genes. COX-2 promoted proliferation of HCC cell lines, and knockdown of YAP antagonized this effect. In addition, our results indicated that EP2 and Wnt/β-Catenin mediate the transcriptional induction of YAP by COX-2. On the other hand, YAP increased COX-2 expression at the level of transcription requiring intact TEAD binding sites in the COX-2 promoter. Collectively, these findings indicated that COX-2 is not only a stimulus of YAP but also a target of Hippo-YAP pathway, thus forming a positive feedback circuit, COX-2-PGE2-EP2-Gαs-β-catenin-YAP-COX-2. In a further study, we showed that inhibition of YAP and COX-2 acted synergistically and more efficiently reduced the growth of HCC cells and tumor formation than either of them alone, suggesting that dual governing of YAP and COX-2 may lead to the discovery of promising therapeutic strategies for HCC patients via blocking this positive feedback loop.
Abbreviations: HCC, hepatocellular carcinoma; HB, hepatoblastoma; TEAD, TEA domain; CTGF, connective tissue growth factor; Cyr61, gene encoding cysteine rich 61; COX, cyclooxygenase; YAP, Yes-associated protein; PGE2, prostaglandin E2; VP, verteporfin; Cel, celecoxib; EP2, prostaglandin E-receptor 2; Gαs, stimulatory G alpha protein; MST 1/2, mammalian STE20-like protein kinase 1/2; LATS1/2, large tumour suppressor 1/2; ChIP, chromatin immunoprecipitation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NF29, neurofibromin 2; ES, embryonic stem; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; TAZ, transcriptional coactivator with PDZ-binding motif; GPCR, G-protein–coupled receptor; AREG, amphiregulin; shRNA, short hairpin RNA; 5SA, mutation of all 5 LATS kinase phosphorylation motifs (S61A, S109A, S127A, S164A, S381A); 5SA/S94A, 5SA mutation and serine 94 changed to alanine which disrupts binding to TEAD transcription factors (mutation S61A, S94A, S109A, S127A, S164A, S381A); WT, wild-type
Introduction
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver in the world and is responsible for roughly one million cancer deaths with a 5-year survival rate of 7%. Majority of HCC patients are identified at a higher stage when curative treatment choices do not take effect. In this case, local regional therapies such as transarterial chemoembolization and drug-eluting beads are the only feasible options. However, HCC is highly tolerant to chemotherapies, and most patients die of ruthless disease relapse [1], [2]. Hence, there are substantial impetus and urgency to discover new HCC diagnostic indicator(s) for early detection, and tumor-specific disease-related proteins as promising curative targets in the handling of HCC. This underlines the necessity to uncover new etiological mechanisms and develop more effective approaches including targeted drugs for the prevention and treatment of HCC.
Cyclooxygenase-2 (COX-2) is a prostaglandin (PG) synthase catalyzing the arachidonic acid to the production of prostaglandins. COX-2 serves as an inducible enzyme and is activated by various insults under pathological conditions [3]. The activation of COX-2 results in formation of its major product, PGE2, which plays a crucial role in modulating many pathophysiological activities [4], [5]. Recent reports show that COX-2 is overexpressed in many solid tumors, including HCC, and plays an important role in tumorigenesis [6]. Thus, COX-2 has become an important drug target for cancer prevention. However, the up- and/or downstream regulators that mediate these COX-2–involved effects remain poorly understood.
The Hippo signaling pathway has important regulatory effects in organ size and cell proliferation. YAP is one of the two main key downstream effectors of the Hippo signaling pathway and is tightly regulated by some serine-threonine kinases such as mammalian STE20-like protein kinase 1/2 (MST 1/2) and large tumor suppressor 1/2 (LATS1/2) [7]. A large body of evidence shows that YAP plays a critical role in cancer development [8], [9]. In addition, YAP is particularly essential for tissue regeneration of the colon after DSS-mediated injury, and hyperactivation of YAP leads to intestinal cancer [10], [11], highly resembling PGE2 phenotypes. This, together with their similar roles in cancer development, raised the possibility that YAP might modulate the function of PGE2.
COX-2 and YAP are shown to be highly associated with HCC and frequently upregulated during tumor formation [2], [3], [12], [13]. However, despite their importance, whether there is a mutual interaction between COX-2 and YAP and how they regulate each other are not clear. In this paper, we reported that the COX-2-PGE2-Gαs-β-Catenin-YAP-COX-2 positive feedback circuit contributes to HCC tumorigenesis in our in vitro and in vivo studies, providing new insights into drug R&D targets for HCC therapy.
Materials and Methods
Cell Lines, Culture, and Reagents
Hep 3B, Hep G2, Bel-7402, HuH7, THLE-3, and HL-7702 cells were obtained from the ATCC and cell bank of Shanghai Institute of Cell Biology (Shanghai, China). Cells were cultured in 75- or 150-cm2 flasks with Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated in a 5% CO2 incubator at 37°C.
Chemicals and Reagents
Dulbecco's modified Eagle medium and fetal bovine serum (Gibco BRL, USA); trypsin, LPS, MTT (Sigma Chemical Co., MO, USA); penicillin and streptomycin (Sunshine Biotechnology, Nanjing, China); and antibodies to YAP, CTGF, Cyr 61, AREG, TEAD1, EP1-EP4, β-catenin, COX-2, MST1, β-catenin siRNA, short hairpin RNA (shRNA) of YAP, COX-2, EP2, MST 1 and HRP-linked goat anti-mouse IgG and horseradish peroxidase (HRP)-linked anti-rabbit IgG were obtained from Santa Cruz (CA, USA). YAP,YAP(5SA), YAP(5SA/S94A) expression plasmids were obtained from Addgene (USA). Doxycycline inducible YAP lentivirus expression plasmid (PIN20YAP) was previously described [14]. EP1-EP4 antibodies, Butaprost, and AH6809 were from Cayman Chemical (Ann Arbor, MI). Celecoxib, verteporfin, and doxycyclin were purchased from Sigma-Aldrich (St. Louis, MO). Other agents were the highest quality available in market.
Cell Viability Assay
Cell viability was measured as described previously [5].
Plasmid Construction and Site-Directed Mutagenesis
The DNA of Cyr61 [nucleotide (nt) position −163 to + 57], CTGF (nt −250 to −1), and COX-2 [nt −800 to −1] promoters was amplified by polymerase chain reaction (PCR) from genomic DNA extracted from human BxPC-3 cells and subsequently cloned into pGL3-basic luciferase reporter vector (Promega). Site-directed mutagenesis was done using the QuickChange Mutagenesis Kit (Stratagene) according to the manufacturer's protocol. COX-2 and EP2 expression plasmids were created as described previously [15].
Immunoprecipitation and Western Blot
The immunoprecipitation was done as described previously [15]. In brief: the cell lysates containing 500 μg protein were incubated with 5 μg primary antibody overnight at 4°C. Fifty microliters of protein A/G plus-agarose (Santa Cruz Biotechnology) was added, and the complex was incubated at 4°C overnight. The beads were washed three times with high salt buffer (1 M Tris-HCl, pH 7.4, 0.50 M NaCl, and 1% Nonidet P-40) and twice with lysis buffer to eliminate nonspecific binding. The immunoprecipitated complexes were released with 2× sample buffer for Western analysis. Western blots are as described [5].
Chromatin Immunoprecipitation (ChIP)–Quantitative PCR (qPCR) Analysis
ChIP was performed with the use of a ChIP-IT Express kit (active motif). In brief, cells were treated with 1% formaldehyde, lysed, and homogenized using a Dounce homogenizer. DNA was shorn by sonication, and the sheared chromatin was incubated with Ig G (Sigma) or YAP/TEAD antibodies followed by qPCR analysis. The amount of ChIP DNA was expressed as fold enrichment relative to input.
Immunofluorescence
This analysis was performed as described previously [15].
Colony Formation Assay
This assay was conducted as described previously [15].
Luciferase Reporter Analysis
This assay was done as described previously [15].
PGE2 Measurement
This analysis was conducted as described previously [5].
RT-qPCR Analysis
Total RNA was isolated from cultured cells and tissues with the use of an RNeasy Mini Kit (Qiagen). Portions of the RNA (1-2 μg) were subjected to RT followed by qPCR analysis with the use of a GeneAmp RNA PCR Core Kit (Applied Biosystems), 2×SYBR Green Pre-mix (Elpisbio), and a 7500 Fast Real-Time PCR machine (Applied Biosystems). The Ct values of target genes were normalized by those for β-actin or glyceraldehyde-3-phosphate dehydrogenase gene.
Xenograft Mouse Model
This analysis was conducted as described previously [15]. Animal protocols were approved by the Institutional Animal Care and Use Committee of the Nanjing Normal University, P.R.C., and conducted in accordance with the Declaration of Helsinki Principles. Four-week-old male nude mice weighing 16 to 20 g were acquired from Shanghai Silaike Laboratory Animals Co. Ltd., Chinese Academy of Sciences. Briefly, cells (5×106) were subcutaneously injected into each nude mouse (nu/nu, male 6 to 8 weeks old) under a sterile environment. After around 10 days of invocation, VP was applied by intraperitoneal injection at 50 mg/kg once every other day for 20 days. Tumor size was measured at indicated time using a caliper, and tumor volume was calculated as 0.5 × L × W2, with L indicating length and W indicating width. About 3 to 5 weeks after injection, tumors were dissected, and specimens from representative tumor tissue were cut with a razor blade and frozen for Western blot analysis.
Knockout Mice
Animal protocols were approved and conducted as those in the above model. A floxed allele of Nf2 (Nf2flox2) has been described previously [16]. In brief, the Cre/loxP recombination system of bacteriophage P1 was used to generate conditional Nf2 knockout mice, and a two-step strategy [17] was utilized to generate ES cell clones carrying the Nf2flox2 mutant allele. The Nf2flox2 allele carried an insertion of two loxP sites in the intronic regions flanking exon. Successfully targeted ES clones were microinjected into C57BL/6 blastocysts. Germline transmission from generated chimeric offspring was confirmed by PCR. To analyze function of YAP in mouse liver, Nf2flox2 was crossed to the Albumin-Cre (Alb-Cre) recombinase to obtain Alb-Cre;Nf2 flox2/flox2 mice. Alb-Cre drives liver-specific Cre expression and achieves efficient deletion at perinatal stage (E18 to P1) in hepatocytes. Three-week-old NF2 knockout mice were administered with VP at 50 mg/kg by intraperitoneal injection once every other day for 4 weeks.
Statistical Analysis
The values are expressed as the means ± SD from different experiments. Unpaired Student’s t test was used to compare two groups, and one-way ANOVA was used to compare three or more groups. P < .05 was considered statistically significant. q value analysis was used to estimate the synergistic effect of the agents. q=EA+B/(EA+EB−EA*EB), where EA represents the effect of A used independently, EB represents the effect of B used independently, and EA+B represents the effect of A and B used together. A q value of >1.15 indicates synergism, a q value of <0.85 indicates antagonism, and a q value of 0.85 to 1.15 indicates additive effect [18].
Results
YAP and COX-2 Associated in HCC Cells
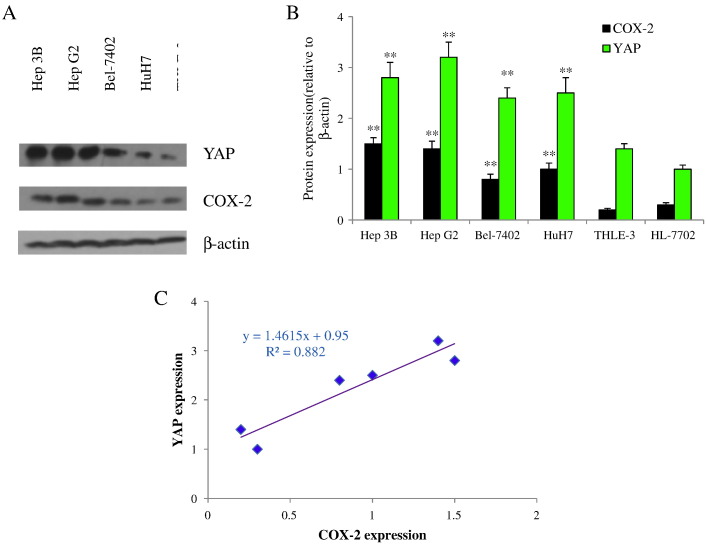
To address YAP and COX-2 expressions and their correlation, Western blot was conducted to detect their expressions in one normal (HL-7702), one immortal (THLE-3) hepatic, and four HCC cell lines. Analysis of the expression of YAP and COX-2 in six human cell lines revealed that YAP and COX-2 were expressed in all the cell lines (Figure 1, A and B). When compared with normal hepatic cell HL-7702 and immortal cell line THLE-3, YAP and COX-2 appeared to be upregulated in a panel of HCC cells including Hep 3B, Hep G2, Bel-7402, and HuH7 as determined by a specific antibody. Furthermore, the highest levels of YAP and COX-2 were evident in Hep 3B and Hep G2 cells, intermediate levels were present in Bel-7402 and HuH7cells, and the lowest levels were present in THLE-3 and HL-7702 cells (Figure 1B). Correlation regression analysis showed that the coefficient of correlation (R2) was 0.882 (Figure 1C). These data indicated that YAP and COX-2 were upregulated and highly associated in HCC cells, raising the possibility that there may be a reciprocal interplay between the two pathways.
Figure 1.
YAP and COX-2 are overexpressed in HCC cells and highly associated. (A) YAP and COX-2 expression in Hep 3B, Hep G2, Bel-7402, HuH7, THLE-3, and HL-7702 cells. (B) The expression levels of YAP and COX-2 were determined as in A and quantified by densitometry. Ratios of YAP or COX-2 over β-actin are expressed as mean ± SD. **P<.01 compared with HL-7702. (C) Data of YAP and COX-2 expressions were analyzed by correlation regression analysis. All data are from at least three independent experiments.
Regulation of YAP Expression by COX-2
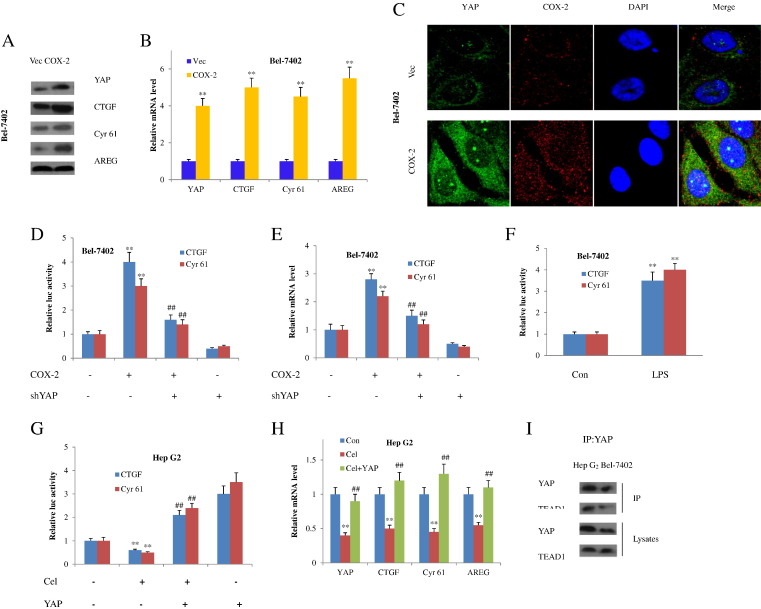
We first evaluated if COX-2 modulates YAP in HCC cells. Overexpression of COX-2 in Bel-7402 cells obviously led to the abundance of YAP mRNA and protein (Figure 2, A and B), which is consistent with the immunofluorescent staining (Figure 2C). Simultaneously, the transcriptional activity of YAP was enhanced as demonstrated by the increased mRNA and protein levels of YAP downstream effectors CTGF, Cyr 61, and AREG (Figure 2, A and B). Then, we sought to investigate if YAP was affected at the transcriptional level. In fact, COX-2 expression produced an activation of a CTGF reporter as well as a Cyr 61 reporter; both are well-established hallmarks of YAP transcriptional ability. In contrast, YAP knockdown blocked YAP downstream gene evocation by COX-2 overexpression (Figure 2, D and E). In addition, LPS was applied to stimulate the COX-2 expression, and we obtained similar results as in forced COX-2 expression (Figure 2F).
Figure 2.
COX-2 increases YAP expression in HCC cells. (A) Western blot analysis of Bel-7402 cells transfected with COX-2–expressing plasmids. (B) Reverse transcription and qPCR assays of samples in panel A. **P<.01 compared with the control vectors. (C) Immunofluorescent staining of YAP and COX-2 in Bel-7402 cells after forced COX-2 expression. Nuclei were stained with DAPI (blue). YAP and COX-2 were shown with green and red color, respectively. (D) Relative CTGF and Cyr 61 luciferase activity in Bel-7402 cells transfected with COX-2–expressing plasmids and/or shYAP. **P<.01 compared with the absence of COX-2 and sh YAP; ##P<.01 compared with the COX-2 alone. (E) Reverse transcription and qPCR assays of CTGF and Cyr 61 in Bel-7402 cells at the presence or absence of COX-2–expressing plasmids or sh YAP. **P<.01 compared with the absence of COX-2 and sh YAP; ##P<.01 compared with the COX-2 alone. (F) Relative CTGF and Cyr 61 luciferase activity in Bel-7402 cells stimulated with LPS. **P<.01 compared with the control group. (G) Relative mRNA level in Hep G2 cells at the presence of celecoxib or celecoxib plus YAP transfection. **P<.01 compared with the control group; ##P<.01 compared with the celecoxib group. (H) Relative CTGF and Cyr 61 luciferase activity in Hep G2 cells treated with celecoxib at the presence or absence of YAP transfection. **P<.01 compared with the absence of celecoxib and YAP; ##P<.01 compared with the celecoxib alone. (I) COX-2 modulates YAP-TEAD1 interaction. Hep G2 and Bel-7402 cells were lysed and immunoprecipitated with YAP or TEAD1 antibodies. YAP or TEAD1 presented in lysates or immunosediments was detected by Western blots. All quantitative data were expressed as mean ± SD (in B, D, E, F, G, and H) from at least three independent experiments.
Consistently, celecoxib, a selective COX-2 inhibitor, inhibited YAP expression and the subsequent CTGF and Cyr 61 reporters’ activation in Hep G2 cells (Figure 2G). As a result, the target gene expressions were also attenuated (Figure 2H). On the contrary, YAP transfection reversed YAP target gene expression decreases caused by COX-2 inhibitor (Figure 2H). Because YAP activates gene expression largely through interaction with TEAD, we next checked whether COX-2 promotes the binding of YAP to TEAD family. By analyzing the immunoprecipitates of YAP or TEAD1 from COX-2–overexpressing or –lower-expressing cells, we found that a high level of COX-2 promoted YAP-TEAD1 interaction and a low level of COX-2 repressed YAP-TEAD1 mutual interplay (Figure 2I).
Involvment of YAP in Functions of COX-2 Pathway
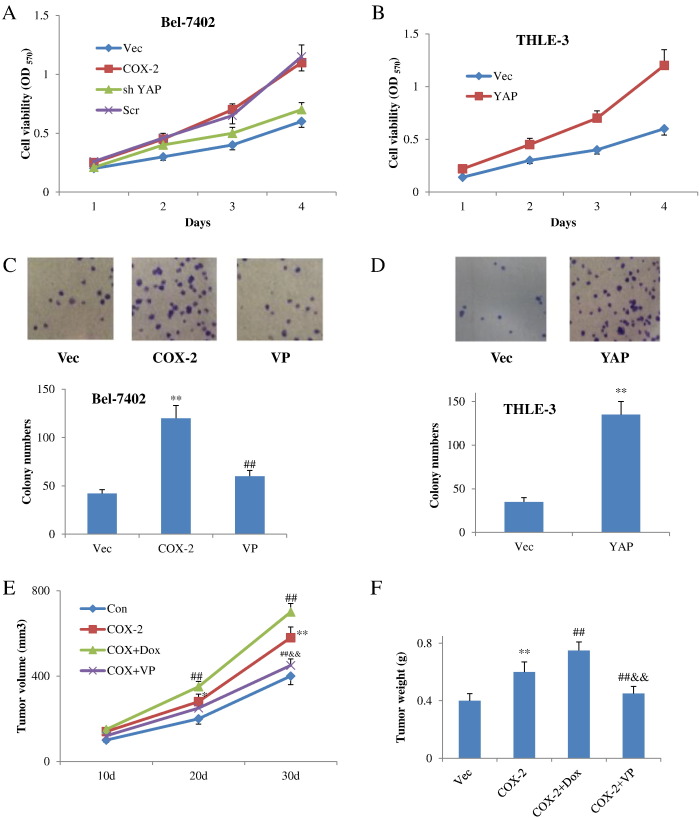
Next, we were interested in the biological functions of YAP in COX-2 pathway, and we examined its role in COX-2–induced overgrowth and colony formation. Importantly, knockdown of YAP impeded overgrowth induced by COX-2–expressing plasmids in Bel-4702 cells (Figure 3A). In contrast, overexpression of YAP promoted cell growth in COX-2–low THLE-3 cells (Figure 3B). Consistently, inhibition of YAP by verteporfin also robustly repressed COX-2–induced colony formation in Bel-7402 cells (Figure 3C), while expression of YAP promoted colony formation in THLE-3 cells (Figure 3D). Moreover, doxycyclin-induced YAP expression promoted tumorigenesis induced by COX-2 overexpression in Hep G2–implanted mice (Figure 3E). On the contrary, YAP inhibitor verteporfin diminished tumor formation induced by COX-2 in these mice (Figure 3F). Together with the above-described findings, YAP plays an important role in COX-2–induced overgrowth and carcinogenesis in vitro and in vivo.
Figure 3.
YAP mediates biological functions of COX-2 pathway. (A) YAP plays a role in COX-2–induced cell proliferation. sh YAP was expressed with COX-2 in Bel-4702 stable cells. Cell viability was determined by MTT assay. (B) Cell viability after YAP transfection in COX-2-low THLE-3 cells. (C) Representative images of colonies in COX-2–expressing Bel-4702 cells with or without verteporfin (upper panel). Representative bar graph demonstrating the colony numbers in COX-2–expressing Bel-4702 cells with or without verteporfin (lower panel). (D) Representative images of colonies in THLE-3 cells transfected with YAP expression plasmids (upper panel). Representative bar graph demonstrating the colony numbers in THLE-3 cells transfected with YAP expression plasmids (lower panel). (E and F) YAP inducer doxycyclin or YAP inhibitor verteporfin in COX-2–induced tumorigenesis in vivo (n=5). *P<.05, **P<.01 compared with the control vector group; ##P<.01 compared with the COX-2 alone. &&P<.01 compared with COX-2 plus doxycycline. All data are from at least three independent experiments.
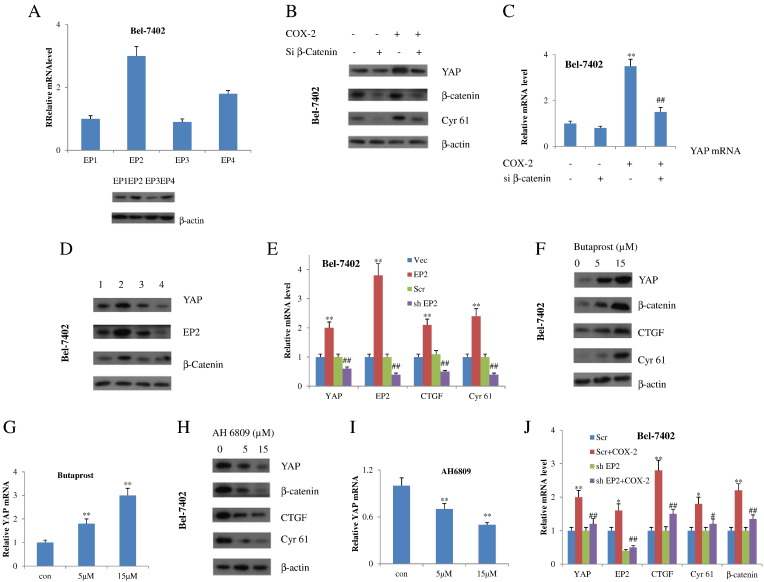
COX-2-Induced YAP Expression Through EP2 Signaling
We next turned to the underlying mechanism through which COX-2 amplifies YAP expression. It has been reported that COX-2 biological actions have been attributed to its catalyzed product PGE2 and its coupling with specific GPCRs, named EP1 to EP4, through idiosyncratic signaling pathways [19]. First, we test whether and to what extent EPs were expressed in HCC cells. Results showed that EP1 to EP4 were all found in Bel-7402 and HuH7 cells. But EP2 has the highest level (Figures 4A, 4SA). Given that PGE2 promotes cancer cell growth through a Gs-Axin-β-catenin signaling axis and Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in cancer cells, we checked whether β-catenin was involved in regulating COX-2–induced YAP expression. In fact, COX-2 failed to upregulate both protein and mRNA levels of YAP in β-catenin–depleted Bel-7402 and HuH7 cells (Figures 4, B and C and 4SB), suggesting that the effect of COX-2 on YAP expression is really mediated by Wnt/β-catenin signaling. We then tested whether EP2 mediates the effect of COX-2 on YAP. Forced expression of EP2 induced an increase of β-catenin as well as YAP expression in both Bel-7402 and HuH7 cells, whereas depletion of EP2 with shRNA had the opposite effect (Figures 4, D and E and 4SC). Furthermore, we utilized EP2 agonists and antagonists to test whether such agents also affect the YAP expression. The EP2 agonist butaprost increased both β-catenin and YAP expression (Figures 4, F and G and 4SD), whereas the EP2 antagonist AH6809 had the contrary effect (Figures 4, H and I and 4SD). Most noticeably, ablation of EP2 diminished the levels of β-catenin, expression of YAP, and YAP target genes induced by COX-2 (Figures 4J and 4SE). Collectively, these data revealed that EP2 and Wnt/β-catenin mediate the transcriptional induction of YAP by COX-2.
Figure 4.
COX-2 promotes expression of β-catenin through EP2 receptor, which positively regulates transcription of YAP. (A) q-RT-PCR (upper) and Western blot (lower) analyses of EP1-EP4 in Bel-7402 cells. (B) Western blot and q-RT-PCR (C) analyses of Bel-7402 cells transfected with si β-catenin or scrambled siRNAs. **P<.01 compared with the absence of COX-2 and si β-catenin. ##P<.01 compared with the COX-2 alone. (D) Western blot and q-RT-PCR (E) analyses of Bel-7402 cells transfected with an EP2-expressing plasmid or an empty vector (control), or with sh EP2 or scrambled (Scr) shRNA. 1: control 2: EP2 3: Scr 4: sh EP2. **P<.01 compared with the control vector group. ##P<.01 compared with the scrambled group. (F) Western blot and q-RT-PCR (G) analyses of Bel-7402 cells at the presence or absence of indicated concentrations of EP2 agonist Butaprost. **P<.01 compared with the control group. (H) Western blot and q-RT-PCR (I) analyses of Bel-7402 cells at the presence or absence of indicated concentrations of EP2 antagonist AH6809. **P<.01 compared with the control group. (J) q-RT-PCR analyses of Bel-7402 cells treated as indicated. *P<.05,**P<.01 compared with the scrambled group. #P<.05, ##P<.01 compared with the sh EP2 group. All quantitative data are expressed as means±SD from at least three independent experiments.
COX-2 Expression Mediated by YAP at A Transcriptional Level
As YAP plays an important role in the development of cancer highly resembling PGE2 phenotypes [10], [20], it raised the possibility that YAP might also regulate the function of COX-2/PGE2 pathway. Therefore, we next checked whether YAP increased COX-2 signaling in HCC cells. As expected, YAP expression upregulated the abundance of mRNAs and proteins of COX-2 in Bel-7402 and HuH7 cells. Meanwhile, treating cells with verteporfin, which is known to disrupt YAP-TEAD binding, greatly reduced COX-2 mRNA levels (Figures 5, A and B and 5SA, SB). Conversely, knockdown of YAP by shRNA in Hep G2 cells resulted in reduction of COX-2 and PGE2 levels (Figure 5, C-E). Moreover, immunofluorescence revealed that knockdown of YAP by shRNA decreased COX-2 expression in Hep G2 cells (Figure 5F). Similar observations were also found in Hep3B cells (Figure 5SC, SD, and SE). Collectively, these studies clearly suggested that YAP increased COX-2 expression in HCC cells.
Figure 5.
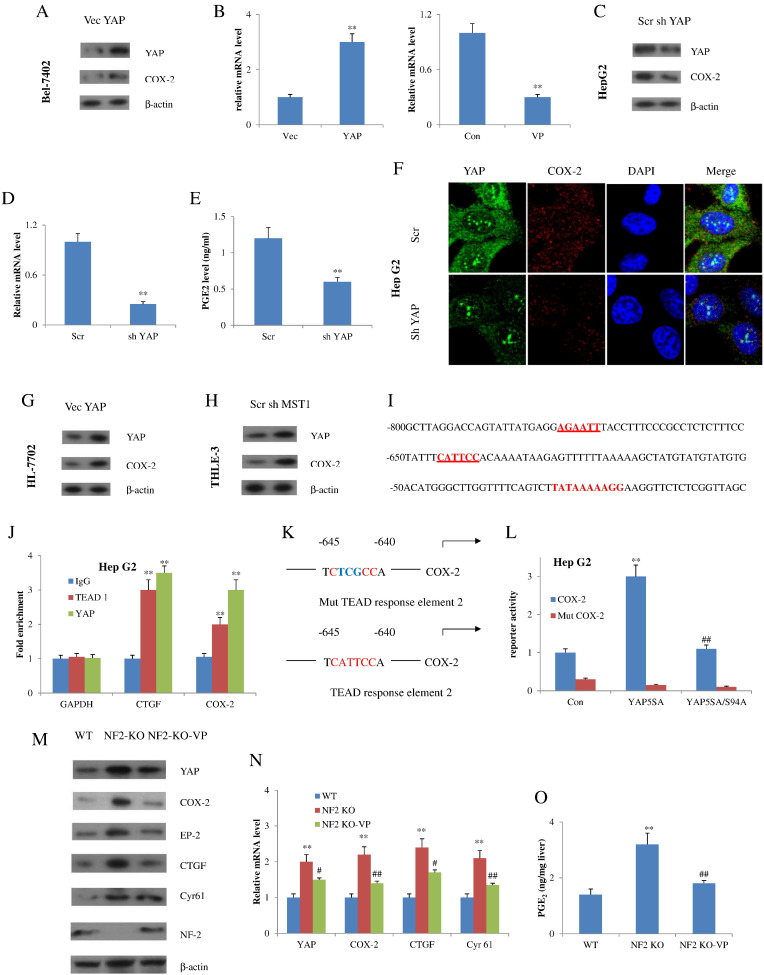
YAP upregulates COX-2 expression in both normal and malignant HCC cells. (A) Bel-7402 cells were transfected with YAP-expressing plasmids. Western blot using antibodies against YAP and COX-2 was performed. (B) q-RT-PCR analyses of COX-2 levels in Bel-7402 cells after YAP transfection (left) or treatment with verteporfin (right). **P<.01 compared with the control vector group. (C) Immunoblotting of YAP and COX-2 was performed in Hep G2 cells with knockdown of YAP shRNA. (D) q-RT-PCR analyses of COX-2 and (E) PGE2 levels in Hep G2 cells after YAP knockdown. **P<.01 compared with the scrambled shRNA group. (F) Immunofluorescence of YAP and COX-2 in Hep G2 cells after YAP knockdown. Nuclei were stained with DAPI (blue). YAP and COX-2 were shown with green and red color, respectively. (G) YAP and COX-2 were detected by Western blot in HL-7702 cells transfected with YAP expression vectors. (H) YAP and COX-2 were detected by Western blot in THLE-3 cells transfected with MST shRNA. (I) Sequence of the COX-2 promoter and two binding sites was identified (bold/underlined) in the promoter regions of human COX-2. (J) ChIP-qPCR analyses of COX-2 promoter regions performed with antibodies to YAP and TEAD1, and corresponding control IgG in Hep G2 cells. Data are expressed as fold enrichment relative to input DNA. CTGF and glyceraldehyde-3-phosphate dehydrogenase were examined as positive and negative controls, respectively. ⁎⁎P<.01 compared with Ig G control group. (K) Luciferase reporter constructs (wild type and mutated) used. (L) Luciferase reporter activity was measured in cells expressing the indicated plasmids. ⁎⁎P<.01 compared with control group; ##P<.01 compared with YAP 5SA group. (M) Immunoblot and (N) reverse transcription qPCR analyses of the livers in the indicated groups. ⁎⁎P<.01 compared with the WT group; #P<.05, ##P<.01 compared with the NF2-KO group. (O) PGE2 content of the liver in WT, NF2-KO, and NF2-KO-VP mice (n=5). ⁎⁎P<.01 compared with the WT group; ##P<.01 compared with the NF2-KO group. All data are shown as means±SD from at least three independent experiments.
To examine if COX-2 expression was mediated by YAP in primary cells, HL-7702 cells were chosen for further assessment. Results showed that HL-7702 cells transfected with YAP-expressing plasmid demonstrated higher COX-2 expression than cells with control vector (Figure 5G). Additionally, Western blot analysis in MST-depleted immortal THLE-3 cells displayed enhanced COX-2 expression compared to the control vector-transfected cells (Figure 5H). Consequently, COX-2 could be upregulated in several types of cells via expression of a constitutively active form of YAP or by stimulation of endogenous YAP protein that resulted from disruption of Hippo pathway upstream members.
Analysis of the human COX-2 promoter sequences reveals two TEAD binding sites located around −778 to −773 (AGAATT) and −645 to −640 (CATTCC) of base pairs upstream of the transcription start site (Figure 5I). TEAD binding COX-2 promoter was then determined by ChIP. Results validated the binding of YAP and TEAD1 at assumed binding sites in the promoter of COX-2 (Figure 5J). To further confirm if COX-2 is a direct downstream gene of YAP-TEAD, we made a luciferase plasmid where the luciferase was activated by COX-2 promoter and then was transfected into Hep G2 cells. The reporter was highly activated by YAP 5SA but not by YAP 5SA/S94A. Remarkably, when the TEAD binding sites were mutated, the reporter failed to be triggered by YAP 5SA (Figure 5, K and L).
We next investigate the modulation of COX-2 and PGE2 by YAP in vivo using Alb-Cre;Nf2 flox2/flox2 mice generated by crossing of Nf2flox2 mice with Albumin-Cre (Alb-Cre) recombinase. In this assay, we tested VP in mice bearing liver-specific knockout of NF2/Merlin, which exhibited liver cancer due to activation of endogenous YAP [21]. The abundance of COX-2 protein and mRNA levels, as well as the PGE2 level of the liver, was reduced in the NF2-KO-VP mice but increased in the NF2-KO mice (Figure 5, M-O). Collectively, these findings indicated that COX-2 is not only a stimulus of YAP but also a target of Hippo-YAP pathway, thus forming a positive feedback circuit, COX-2-PGE2-EP2-Gαs-β-catenin-YAP-COX-2.
Promotion of Cell Growth and Tumorigenesis by A Combination of YAP and COX-2
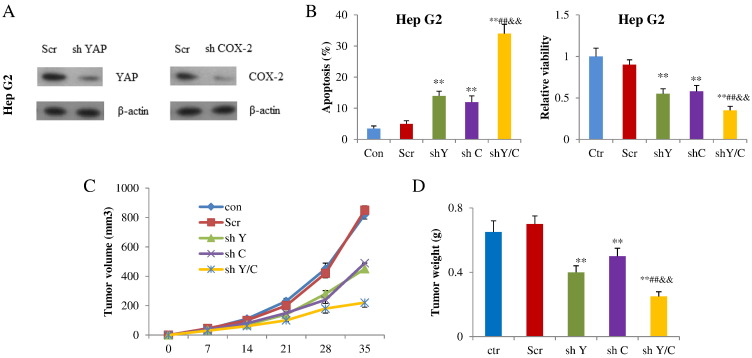
Having shown that COX-2 signaling and YAP form a positive regulatory loop, this prompted us to explore whether YAP and COX-2 might synergize to promote HCC cell growth and tumor formation. First, we assessed the effects of shRNA targeting YAP and COX-2 on apoptosis and cell viability in Hep G2 and Hep 3B cells. The shRNA targeting YAP or COX-2 substantially attenuated expression of the target genes compared with the control shRNA (Figure 6A). Then, the Hep G2 cells were further subjected to flow cytometry and MTT assays. Transfection with shRNA targeting YAP increased the apoptosis percentage from 5% to 14% in Hep G2 cells and 6% to 16% in Hep 3B cell cultures. The shRNA targeting COX-2 had similar effects and increased apoptosis in Hep G2 and Hep 3B cells too. The cotransfection of these two shRNAs synergistically increased apoptosis in Hep G2 and Hep 3B cells. We also found that both shRNAs had synergistic effects on decreasing cell viability in Hep G2 and Hep 3B cells (Figures 6B, 6S). Finally, we found that sh YAP and sh COX-2 suppressed tumor volume and weight when compared with control group, while combination of sh YAP and sh COX-2 exhibited advantages over either shYAP or shCOX-2 alone in inducing tumor volume as well as tumor weight (Figure 6, C and D). Based on these results, YAP and COX-2 play synergistic roles in regulating HCC cell growth and tumor development.
Figure 6.
YAP and COX-2 acted synergistically in HCC cell growth and tumorigenesis. (A) Western blot analysis of YAP and COX-2 after shYAP or shCOX-2 was introduced in Hep G2 cells. (B) Apoptosis and cell viability of Hep G2 cells after shYAP and/or shCOX-2 was introduced. (C) Tumor volume and weight (D) were decreased after shYAP and/or shCOX-2 transfection (n=5). **P<.01 compared with Scr; ##P<.01 compared with shYAP; &&P<.01 compared with shCOX-2. All quantitative data are shown as means ± SD from at least three independent experiments.
Discussion
HCC is publicly recognized as the sixth most prevalent cancer all over the world. It is responsible for approximately one million deaths each year, mainly in underdeveloped and developing countries. However, the treatment of HCC is still difficult for therapists. Therefore, broadening our knowledge of molecular signaling including proteins or biomarkers responsible for tumorigenesis or tumor development is crucial for developing novel therapeutic strategies to overcome HCC [22].
COX-2 and YAP are often found to be highly associated with HCC and frequently upregulated in HCC cells or tissues, but their mutual regulative mechanisms remain unclear. In this study, we found that COX-2 mediates HCC carcinogenesis by activating YAP signaling. Results demonstrated that COX-2 augments YAP mRNA and protein levels through the Wnt/β-catenin mechanism. Additionally, we found that COX-2 is a direct target gene of YAP. YAP induces COX-2 expression and transcription in multiple cell systems at the level of transcription requiring intact TEAD binding sites in the COX-2 promoter. We think that this positive feedback circuit plays an important role in HCC tumorigenesis and development. These findings provide a significant insight into the mechanism of HCC formation and the reciprocal regulatory effects between COX-2/PGE2 and Hippo-YAP pathway.
Feedback loops are ordinarily found in developmental progress, which keep a developmental action silent in fluctuating background signals but trigger a robust response upon real stimuli at the right time and place [23]. Feedback loops also exist in many physiological regulatory progresses, such as blood pressure, heart rate, urinary excretion, and so on. For tumor pathological regulatory pathways, feedback loops are also commonly seen [24], [25], [26]. But for YAP pathway, although it is identified as an important transcriptional regulator, specific feedback loops with YAP as a component are rarely reported. In this paper, our study of COX-2 in the Hippo pathway gives such an example. Through EP2 and Wnt/β-catenin signaling, COX-2 and its product PGE2 activate the YAP expression and downstream effectors such as CTGF and Cyr 61, resulting in tumor growth. Another example is YAP-miR-130a-VGLL4 positive regulatory loop, which sustains role of the Hippo pathway in developmental and tumor progress. Such mechanisms may explain YAP activation in many cancers without the regulation of YAP dephosphorylation or nuclear localization [23].
YAP is involved in various cancers and now is a hot topic in cancer research. A large number of studies have focused on the YAP phosphorylation or localization. But only disorder of this mechanism is not enough to explain YAP activation in many cancers [27], [28]. For example, most of the immunohistochemistry and microarray data in cancer specimen show increase in both cytosol and nuclear YAP abundance rather than focused nuclear localization of YAP [20], [29]. This phenomenon also exists in our cultured cells. Besides, high density of cell-cell contact retention of YAP in cytoplasm was not observed in many occasions. Recently, it is reported that the mRNA level of YAP (and TAZ) sufficiently correlates with target gene expression and is well associated with cancer progression [11], [29]. Together with our results in this paper, the importance of transcriptional regulation of YAP should be well addressed. Here, our data are consistent with the idea that the excessive expression of YAP is actually a more important factor in tumorigenesis. Another thing worthy of being mentioned is that cytoplasm YAP may affect tumor formation or growth directly regardless of the nuclear translocation. For instance, in our unpublished data, we found that cytoplasm YAP was able to promote dissociation of p65 with IκB and its translocation to the nucleus to control the expressions of target genes in tumorigenesis. Finally, we found during the search for anti-YAP drug candidates that some of the natural products did not affect the YAP translocation or mRNA expression but the protein levels. This might highlight the important role of YAP degradation in regulating Hippo-YAP pathway. Thus, our findings broaden the knowledge about the mechanisms regarding YAP activation in cancers.
It has been reported recently that Hippo-YAP pathway is a downstream of GPCR signaling [30]. GPCRs differentially regulate YAP or TAZ phosphorylation through Gα-Rho-actin-Lats1/2-YAP/TAZ axis. For EP2 receptor, it is a Gαs-linked GPCR, which actually decreases YAP phosphorylation and activates YAP downstream target genes [31], [32], [33]. But in our study, we did not observe that this pathway was validated. Substitutively, we found that COX-2 failed to upregulate both protein and mRNA levels of YAP in β-catenin–depleted cancer cells, suggesting that the effect of COX-2 on YAP expression is really regulated via Wnt/β-catenin signaling. Our results suggest the complexity of Hippo-YAP regulation, and the exact mechanism might be connected to different cell types and the environmental context surrounding them.
Because both YAP and COX-2 participate in HCC formation and development, we finally wanted to know whether YAP and COX-2 had synergistic performance in keeping tumor growth. Results showed that combination of sh YAP and sh COX-2 exhibited advantages over either shYAP or shCOX-2 alone in inducing apoptosis and reducing viability of Hep G2 cells as well as tumor formation. These results point to the idea that targeting YAP and COX-2 would be more efficacious than single inhibition in preventing tumor growth regarding YAP/COX-2 high expression, and a dual YAP and COX-2 inhibitor could be a novel drug candidate for successful HCC treatment. Currently, although tremendous progress has been made toward understanding the molecular mechanism underlying HCC development and treatment, the drug resistance is still an unconquerable barrier for successful survival. Recent evidence shows that COX-2 is overexpressed in many solid tumors, including HCC, and is involved in drug resistance and poor prognosis [6]. Meanwhile, a large body of evidence shows that YAP/TAZ has been shown to mediate resistance to chemotherapy in human cancers [8], [9], [34]. The mechanism described in this paper might open a window for overcoming the notorious drug resistance. Inhibitors of YAP may be synergistic with COX-2 inhibitors in sensitizing cancer cells to drugs by disrupting this positive feedback circuit.
In conclusion, YAP and COX-2 forms a positive loop and are synergistic in modulating HCC cell growth and tumor formation, suggesting that dual governing of YAP and COX-2 may lead to the discovery of promising therapeutic strategies for HCC patients.
Acknowledgments
Acknowledgements
This work was supported by Project 81773948 supported by the National Natural Science Foundation of China, the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (SRF for ROCS, SEM, 2015311), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and NSFC for Talents Training in Basic Science (grant nos. J1103507, J1210025).
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2017.12.004.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wickline ED, Du Y, Stolz DB, Kahn M, Monga SP. γ-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer afterβ-catenin knockdown. Neoplasia. 2013;15(4):421–434. doi: 10.1593/neo.122098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang TC, Man S, Xu P, Francia G, Hashimoto K, Emmenegger U, Kerbel RS. Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia. 2010;12(11):928–940. doi: 10.1593/neo.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Qu L, Tao H. Cyclo-oxygenase 2 up-regulates the effect of multidrug resistance. Cell Biol Int. 2009;34:21–25. doi: 10.1042/CBI20090129. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J, Du YF, Xiao ZY, Pan LL, Li W, Huan L, Gong ZN, Wei SH, Huang SQ, Xun W. Growth inhibitory effect of KYKZL-1 on Hep G2 cells via inhibition of AA metabolites and caspase-3 pathway and cell cycle arrest. Toxicol Appl Pharmacol. 2014;274:96–106. doi: 10.1016/j.taap.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Fantappie O, Solazzo M, Lasagna N, Platini F, Tessitore L, Mazzanti R. P-glycoprotein mediates celecoxib-induced apoptosis in multiple drug-resistant cell lines. Cancer Res. 2007;67:4915–4923. doi: 10.1158/0008-5472.CAN-06-3952. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 12.Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–1517. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu G, Wang J, Wu F, Wang N, Zhou W, Wang Q, Pan W, Ao G, Yang J. YAP and 14-3-3gamma are involved in HS-OA–induced growth inhibition of hepatocellular carcinoma cells: a novel mechanism for hydrogen sulfide releasing oleanolic acid. Oncotarget. 2016;7:52150–52165. doi: 10.18632/oncotarget.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- 17.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 18.Dai TJ. Quantitative analysis of the combination of drugs. Chin Pharmacol Bull. 1998;14:479–504. [Google Scholar]
- 19.Breyer RM, Kennedy CR, Zhang Y, Breyer MD. Structure-function analyses of eicosanoid receptors. Physiologic and therapeutic implications. Ann N Y Acad Sci. 2000;905:221–231. doi: 10.1111/j.1749-6632.2000.tb06552.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 23.Shen S, Guo X, Yan H, Lu Y, Ji X, Li L, Liang T, Zhou D, Feng XH, Zhao JC. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25:997–1012. doi: 10.1038/cr.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau TS, Chan LK, Wong EC, Hui CW, Sneddon K, Cheung TH, Yim SF, Lee JH, Yeung CS, Chung TK. A loop of cancer-stroma-cancer interaction promotes peritoneal metastasis of ovarian cancer via TNFalpha-TGFalpha-EGFR. Oncogene. 2017;36:3576–3587. doi: 10.1038/onc.2016.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui CP, Wong CC, Kai AK, Ho DW, Lau EY, Tsui YM, Chan LK, Cheung TT, Chok KS, Chan AC. SENP1 promotes hypoxia-induced cancer stemness by HIF-1alpha deSUMOylation and SENP1/HIF-1alpha positive feedback loop. Gut. 2017;0:1–11. doi: 10.1136/gutjnl-2016-313264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray LB. Regulator loop enabling cancer cell growth. Science. 2017;355:491–492. doi: 10.1126/science.355.6324.491-b. [DOI] [PubMed] [Google Scholar]
- 27.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lee S, Lim DS. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA. Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material