Abstract
Background
ESBL-producing Enterobacteriaceae (ESBLPE) are increasing in prevalence worldwide and are more difficult to treat than non-ESBLPE. Their prevalence in the UK general population is unknown, as the only previous UK ESBLPE faecal colonization study involved patients with diarrhoea.
Objectives
To estimate the prevalence of CTX-M ESBLPE faecal colonization in the general adult population of England in 2014, and investigate risk factors.
Methods
A stratified random sample of 58 337 registered patients from 16 general practices within four areas of England were invited to participate by returning faeces specimens and self-completed questionnaires. Specimens were tested for ESBLPE and carbapenemase-producing Enterobacteriaceae (CPE).
Results
2430 individuals participated (4% of those invited). The estimated prevalence of colonization with CTX-M ESBLPE in England was 7.3% (95% CI 5.6%–9.4%) (Shropshire 774 participants, 4.9% colonization; Southampton City 740 participants, 9.2%; Newham 612 participants, 12.7%; Heart of Birmingham 234 individuals, 16.0%) and was particularly high in: those born in Afghanistan (10 participants, 60.0% colonization, 95% CI 29.7%–84.2%); those born on the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) (259 participants, 25.0% colonization, 95% CI 18.5%–32.9%); travellers to South Asia (India, Pakistan, Bangladesh, Sri Lanka or Nepal) in the last year (140 participants, 38.5% colonization, 95% CI 27.8%–50.5%); and healthcare domestics (8 participants, unweighted 37.5% colonization, 95% CI 8.5%–75.5%). Risk factors identified included: being born in the Indian subcontinent (aOR 5.4, 95% CI 3.0–9.7); travel to South Asia (aOR 2.9, 95% CI 1.8–4.8) or to Africa, China, South or Central America, South East or Pacific Asia or Afghanistan (aOR 2.6, 95% CI 1.7–4.1) in the last year; and working as a healthcare domestic (aOR 6.2, 95% CI 1.3–31). None of the 48 participants who took co-amoxiclav in the last year was colonized with CTX-M ESBLPE. blaCTX-M-15 accounted for 66% of CTX-M ESBLPE positives. 0.1% (two participants) were colonized with CPE.
Conclusions
CTX-M ESBLPE are established in the general population in England and prevalence is particularly high in people from certain countries of birth or with recent travel. We recommend that these findings be taken into account in guidance on the empirical management of patients presenting with a likely Enterobacteriaceae infection.
Introduction
Extensive overuse of antibiotics worldwide has led to increasing prevalence of antibiotic-resistant Gram-negative bacteria (mainly Escherichia coli) that produce ESBLs; 85%–90% of these being CTX-M genotypes.1–4 Carriage is particularly high in South Asia.4 Between 2010 and 2015 total E.coli bloodstream and urine infections in England have continued to rise and, moreover, resistance in E.coli to important hospital antibiotics such as co-amoxiclav and piperacillin/tazobactam rose significantly making treatment more difficult.5,6 ESBL-producing Enterobacteriaceae (ESBLPE) are opportunistic pathogens with large bowel colonization typically preceding an ESBLPE infection,7,8 so we believe that understanding the prevalence of faecal colonization overall and for certain sections of the general population will help inform empirical antibiotic guidance. Recent European studies indicate that travellers to countries outside Europe have an up to 10-fold higher prevalence of ESBLPE faecal colonization than the local population.3,9,10 The prevalence of CTX-M ESBLPE in diagnostic faecal specimens in a UK laboratory in 2010 was double in Middle Eastern/South Asian patients (22.8%) compared with Europeans (8.1%).11 Studies in returning European travellers have found that travel to South Asia was the most important risk factor,12 while antibiotic use10,12,13 and travellers’ diarrhoea were other possible risk factors for ESBLPE acquisition. Travel acquisition is important as between 1% and 8% of returning travellers are hospitalized,14,15 equating to 3.1–24.8 million Europeans each year.16 Importantly, there are no studies of the colonization of ESBLPE in the UK general population.
This study aimed to estimate the prevalence of colonization with CTX-M ESBLPE across different sections of the adult general population of England in 2014, including different ethnic groups, and to investigate the potential risk factors for their carriage.
Methods
The study was undertaken in four NHS Primary Care Trusts (PCTs) in England. PCTs were state-funded and commissioned primary medical care from general practices in England until 2015; this has now been taken over by Clinical Commissioning Groups. All the UK population are registered with, or have access to, a general medical practice whom they consult for primary care. The four PCTs were purposively selected to capture the UK ethnic diversity: Newham (London, highest ethnic diversity), Heart of Birmingham (predominantly Asian), Shropshire (rural, mostly white British) and Southampton City (mixed ethnicity). Three to five willing Primary Care Research Network practices from each PCT were non-randomly selected to broadly represent each area with respect to ethnicity and deprivation.
Individuals aged ≥ 18 years in selected practices were stratified by record of ethnicity (white, Asian, black, other/mixed or unknown), sex and age. Within each stratum individuals were randomly selected in 2013 and 2014 to receive an invitation letter. Respondents were sent study information, a faeces sample collection kit (not rectal swab), £5 incentive offer and a questionnaire (Figure S1, available as Supplementary data at JAC Online) (including questions on age, ethnic group, country of birth, employment, household characteristics, hospitalization, antibiotic use, travel abroad in past year and diet). Those not returning kits received a telephone reminder. Respondents were asked to collect scoops of faeces from both ends and the middle of their faeces sample and place them in a sterile container, keep the container cool and return by first-class post within 24 h to a central laboratory. As sampling progressed, faeces returns were monitored and invitations to reach Asian, black and younger age group sample sizes increased as necessary, including direct approach by general practice receptionists to individuals in some ethnic groups. For some practices all individuals within a given stratum were invited.
Laboratory analysis
Faecal samples were screened for ESBLPE, using direct culture on selective medium (Brilliance™ ESBL agar, Oxoid Ltd) for 24 h. To increase sensitivity, all samples were enriched as well as directly plated; 20 mg of each faecal sample was incubated for 24 h in 10 mL of brain heart infusion (BHI) broth containing 1 mg/L cefpodoxime17 and subcultured onto BrillianceTM agar as before.17,18 Oxidase-negative presumptive colonies of ESBLPE were defined as: +, 1–10 cfu; ++, 10–100 cfu; and +++, ≥100 cfu. One colony from each different colony morphology, from each plate of Brilliance agar, was identified using MALDI-TOF MS (Bruker UK Ltd) and tested for the blaCTX-M gene using multiplex PCR.19 Full-length gene amplification and sequencing identified blaCTX-M genotypes (Table 1). A 10 μg ertapenem disc was placed on all selective plates to detect potential carbapenemase-producing Enterobacteriaceae (CPE).20 Colonies growing in the zone of inhibition were tested by PCR for CPE genes.21
Table 1.
List of PCR and sequencing primers
| CTX-M group | Name | Primer sequence | Location |
|---|---|---|---|
| 8 and 25/26 | 5′CTXM26 | TTG ATT AAC TAC AAC CCC AT | CTX-M-26 (313–332) |
| 3′CTXM26 | GAT ATC ATT CGT CGT ACC AT | CTX-M-26 (747–728) | |
| 5′CTXMF26-1 | CTC TGC GCA ATC TGA CGT TG | CTX-M-26 (575–594) | |
| 5′CTXMF26-2 | AAG GCG GGC GAT GTT AAT GA | CTX-M-26 (18–37) | |
| 3′CTXMR26-1 | GCC AAT CGT ACG GGC AAA TG | CTX-M-26 (477–458) | |
| 1 | ISEcp1 | AAA AAT GAT TGA AAG GTG GT | ISEcp1 (−149 to −128) |
| 3′CTXM-1R | ATA CAT CGC GAC GGC TTT CT | CTX-M-1 (838–819) | |
| 5′G1S1 | ATG GTT AAA AAA TCA CTG CG | CTX-M-15 (1–20) | |
| 9 | 5′G9full | GAA TAC TGA TGT AAC ACG GAT | CTX-M-9 (−40 to −22) |
| 3′G9full | AGT TAC AGC CCT TCG GCG AT | CTX-M-9 (859–878) |
Data analysis
To estimate the prevalence of ESBLPE colonization for adults living in England in 2014 we used post-stratification weights based on the 2011 national census and number of eligible individuals at selected practices. To estimate the prevalence for each GP practice and PCT we used sampling weights based on the numbers of eligible individuals in each group at each practice. A new variable ‘region of origin’ was derived mainly from ethnic group and country of birth. Multivariable logistic regression models were used to control for country of birth and region of origin (if born in the UK) (Table 2). Based on this preliminary analysis, factors that were associated with an increased risk of colonization were considered in further analysis. We also considered the strength of evidence and the number of missing values, and the evidence from other studies; we did not follow an automated model selection process. The final multivariable model for colonization with CTX-M ESBLPE included country of birth and region of origin (if born in the UK) as a factor variable with eight categories, the base category of which was ‘born in some country not including the UK, India, Pakistan or Bangladesh (IPB), Sri Lanka, Afghanistan or the Middle East’ (Table 3). From the final model we estimated the adjusted ORs (aORs) for each risk factor, the percentage of carriers attributable to each risk factor (for example, travel to India) and, for groups of risk factors (for example, travel abroad in past year), the population attributable fraction (PAF). The PAF is dependent on both the aOR and the probability of being exposed to the factor.
Table 2.
Prevalence of colonization with blaCTX-M ESBLPE in different sections of the adult population of England in 2014
| Factor | No. of specimens | No. of blaCTX-M ESBLPE- positive specimens | blaCTX-M ESBLPE- positive (unweighted %) | Prevalence [95% CI] (weighted %)a | Test for a difference in prevalence between the groups of each factor (P value) | OR adjusted forb (country of birth and region of origin if born in the UK) (aOR) [95% CI] | Test for the effect of each factor after adjustment for country of birth and region of origin if born in the UK (P value) |
|---|---|---|---|---|---|---|---|
| Overall | 2427 | 208 | 8.6 | 7.3 [5.6, 9.4] | |||
| Practice/Medical Centre | |||||||
| Lathom Road | 129 | 12 | 9.3 | 11.1 [6.0, 19.6] | 0.02 | 1 | 0.74 |
| Stratford Village | 230 | 18 | 7.8 | 9.0 [4.6, 17.0] | 1.1 [0.5, 2.3] | ||
| St Bartholomew’s | 224 | 29 | 12.9 | 15.6 [9.3, 24.9] | 1.6 [0.8, 3.3] | ||
| Greet Medical Practice | 47 | 11 | 23.4 | 11.5 [5.6, 22.1] | 1.7 [0.6, 4.4] | ||
| High Trees | 89 | 13 | 14.6 | 18.6 [6.4, 43.5] | 1.8 [0.7, 4.1] | ||
| Khattak Memorial | 46 | 11 | 23.9 | 18.9 [8.9, 35.9] | 1.2 [0.4, 3.6] | ||
| Alma | 169 | 16 | 9.5 | 11.7 [6.0, 21.7] | 1.3 [0.6, 2.9] | ||
| Aldermoor | 365 | 23 | 6.3 | 6.8 [4.4, 10.5] | 1.1 [0.5, 2.4] | ||
| Church Stretton | 242 | 15 | 6.2 | 6.5 [3.8, 10.8] | 1.2 [0.5, 2.8] | ||
| Plas Ffynnon | 323 | 13 | 4.0 | 3.7 [2.2, 6.4] | 0.7 [0.3, 1.7] | ||
| The Caxton | 209 | 9 | 4.3 | 4.8 [2.4, 9.1] | 0.8 [0.3, 2.1] | ||
| Mulberry House | 185 | 17 | 9.2 | 9.5 [5.2, 16.8] | 1.7 [0.7, 3.9] | ||
| City Road | 52 | 6 | 11.5 | 15.5 [4.9, 39.7] | 1.0 [0.3, 2.7] | ||
| Nicholstown | 70 | 7 | 10.0 | 24.0 [9.7, 48.3] | 1.0 [0.3, 2.7] | ||
| Barking Road | 29 | 6 | 20.7 | 17.2 [6.2, 39.6] | 1.4 [0.4, 5.4] | ||
| St Deny’s | 21 | 2 | 9.5 | 6.7 [1.2, 29.6] | 1.8 [0.4, 8.8] | ||
| PCT | |||||||
| Newham | 612 | 65 | 10.6 | 12.7 [9.1, 17.4] | 0.001 | 1 | 0.33 |
| Heart of Birmingham teaching | 234 | 41 | 17.5 | 16.0 [10.2, 24.2] | 1.1 [0.7, 1.8] | ||
| Shropshire County | 774 | 37 | 4.8 | 4.9 [3.4, 7.0] | 0.7 [0.4, 1.2] | ||
| Southampton City | 740 | 58 | 7.8 | 9.2 [6.1, 13.7] | 1.0 [0.6, 1.5] | ||
| Age group (years) | |||||||
| 18–39 | 562 | 53 | 9.4 | 7.6 [5.5, 10.5] | 0.54 (test for trend: P = 0.30) | 1 | 0.84 (test for trend: P = 0.77) |
| 40–49 | 356 | 39 | 11.0 | 5.6 [3.5, 8.8] | 1.1 [0.7, 1.8] | ||
| 50–59 | 435 | 33 | 7.6 | 6.5 [4.3, 9.8] | 0.9 [0.6, 1.5] | ||
| 60–69 | 566 | 42 | 7.4 | 7.1 [5.2, 9.6] | 1.1 [0.7, 1.7] | ||
| 70–79 | 366 | 32 | 8.7 | 7.3 [5.0, 10.5] | 1.3 [0.8, 2.1] | ||
| 80–100 | 142 | 9 | 6.3 | 3.7 [1.6, 8.4] | 0.8 [0.4, 1.8] | ||
| Gender | |||||||
| male | 1052 | 106 | 10.1 | 7.3 [5.5, 9.5] | 0.40 | 1.2 [0.9, 1.6] | 0.26 |
| female | 1378 | 102 | 7.4 | 6.3 [5.0, 7.8] | 1 | ||
| GP record of whether antibiotic used in the year before | |||||||
| no | 1641 | 132 | 8.0 | 7.0 [5.6, 8.6] | 0.48 | 1 | 0.64 |
| yes | 786 | 76 | 9.7 | 6.1 [4.6, 8.1] | 1.1 [0.8, 1.5] | ||
| Born in the UK? | |||||||
| UK | 1596 | 103 | 6.5 | 5.9 [4.6, 7.4] | <0.001 | ||
| other | 760 | 98 | 12.9 | 11.4 [8.9, 14.4] | |||
| Country of birth | |||||||
| UK | 1596 | 103 | 6.5 | 5.9 [4.6, 7.4] | <0.001 | ||
| Ireland | 24 | 1 | 4.2 | 4.2 [0.6, 24.5] | |||
| India | 136 | 33 | 24.3 | 28.7 [18.8, 41.2] | |||
| Pakistan | 81 | 21 | 25.9 | 18.6 [10.5, 30.8] | |||
| Bangladesh | 34 | 8 | 23.5 | 23.5 [11.8, 41.3] | |||
| Sri Lanka | 8 | 2 | 25.0 | 25.0 [7.2, 59.0] | |||
| Nepal | 4 | 0 | 0 | 0 [0, 60.2] | |||
| Afghanistan | 10 | 6 | 60.0 | 60.0 [29.7, 84.2] | |||
| Africa | 134 | 6 | 4.5 | 5.8 [2.2, 14.5] | |||
| Australasia | 7 | 1 | 14.3 | 9.5 [1.2, 48.0] | |||
| Caribbean | 76 | 7 | 9.2 | 7.6 [3.7, 15.1] | |||
| China | 17 | 0 | 0 | 0 [0, 19.5] | |||
| Eastern Europe | 71 | 2 | 2.8 | 3.1 [0.6, 15.1] | |||
| Middle East | 18 | 3 | 16.7 | 15.5 [4.7, 40.5] | |||
| North America | 10 | 1 | 10.0 | 8.3 [1.0, 44.6] | |||
| South or Central America | 13 | 0 | 0 | 0 [0, 24.7] | |||
| South East or Pacific Asia | 31 | 3 | 9.7 | 13.2 [4.0, 35.6] | |||
| Western Europe (excl. UK and Ireland) | 67 | 3 | 4.5 | 5.7 [1.5, 19.5] | |||
| Mauritius | 10 | 0 | 0 | 0 [0, 30.8] | |||
| Country of birth among the Indian, Pakistani or Bangladeshi (IPB) ethnic group | |||||||
| Asian-IPB; born in UK | 46 | 8 | 17.4 | 18.5 [7.5, 39.1] | <0.001 | ||
| Asian-IPB; born in India | 128 | 31 | 24.2 | 29.5 [19.2, 42.4] | |||
| Asian-IPB; born in Pakistan | 78 | 20 | 25.6 | 17.8 [9.8, 30.2] | |||
| Asian-IPB; born in Bangladesh | 34 | 8 | 23.5 | 23.5 [11.8, 41.3] | |||
| Asian-IPB; born in Africa | 17 | 0 | 0 | 0 [0, 19.5] | |||
| Asian-IPB; born in some other country | 6 | 2 | 33.3 | 33.1 [6.1, 78.9] | |||
| not Asian-IPB | 2090 | 136 | 6.5 | 5.9 [4.8, 7.2] | |||
| Ethnic groupc | |||||||
| White-British | 1532 | 92 | 6.0 | 5.7 [4.5, 7.2] | <0.001 | ||
| White-Irish | 30 | 1 | 3.3 | 3.3 [0.5, 20.2] | |||
| White-Gypsy or Irish Traveller | 2 | 0 | 0 | 0 [0, 84.2] | |||
| White-Other | 169 | 9 | 5.3 | 5.1 [2.2, 11.1] | |||
| Mixed-White and Black Caribbean | 6 | 0 | 0 | 0 [0, 45.9] | |||
| Mixed-White and Black African | 6 | 0 | 0 | 0 [0, 45.9] | |||
| Mixed-White and Asian | 16 | 2 | 12.5 | 12.5 [3.1, 38.6] | |||
| Mixed-Other | 22 | 1 | 4.5 | 4.5 [0.6, 26.2] | |||
| Asian-Indian | 183 | 38 | 20.8 | 26.1 [17.6, 36.8] | |||
| Asian-Pakistani | 114 | 26 | 22.8 | 17.4 [10.2, 28.0] | |||
| Asian-Bangladeshi | 40 | 8 | 20.0 | 20.0 [9.9, 36.3] | |||
| Asian-Chinese | 23 | 0 | 0 | 0 [0, 14.8] | |||
| Asian-Other | 68 | 14 | 20.6 | 20.6 [11.7, 33.6] | |||
| Black-African | 100 | 5 | 5.0 | 5.0 [2.2, 11.3] | |||
| Black-Caribbean | 96 | 10 | 10.4 | 11.2 [6.2, 19.4] | |||
| Black-Other | 3 | 0 | 0 | 0 [0, 70.8] | |||
| Arab | 7 | 1 | 14.3 | 14.3 [2.0, 58.1] | |||
| Other | 10 | 1 | 10.0 | 10.0 [1.4, 46.8] | |||
| Region of origind | |||||||
| UK | 1505 | 87 | 5.8 | 5.5 [4.3, 7.0] | <0.001 | ||
| Ireland | 38 | 2 | 5.3 | 4.8 [1.2, 17.3] | |||
| India | 192 | 39 | 20.3 | 24.4 [16.4, 34.7] | |||
| Pakistan | 119 | 28 | 23.5 | 18.6 [11.4, 28.9] | |||
| Bangladesh | 41 | 9 | 22.0 | 22.6 [11.8, 38.9] | |||
| Sri Lanka | 8 | 2 | 25.0 | 25.0 [7.2, 59.0] | |||
| Nepal | 5 | 0 | 0 | 0 [0, 52.2] | |||
| Afghanistan | 10 | 6 | 60.0 | 60.0 [29.7, 84.2] | |||
| Africa | 118 | 6 | 5.1 | 6.6 [2.5, 16.4] | |||
| Australasia | 7 | 1 | 14.3 | 9.5 [1.2, 47.8] | |||
| Caribbean | 118 | 11 | 9.3 | 8.2 [3.9, 16.5] | |||
| China | 17 | 0 | 0 | 0 [0, 19.5] | |||
| Eastern Europe | 77 | 2 | 2.6 | 2.8 [0.5, 13.6] | |||
| Middle East | 16 | 2 | 12.5 | 12.3 [2.8, 41.2] | |||
| North America | 9 | 1 | 11.1 | 11.7 [1.4, 55.7] | |||
| South or Central America | 7 | 1 | 14.3 | 7.1 [1.0, 37.4] | |||
| South East or Pacific Asia | 31 | 3 | 9.7 | 14.5 [4.7, 36.7] | |||
| Western Europe (excl. UK and Ireland) | 77 | 7 | 9.1 | 10.8 [4.6, 23.5] | |||
| Mixed | 12 | 0 | 0 | 0 [0, 26.5] | |||
| Mauritius/Seychelles | 8 | 0 | 0 | 0 [0, 36.9] | |||
| Combination of region of origind and country of birth with 11 groups | |||||||
| born in UK; UK origin | 1459 | 85 | 5.8 | 5.6 [4.4, 7.1] | <0.001 | ||
| born in UK; Asia-IPB origin | 52 | 8 | 15.4 | 15.7 [6.3, 33.9] | |||
| born in UK; Caribbean origin | 32 | 4 | 12.5 | 11.9 [3.3, 34.8] | |||
| born in UK; Other origin | 45 | 5 | 11.1 | 8.1 [3.0, 20.2] | |||
| born in India | 136 | 33 | 24.3 | 28.5 [18.7, 41.0] | |||
| born in Pakistan | 81 | 21 | 25.9 | 18.6 [10.5, 30.7] | |||
| born in Bangladesh | 34 | 8 | 23.5 | 23.5 [11.8, 41.3] | |||
| born in Sri Lanka | 8 | 2 | 25.0 | 25.0 [7.2, 59.0] | |||
| born in Afghanistan | 10 | 6 | 60.0 | 60.0 [29.7, 84.2] | |||
| born in the Middle East | 18 | 3 | 16.7 | 15.5 [4.7, 40.5] | |||
| born in some other country | 464 | 24 | 5.2 | 5.3 [3.3, 8.5] | |||
| Combination of region of origind and country of birth with 8 groups | |||||||
| born in UK; UK origin | 1459 | 85 | 5.8 | 5.6 [4.4, 7.1] | <0.001 | ||
| born in UK; Asia-IPB origin | 52 | 8 | 15.4 | 15.7 [6.3, 33.9] | |||
| born in UK; Caribbean origin | 32 | 4 | 12.5 | 11.9 [3.3, 34.8] | |||
| born in UK; Other origin | 45 | 5 | 11.1 | 8.1 [3.0, 20.2] | |||
| born in India, Pakistan, Bangladesh or Sri Lanka | 259 | 64 | 24.7 | 25.0 [18.5, 32.9] | |||
| born in Afghanistan | 10 | 6 | 60.0 | 60.0 [29.7, 84.2] | |||
| born in the Middle East | 18 | 3 | 16.7 | 15.5 [4.7, 40.5] | |||
| born in some other country | 464 | 24 | 5.2 | 5.3 [3.3, 8.5] | |||
| Do you work in a healthcare setting? | |||||||
| no | 2074 | 178 | 8.6 | 6.6 [5.5, 8.0] | 0.60 | 1 | 0.89 |
| yes | 297 | 23 | 7.7 | 7.7 [4.6, 12.6] | 1.0 [0.6, 1.7] | ||
| Type of healthcare worker | |||||||
| nurse | 86 | 6 | 7.0 | 5.7 [2.1, 14.5] | 0.39 | 0.8 [0.3, 2.1] | 0.25 |
| care assistant | 59 | 2 | 3.4 | 8.9 [2.2, 29.9] | 0.4 [0.1, 1.9] | ||
| doctor | 30 | 4 | 13.3 | 18.0 [6.6, 40.5] | 1.6 [0.6, 4.6] | ||
| domestic | 8 | 3 | 37.5 | 20.2 [3.9, 61.1] | 6.0 [1.1, 33.3] | ||
| other work in healthcare (incl. unsp.) | 114 | 8 | 7.0 | 5.6 [2.3, 13.1] | 1.1 [0.5, 2.3] | ||
| not working in healthcare | 2074 | 178 | 8.6 | 6.6 [5.5, 8.0] | 1 | ||
| Type of healthcare worker | |||||||
| hands-on healthcare worker | 175 | 12 | 6.9 | 8.7 [4.4, 16.5] | 0.14 | 0.8 [0.4, 1.6] | 0.53 |
| hands-off healthcare worker (incl. unsp.) | 122 | 11 | 9.0 | 6.3 [2.9, 13.2] | 1.4 [0.7, 2.6] | ||
| not working in healthcare | 2074 | 178 | 8.6 | 6.6 [5.5, 8.0] | 1 | ||
| Does your work involve contact with animals? | |||||||
| no | 2219 | 192 | 8.7 | 6.8 [5.6, 8.1] | 0.93 | 1 | 0.75 |
| yes | 85 | 6 | 7.1 | 6.5 [2.8, 14.4] | 1.2 [0.5, 2.7] | ||
| Type of work involving contact with animals | |||||||
| farm work (incl. meat prep.) | 48 | 5 | 10.4 | 9.7 [3.9, 22.6] | 0.76 | 1.8 [0.7, 4.5] | 0.33 |
| veterinary work | 13 | 0 | 0 | 0 [0, 24.7] | 0 | ||
| other work with animals (incl. unsp.) | 24 | 1 | 4.2 | 2.8 [0.4, 17.8] | 0.7 [0.1, 5.4] | ||
| not working with animals | 2219 | 192 | 8.7 | 6.8 [5.6, 8.1] | 1 | ||
| Have you been hospitalized in the past 12 months? | |||||||
| no | 2126 | 174 | 8.2 | 6.6 [5.4, 8.0] | 0.63 | 1 | 0.73 |
| yes | 240 | 24 | 10.0 | 7.6 [4.4, 12.9] | 1.1 [0.7, 1.7] | ||
| Have you taken any antibiotics in the past 12 months? | |||||||
| no | 1427 | 111 | 7.8 | 6.8 [5.4, 8.6] | 0.96 | 1 | 0.88 |
| yes | 777 | 73 | 9.4 | 6.8 [5.1, 8.9] | 1.0 [0.7, 1.4] | ||
| Have you taken any amoxicillin in the past 12 months? | |||||||
| no | 1835 | 147 | 8.0 | 6.8 [5.5, 8.4] | 0.90 | 1 | 0.59 |
| yes | 369 | 37 | 10.0 | 6.6 [4.5, 9.7] | 0.9 [0.6, 1.3] | ||
| Have you taken any trimethoprim in the past 12 months? | |||||||
| no | 2158 | 181 | 8.4 | 6.8 [5.7, 8.2] | 0.64 | 1 | 0.82 |
| yes | 46 | 3 | 6.5 | 5.3 [1.7, 15.0] | 0.9 [0.3, 2.8] | ||
| Have you taken any erythromycin in the past 12 months? | |||||||
| no | 2157 | 179 | 8.3 | 6.7 [5.6, 8.1] | 0.43 | 1 | 0.57 |
| yes | 47 | 5 | 10.6 | 10.3 [3.6, 26.1] | 1.3 [0.5, 3.5] | ||
| Have you taken any clarithromycin in the past 12 months? | |||||||
| no | 2178 | 181 | 8.3 | 6.8 [5.7, 8.2] | 0.83 | 1 | 0.67 |
| yes | 26 | 3 | 11.5 | 5.9 [1.6, 19.3] | 1.3 [0.4, 5.0] | ||
| Have you taken any co-amoxiclav in the past 12 months? | |||||||
| no | 2156 | 184 | 8.5 | 7.0 [5.8, 8.4] | 0.03 | 1 | 0.004 |
| yes | 48 | 0 | 0 | 0 [0, 7.4] | 0 | ||
| Have you taken any ciprofloxacin in the past 12 months? | |||||||
| no | 2189 | 180 | 8.2 | 6.8 [5.6, 8.1] | 0.61 | 1 | 0.03 |
| yes | 15 | 4 | 26.7 | 9.5 [2.5, 30.1] | 3.0 [1.1, 8.1] | ||
| Have you taken any cefalexin in the past 12 months? | |||||||
| no | 2177 | 180 | 8.3 | 6.8 [5.6, 8.1] | 0.34 | 1 | 0.31 |
| yes | 27 | 4 | 14.8 | 11.4 [3.8, 29.3] | 1.8 [0.6, 5.8] | ||
| Have you taken any other antibiotics in the past 12 months? | |||||||
| no | 2082 | 177 | 8.5 | 6.8 [5.6, 8.2] | 0.97 | 1 | 0.26 |
| yes | 122 | 7 | 5.7 | 6.7 [2.5, 16.7] | 0.6 [0.3, 1.4] | ||
| Have you taken co-amoxiclav, ciprofloxacin or cefalexin in the past 12 months? | |||||||
| no | 2119 | 177 | 8.4 | 6.9 [5.7, 8.3] | 0.22 | 1 | 0.76 |
| yes | 85 | 7 | 8.2 | 4.1 [1.7, 9.4] | 0.9 [0.4, 1.9] | ||
| Do you currently have a urinary catheter? | |||||||
| no | 2275 | 192 | 8.4 | 6.8 [5.7, 8.2] | 0.27 | 1 | 0.62 |
| yes | 15 | 2 | 13.3 | 15.5 [3.3, 49.3] | 1.4 [0.4, 5.5] | ||
| Do you regularly eat beef? | |||||||
| no | 841 | 92 | 10.9 | 7.5 [5.8, 9.7] | 0.39 | 1 | 0.87 |
| yes | 1517 | 105 | 6.9 | 6.5 [5.1, 8.2] | 1.0 [0.7, 1.4] | ||
| Do you regularly eat pork/ham/bacon? | |||||||
| no | 558 | 76 | 13.6 | 11.4 [8.5, 15.1] | <0.001 | 1 | 0.36 |
| yes | 1681 | 103 | 6.1 | 5.8 [4.6, 7.3] | 0.8 [0.6, 1.2] | ||
| Do you regularly eat lamb? | |||||||
| no | 940 | 66 | 7.0 | 6.5 [4.8, 8.7] | 0.68 | 1 | 0.31 |
| yes | 1291 | 121 | 9.4 | 7.1 [5.6, 8.9] | 1.2 [0.9, 1.7] | ||
| Do you regularly eat chicken? | |||||||
| no | 232 | 22 | 9.5 | 7.5 [4.6, 12.1] | 0.67 | 1 | 0.68 |
| yes | 2105 | 176 | 8.4 | 6.7 [5.5, 8.1] | 1.1 [0.7, 1.9] | ||
| Do you regularly eat fish/seafood? | |||||||
| no | 295 | 33 | 11.2 | 9.4 [6.0, 14.3] | 0.10 | 1 | 0.59 |
| yes | 2001 | 157 | 7.8 | 6.3 [5.1, 7.6] | 0.9 [0.6, 1.4] | ||
| Do you regularly eat salad products? | |||||||
| no | 121 | 16 | 13.2 | 9.1 [5.2, 15.4] | 0.30 | 1 | 0.11 |
| yes | 2207 | 179 | 8.1 | 6.7 [5.5, 8.1] | 0.6 [0.4, 1.1] | ||
| Not regularly eating meat | |||||||
| no | 2172 | 178 | 8.2 | 6.6 [5.4, 8.0] | 0.24 | 1 | 0.89 |
| yes | 176 | 20 | 11.4 | 9.2 [5.5, 15.1] | 1.0 [0.6, 1.8] | ||
| Not regularly eating meat, fish or seafood (vegetarian) | |||||||
| no | 2251 | 186 | 8.3 | 6.6 [5.5, 8.0] | 0.25 | 1 | 0.55 |
| yes | 105 | 12 | 11.4 | 10.0 [5.0, 18.8] | 0.8 [0.4, 1.6] | ||
| In the past year, have you spent time in any country outside the UK? | |||||||
| no | 1142 | 75 | 6.6 | 4.6 [3.3, 6.3] | <0.001 | 1 | <0.001 |
| yes | 1234 | 127 | 10.3 | 8.8 [7.1, 10.8] | 2.0 [1.5, 2.8] | ||
| Have you been hospitalized abroad in the last year? | |||||||
| no | 2364 | 199 | 8.4 | 6.6 [5.5, 8.0] | <0.001 | 1 | 0.04 |
| yes | 12 | 3 | 25.0 | 37.7 [13.1, 70.8] | 3.6 [1.1, 12.2] | ||
| Have you had diarrhoea abroad in the last year? | |||||||
| no | 2239 | 186 | 8.3 | 6.5 [5.4, 7.8] | 0.09 | 1 | 0.03 |
| yes | 137 | 16 | 11.7 | 11.2 [6.1, 19.7] | 1.8 [1.0, 3.2] | ||
| Have you been abroad to India in the last year? | |||||||
| no | 2279 | 174 | 7.6 | 6.2 [5.1, 7.5] | <0.001 | 1 | 0.001 |
| yes | 97 | 28 | 28.9 | 33.7 [22.6, 47.0] | 3.2 [1.7, 6.2] | ||
| Have you been abroad to Pakistan in the last year? | |||||||
| no | 2352 | 191 | 8.1 | 6.6 [5.5, 7.8] | <0.001 | 1 | 0.007 |
| yes | 24 | 11 | 45.8 | 48.6 [24.8, 73.1] | 3.6 [1.4, 9.1] | ||
| Have you been abroad to Bangladesh in the last year? | |||||||
| no | 2365 | 200 | 8.5 | 6.7 [5.6, 8.1] | 0.16 | 1 | 0.93 |
| yes | 11 | 2 | 18.2 | 17.4 [4.2, 50.1] | 0.9 [0.2, 4.9] | ||
| Have you been abroad to Sri Lanka in the last year? | |||||||
| no | 2367 | 199 | 8.4 | 6.7 [5.6, 8.0] | <0.001 | 1 | 0.02 |
| yes | 9 | 3 | 33.3 | 39.8 [12.4, 75.6] | 6.4 [1.3, 31.4] | ||
| Have you been abroad to Nepal in the last year? | |||||||
| no | 2373 | 201 | 8.5 | 6.6 [5.5, 7.9] | <0.001 | 1 | 0.11 |
| yes | 3 | 1 | 33.3 | 76.8 [17.9, 98.0] | 8.9 [0.6, 133] | ||
| Have you been abroad to South Asia (India, Pakistan, Bangladesh, Sri Lanka or Nepal) in the last year? | |||||||
| no | 2236 | 158 | 7.1 | 5.8 [4.7, 7.1] | <0.001 | 1 | <0.001 |
| yes | 140 | 44 | 31.4 | 38.5 [27.8, 50.5] | 3.3 [2.0, 5.6] | ||
| Have you been abroad to Afghanistan in the last year? | |||||||
| no | 2374 | 201 | 8.5 | 6.7 [5.6, 8.1] | 0.43 | 1 | 0.22 |
| yes | 2 | 1 | 50.0 | 17.1 [1.3, 76.1] | 3.5 [0.5, 25.2] | ||
| Have you been abroad to Africa in the last year? | |||||||
| no | 2235 | 184 | 8.2 | 6.2 [5.1, 7.5] | <0.001 | 1 | 0.006 |
| yes | 141 | 18 | 12.8 | 16.4 [9.4, 27.0] | 2.2 [1.2, 3.7] | ||
| Have you been abroad to Australasia in the last year? | |||||||
| no | 2341 | 200 | 8.5 | 6.7 [5.6, 8.1] | 0.75 | 1 | 0.94 |
| yes | 35 | 2 | 5.7 | 8.6 [1.9, 31.5] | 0.9 [0.2, 4.0] | ||
| Have you been abroad to the Caribbean in the last year? | |||||||
| no | 2319 | 197 | 8.5 | 6.7 [5.6, 8.0] | 0.49 | 1 | 0.50 |
| yes | 57 | 5 | 8.8 | 9.8 [3.3, 25.5] | 1.4 [0.5, 3.9] | ||
| Have you been abroad to China in the last year? | |||||||
| no | 2352 | 198 | 8.4 | 6.6 [5.5, 7.9] | 0.03 | 1 | 0.06 |
| yes | 24 | 4 | 16.7 | 22.9 [7.1, 53.5] | 2.9 [0.9, 9.1] | ||
| Have you been abroad to Eastern Europe in the last year? | |||||||
| no | 2127 | 187 | 8.8 | 6.9 [5.7, 8.3] | 0.51 | 1 | 0.77 |
| yes | 249 | 15 | 6.0 | 5.7 [3.3, 9.7] | 0.9 [0.5, 1.6] | ||
| Have you been abroad to the Middle East in the last year? | |||||||
| no | 2329 | 195 | 8.4 | 6.7 [5.6, 8.0] | 0.29 | 1 | 0.22 |
| yes | 47 | 7 | 14.9 | 10.6 [4.5, 23.0] | 1.7 [0.7, 3.8] | ||
| Have you been abroad to North America in the last year? | |||||||
| no | 2218 | 186 | 8.4 | 6.4 [5.3, 7.7] | 0.07 | 1 | 0.06 |
| yes | 158 | 16 | 10.1 | 11.3 [6.2, 19.8] | 1.7 [1.0, 3.0] | ||
| Have you been abroad to South or Central America in the last year? | |||||||
| no | 2347 | 197 | 8.4 | 6.6 [5.5, 7.9] | 0.05 | 1 | 0.04 |
| yes | 29 | 5 | 17.2 | 19.4 [6.3, 46.4] | 3.1 [1.1, 9.2] | ||
| Have you been abroad to South East or Pacific Asia in the last year? | |||||||
| no | 2309 | 191 | 8.3 | 6.4 [5.3, 7.7] | 0.003 | 1 | 0.001 |
| yes | 67 | 11 | 16.4 | 17.4 [9.0, 31.1] | 3.3 [1.6, 6.5] | ||
| Have you been abroad to a country in Western Europe (not incl. UK and Ireland) in the last year? | |||||||
| no | 1607 | 144 | 9.0 | 6.4 [5.1, 7.9] | 0.41 | 1 | 0.19 |
| yes | 769 | 58 | 7.5 | 7.4 [5.5, 10.0] | 1.3 [0.9, 1.8] | ||
| Have you been abroad to Maldives, Mauritius or Seychelles in the last year? | |||||||
| no | 2358 | 202 | 8.6 | 6.8 [5.7, 8.1] | 0.39 | 1 | 0.14 |
| yes | 18 | 0 | 0 | 0 [0, 18.5] | 0 | ||
| Have you been abroad to: Australia, the Caribbean, Eastern Europe, the Middle East, North America, Western Europe (not incl. UK and Ireland), Maldives, Mauritius or Seychelles in the last year? | |||||||
| no | 1335 | 119 | 8.9 | 6.0 [4.7, 7.7] | 0.20 | 1 | 0.03 |
| yes | 1041 | 83 | 8.0 | 7.6 [5.9, 9.8] | 1.4 [1.0, 2.0] | ||
| Have you been abroad to: Africa, China, South or Central America, South East or Pacific Asia or Afghanistan in the last year? | |||||||
| no | 2125 | 166 | 7.8 | 5.6 [4.6, 6.8] | <0.001 | 1 | <0.001 |
| yes | 251 | 36 | 14.3 | 16.6 [11.3, 23.7] | 2.8 [1.8, 4.3] | ||
| Number of adults living in respondent’s household (incl. respondent) | |||||||
| 1 | 413 | 29 | 7.0 | 6.1 [3.8, 9.5] | 0.02 (test for trend: P = 0.06) | 1 | 0.65 (test for trend: P = 0.96) |
| 2 | 1177 | 87 | 7.4 | 5.9 [4.6, 7.5] | 1.0 [0.6, 1.6] | ||
| 3 | 406 | 39 | 9.6 | 8.3 [5.3, 13.0] | 1.1 [0.6, 1.8] | ||
| 4 | 208 | 19 | 9.1 | 6.1 [3.5, 10.6] | 0.8 [0.4, 1.6] | ||
| 5 | 86 | 15 | 17.4 | 18.8 [9.8, 32.9] | 1.7 [0.8, 3.5] | ||
| 6+ | 67 | 10 | 14.9 | 7.6 [3.0, 17.9] | 0.9 [0.3, 2.1] | ||
| Number of children aged 5–17 years living in respondent’s household | |||||||
| 0 | 1888 | 152 | 8.1 | 6.9 [5.6, 8.4] | 0.18 (test for trend: P = 0.77) | 1 | 0.24 (test for trend: P = 0.47) |
| 1 | 245 | 15 | 6.1 | 3.6 [1.8, 7.0] | 0.6 [0.3, 1.0] | ||
| 2 | 156 | 20 | 12.8 | 9.5 [5.1, 17.1] | 1.1 [0.6, 1.9] | ||
| 3+ | 68 | 12 | 17.6 | 8.1 [3.4, 17.9] | 0.8 [0.4, 1.6] | ||
| Number of children aged 0–4 years living in respondent’s household | |||||||
| 0 | 2078 | 165 | 7.9 | 6.4 [5.2, 7.8] | 0.12 (test for trend: P = 0.28) | 1 | 0.85 (test for trend: P = 0.24) |
| 1 | 210 | 25 | 11.9 | 9.9 [6.1, 15.6] | 1.0 [0.6, 1.7] | ||
| 2+ | 69 | 9 | 13.0 | 4.6 [1.9, 10.4] | 1.2 [0.6, 2.3] | ||
| Number of children aged 0–17 years living in respondent’s household | |||||||
| 0 | 1719 | 132 | 7.7 | 6.5 [5.2, 8.1] | 0.96 (test for trend: P = 0.44) | 1 | 0.78 (test for trend: P = 0.96) |
| 1 | 291 | 23 | 7.9 | 7.0 [4.2, 11.6] | 0.8 [0.5, 1.3] | ||
| 2 | 246 | 27 | 11.0 | 7.1 [4.1, 12.0] | 1.0 [0.6, 1.6] | ||
| 3+ | 101 | 17 | 16.8 | 7.9 [3.9, 15.1] | 0.9 [0.5, 1.6] | ||
| Number of people living in respondent’s household | |||||||
| 1 | 377 | 27 | 7.2 | 5.8 [3.6, 9.4] | 0.07 (test for trend: P = 0.06) | 1 | 0.71 (test for trend: P = 1.00) |
| 2 | 901 | 64 | 7.1 | 6.2 [4.8, 8.1] | 1.0 [0.6, 1.7] | ||
| 3 | 393 | 30 | 7.6 | 7.2 [4.3, 11.8] | 0.9 [0.5, 1.6] | ||
| 4 | 317 | 21 | 6.6 | 4.6 [2.5, 8.2] | 0.7 [0.4, 1.4] | ||
| 5 | 183 | 25 | 13.7 | 9.6 [6.0, 15.1] | 1.2 [0.7, 2.3] | ||
| 6 | 91 | 14 | 15.4 | 13.2 [6.1, 26.4] | 1.0 [0.4, 2.2] | ||
| 7 | 46 | 10 | 21.7 | 19.0 [8.0, 38.7] | 1.5 [0.6, 3.8] | ||
| 8+ | 49 | 8 | 16.3 | 6.6 [2.9, 14.0] | 0.7 [0.3, 1.7] | ||
| Do you have any cats living in your house or flat? | |||||||
| no | 1939 | 169 | 8.7 | 6.9 [5.7, 8.4] | 0.70 | 1 | 0.74 |
| yes | 422 | 32 | 7.6 | 6.3 [4.1, 9.7] | 1.1 [0.7, 1.6] | ||
| Do you have any dogs living in your house or flat? | |||||||
| no | 1989 | 178 | 8.9 | 7.1 [5.9, 8.6] | 0.20 | 1 | 0.88 |
| yes | 362 | 22 | 6.1 | 5.0 [3.0, 8.3] | 1.0 [0.6, 1.7] | ||
| Do you have any rabbits living in your house or flat? | |||||||
| no | 2121 | 183 | 8.6 | 6.5 [5.4, 7.8] | 0.94 | 1 | 0.79 |
| yes | 45 | 3 | 6.7 | 6.8 [1.9, 21.6] | 0.8 [0.2, 3.0] | ||
| Do you have any guinea pigs living in your house or flat? | |||||||
| no | 2091 | 182 | 8.7 | 6.6 [5.5, 7.9] | 0.23 | 1 | 0.21 |
| yes | 36 | 1 | 2.8 | 0.9 [0.1, 6.4] | 0.3 [0.04, 2.0] | ||
| Do you have any hamsters living in your house or flat? | |||||||
| no | 2092 | 183 | 8.7 | 6.7 [5.5, 8.1] | 0.23 | 1 | 0.43 |
| yes | 30 | 1 | 3.3 | 2.2 [0.3, 14.1] | 0.5 [0.08. 3.0] | ||
| Do you live in a nursing home, care home or residential home? | |||||||
| no | 2348 | 200 | 8.5 | 6.8 [5.7, 8.1] | 1.00 | 1 | 0.14 |
| yes | 9 | 0 | 0 | 0 [0, 33.6] | 0 | ||
| Is there anyone in your household who works in a healthcare setting? | |||||||
| no | 2045 | 173 | 8.5 | 6.7 [5.5, 8.1] | 0.97 | 1 | 0.81 |
| yes | 287 | 23 | 8.0 | 6.8 [4.0, 11.3] | 0.9 [0.6, 1.5] | ||
| Is there anyone in your household whose work involves contact with animals? | |||||||
| no | 2225 | 189 | 8.5 | 6.9 [5.7, 8.3] | 0.39 | 1 | 0.76 |
| yes | 91 | 6 | 6.6 | 4.7 [2.0, 10.9] | 1.1 [0.5, 2.7] | ||
| Is there anyone in your household who has been hospitalized in the past year? | |||||||
| no | 2096 | 178 | 8.5 | 6.9 [5.7, 8.3] | 0.61 | 1 | 0.40 |
| yes | 250 | 21 | 8.4 | 5.9 [3.2, 10.5] | 0.8 [0.5, 1.4] | ||
| Is there anyone in your household who has taken antibiotics in the past year? | |||||||
| no | 1546 | 114 | 7.4 | 6.4 [5.1, 8.1] | 0.91 | 1 | 0.57 |
| yes | 737 | 73 | 9.9 | 6.6 [4.9, 8.9] | 1.1 [0.8, 1.5] | ||
| Is there anyone in your household who has spent time abroad in the last year? | |||||||
| no | 1318 | 96 | 7.3 | 5.6 [4.3, 7.3] | 0.03 | 1 | <0.001 |
| yes | 1017 | 103 | 10.1 | 8.3 [6.5, 10.5] | 1.8 [1.3, 2.4] | ||
| Housemate been to India in the last year | |||||||
| no | 2251 | 178 | 7.9 | 6.5 [5.4, 7.8] | <0.001 | 1 | 0.01 |
| yes | 83 | 21 | 25.3 | 24.9 [14.5, 39.2] | 2.4 [1.3, 4.6] | ||
| Housemate been to Pakistan in the last year | |||||||
| no | 2314 | 195 | 8.4 | 6.8 [5.7, 8.1] | 0.21 | 1 | 0.85 |
| yes | 20 | 4 | 20.0 | 15.6 [3.9, 45.5] | 1.1 [0.3, 3.6] | ||
| Housemate been to Bangladesh in the last year | |||||||
| no | 2324 | 199 | 8.6 | 6.8 [5.7, 8.2] | 1.00 | 1 | 0.02 |
| yes | 10 | 0 | 0 | 0 [0, 30.8] | 0 | ||
| Housemate been to Sri Lanka in the last year | |||||||
| no | 2326 | 197 | 8.5 | 6.8 [5.6, 8.1] | 0.06 | 1 | 0.13 |
| yes | 8 | 2 | 25.0 | 26.6 [5.5, 69.5] | 3.7 [0.7, 19.6] | ||
| Housemate been to the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) in the last year | |||||||
| no | 2214 | 172 | 7.8 | 6.4 [5.3, 7.7] | <0.001 | 1 | 0.05 |
| yes | 120 | 27 | 22.5 | 22.7 [14.2, 34.1] | 1.7 [1.0, 3.0] | ||
| Housemate been to Afghanistan in the last year | |||||||
| no | 2332 | 199 | 8.5 | 6.8 [5.7, 8.2] | 1.00 | 1 | 0.39 |
| yes | 2 | 0 | 0 | 0 [0, 84.2] | 0 | ||
| Housemate been to Africa in the last year | |||||||
| no | 2239 | 190 | 8.5 | 6.6 [5.5, 8.0] | 0.20 | 1 | 0.27 |
| yes | 95 | 9 | 9.5 | 11.5 [5.0, 24.3] | 1.5 [0.7, 3.0] | ||
| Housemate been to Australasia in the last year | |||||||
| no | 2304 | 197 | 8.6 | 6.7 [5.6, 8.0] | 0.43 | 1 | 0.86 |
| yes | 30 | 2 | 6.7 | 12.5 [2.5, 44.0] | 1.1 [0.3, 4.9] | ||
| Housemate been to the Caribbean in the last year | |||||||
| no | 2288 | 196 | 8.6 | 6.8 [5.7, 8.1] | 0.66 | 1 | 0.85 |
| yes | 46 | 3 | 6.5 | 8.9 [2.6, 26.9] | 1.1 [0.3, 3.8] | ||
| Housemate been to China in the last year | |||||||
| no | 2313 | 196 | 8.5 | 6.7 [5.6, 8.0] | 0.07 | 1 | 0.16 |
| yes | 21 | 3 | 14.3 | 20.1 [5.6, 51.4] | 2.6 [0.7, 9.9] | ||
| Housemate been to Eastern Europe in the last year | |||||||
| no | 2126 | 185 | 8.7 | 6.9 [5.7, 8.2] | 0.86 | 1 | 0.79 |
| yes | 208 | 14 | 6.7 | 6.5 [3.4, 12.1] | 1.1 [0.6, 1.9] | ||
| Housemate been to the Middle East in the last year | |||||||
| no | 2291 | 189 | 8.2 | 6.7 [5.6, 8.0] | 0.02 | 1 | 0.03 |
| yes | 43 | 10 | 23.3 | 16.9 [7.5, 33.6] | 2.5 [1.1, 5.6] | ||
| Housemate been to North America in the last year | |||||||
| no | 2192 | 185 | 8.4 | 6.8 [5.6, 8.1] | 0.70 | 1 | 0.15 |
| yes | 142 | 14 | 9.9 | 7.6 [4.2, 13.4] | 1.6 [0.9, 2.8] | ||
| Housemate been to South or Central America in the last year | |||||||
| no | 2309 | 196 | 8.5 | 6.8 [5.7, 8.1] | 0.77 | 1 | 0.16 |
| yes | 25 | 3 | 12.0 | 8.1 [2.5, 23.4] | 2.4 [0.7, 8.2] | ||
| Housemate been to South East or Pacific Asia in the last year | |||||||
| no | 2285 | 191 | 8.4 | 6.5 [5.4, 7.8] | 0.01 | 1 | 0.006 |
| yes | 49 | 8 | 16.3 | 17.9 [8.4, 34.1] | 3.1 [1.4, 7.1] | ||
| Housemate been to Western Europe (not incl. UK and Ireland) in the last year | |||||||
| no | 1708 | 149 | 8.7 | 6.8 [5.5, 8.3] | 0.91 | 1 | 0.11 |
| yes | 626 | 50 | 8.0 | 6.9 [4.9, 9.7] | 1.4 [0.9, 1.9] | ||
| Housemate been to another region of the world in the last year | |||||||
| no | 2317 | 198 | 8.5 | 6.8 [5.7, 8.1] | 0.92 | 1 | 0.81 |
| yes | 17 | 1 | 5.9 | 7.5 [1.1, 37.7] | 0.8 [0.1, 5.5] | ||
Also shown are the ORs for potential risk factors for colonization after adjustment for the person’s country of birth and the person’s region of origin if born in the UK.
To estimate the prevalence of ESBLPE colonization for adults living in England in 2014 we used weights based on the 2011 national census and the number of eligible individuals at the selected practices. Weights for ethnic group, age group and sex were calculated for all individuals based on the census data alone. To estimate the prevalence for each GP practice and PCT we used weights based on the numbers of eligible individuals in each group at each practice.
Categories of this factor variable referred to in this table include: Born in the UK: UK origin; Born in the UK: India, Pakistan or Bangladesh origin; Born in the UK: Caribbean origin; Born in the UK: other origin; Born in India; Born in Pakistan; Born in Bangladesh; Born in Sri Lanka; Born in Afghanistan; Born in the Middle East; and Born in some other country.
Ethnic group was self-declared by each participant when completing the research questionnaire.
Region of origin/origin is derived from the self-declared ethnic group and country of birth.
Table 3.
Final multivariate model for colonization with CTX-M ESBLPE
| Risk factor | Categories | Number of people exposed to the risk factor in the model (n = 2319) | aOR [95% CI]; P value | Percentage PAF [95% CI] | Percentage PAF [95% CI] | |
|---|---|---|---|---|---|---|
| Country of birth and region of origina if born in the UK | Born in the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) | 256 | 5.4 [3.0, 9.7]; <0.001 | 23.8 [15.9, 30.9] | 27.7 [19.5, 35.1] | |
| Born in Afghanistan | 9 | 46.0 [9.6, 218]; <0.001 | 2.8 [1.3, 4.3] | |||
| Born in the Middle East | 18 | 4.7 [1.3, 17.0]; 0.02 | 1.1 [−0.4, 2.6] | |||
| Born in the UK and of UK origin | 1451 | 1.3 [0.8, 2.1]; 0.24 | 9.9 [−7.1, 24.1] | 15.3 [−3.5, 30.6] | ||
| Born in the UK and of IPB origin | 52 | 3.8 [1.5, 9.2]; 0.004 | 2.8 [0.1, 5.4] | |||
| Born in the UK and of Caribbean origin | 32 | 3.4 [1.0, 10.9]; 0.04 | 1.4 [−0.6, 3.3] | |||
| Born in the UK and of some other origin or of mixed origin | 45 | 2.2 [0.7, 6.5]; 0.17 | 1.3 [−1.0, 3.5] | |||
| Compared with (Reference category): Born in some country other than UK, IPB, Sri Lanka, Afghanistan or the Middle East | 464 | Reference | Reference | Reference | ||
| Travel abroad in the past year | South Asia | 133 | 2.9 [1.8, 4.8]; <0.001 | 12.1 [5.9, 7.8] | 27.9 [16.0, 38.1] | |
|
||||||
|
||||||
|
||||||
| Compared with (Reference category): No travel to South Asia | 2186 | Reference | Reference | |||
| Countries outside Asia with higher risk | 246 | 2.6 [1.7, 4.1]; <0.001 | 9.9 [4.3, 15.1] | |||
|
||||||
|
||||||
|
||||||
| Compared with (Reference category): No travel to countries outside Asia with higher risk | 2073 | Reference | Reference | |||
| Other countries | 1021 | 1.3 [0.9, 1.8]; 0.15 | 7.9 [−3.5, 18.1] | |||
|
||||||
|
||||||
|
||||||
|
||||||
| Compared with (Reference category): No travel to Other countries | 1298 | Reference | Reference | |||
| Domestic work in a healthcare setting | Yes | 8 | 6.2 [1.3, 31]; 0.03 | 1.1 [−0.2, 2.3] | ||
| Compared with (Reference category): No | 2311 | Reference | Reference | |||
Region of origin/origin is derived from the self-declared ethnic group and country of birth.
Among participants colonized with CTX-M ESBLPE we calculated the percentage colonized with a particular blaCTX-M genotype, and the percentage belonging to particular ethnic groups among those who were carriers of a particular blaCTX-M genotype.
Ethics
Approval for the study was obtained from the NRES Committee South West - Frenchay, Bristol, UK (13/SW/0017). The data we collected from GP practices were anonymous.
Results
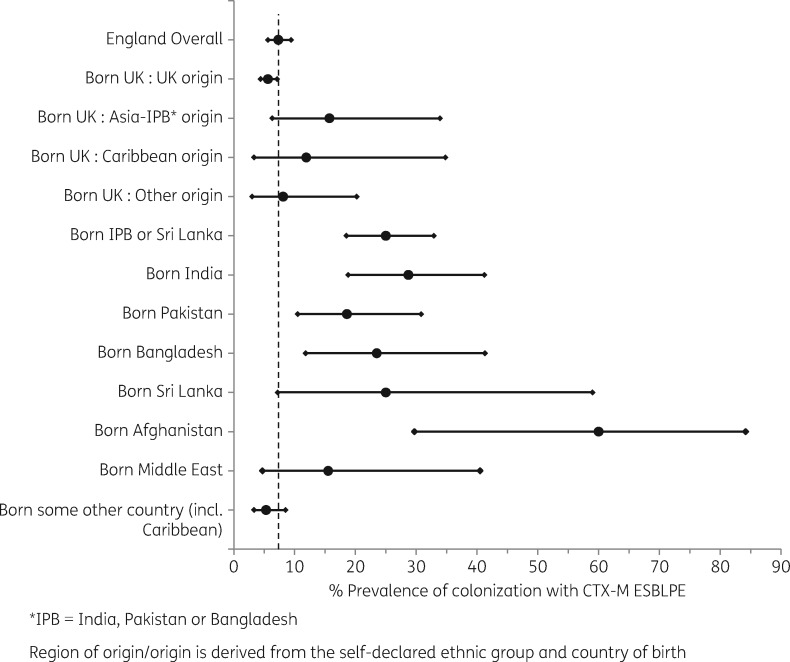
Of 76 154 adult individuals registered in 16 GP practices, 58 337 were invited to participate; 3389 (5.8%) expressed interest and were sent a faeces kit, and 2331 (4.0%) returned a sample. A further 99 individuals invited by general practice receptionists participated, making a total of 2430 participants. The number of stool specimens received from participants in each section of the adult population in England, the number of specimens positive for ESBLPE, and the unweighted and weighted percentage positive for ESBLPE are shown in Table 2. The estimated prevalence of colonization with CTX-M ESBLPE in adults living in England in 2014 was 7.3% (95% CI 5.6%–9.4%). Of the four PCTs, Heart of Birmingham teaching PCT (234 participants) had the highest estimated prevalence at 16.0% (95% CI 10.2%–24.2%) [Newham 612 participants, 12.7% (95% CI 9.1%–17.4%); Southampton City 740 participants, 9.2% (95% CI 6.1%–13.7%); and Shropshire County 774 participants, 4.9% (95% CI 3.4%–7.0%)]. There was no evidence that estimated prevalence differed by age or sex. There were high estimated prevalences for participants born in India (136 participants, 28.7% prevalence, 95% CI 18.8%–41.2%), Pakistan (81 participants, 18.6% prevalence, 95% CI 10.5%–30.8%), Bangladesh (34 participants, 23.5% prevalence, 95% CI 11.8%–41.3%), Sri Lanka (8 participants, 25.0% prevalence, 95% CI 7.2%–59.0%), Afghanistan (10 participants, 60.0% prevalence, 95% CI 29.7%–84.2%) and the Middle East (18 participants, 15.5% prevalence, 95% CI 4.7%–40.5%) (Figure 1). The overall estimated prevalence for those born in the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) combined (259 participants) was 25.0%, (95% CI 18.5%–32.9%); differences between these four countries were non-significant (P = 0.65). The estimated prevalence for those born in the UK with an IPB region of origin was 15.7% (52 participants), while for those born in the UK with a UK region of origin (1459 participants) it was 5.6% (95% CI 4.4%–7.1%, P = 0.03). However there was a low estimated prevalence for those born in Africa and of the IPB ethnic group (17 participants, 0% prevalence, 95% CI 0%–19.5%).
Figure 1.
Prevalence of colonization with CTX-M ESBLPE by country of birth and region of origin if born in the UK (with 95% CI). Adults from the general population of England in 2014. Dotted line is the estimated 2014 prevalence in England.
Overall estimated prevalence of ESBLPE was the same in those reporting taking any antibiotic in the last year (777 participants, 6.8% prevalence 95% CI 5.1%–8.9%) or not (1427 participants, 6.8% prevalence 95% CI 5.4%–8.6%). None of 48 participants who reported having taken any co-amoxiclav in the past 12 months carried ESBLPE (0% prevalence, 95% CI 0%–7.4%, P = 0.03). Estimated prevalence of ESBLPE in the 15 participants who had taken ciprofloxacin was 9.5%, (95% CI 2.5%–30.1%, P = 0.61).
Two other groups with high estimated prevalence were those who had travelled to South Asia (India, Pakistan, Bangladesh, Sri Lanka or Nepal) in the last year (140 participants, 38.5% prevalence, 95% CI 27.8%–50.5%) and those working as a domestic in a healthcare setting [8 participants, unweighted prevalence 37.5%, 95% CI 8.5%–75.5% (3/8; 1 black African, CTX-M-15, no travel abroad; 1 white British, CTX-M-15, travelled to India with partner for 12 days; 1 Asian Indian, CTX-M-27, travelled to India alone for 42 days)]. If someone else in the participant’s household had been to India, Bangladesh, the Middle East, the Indian subcontinent or South East or Pacific Asia, this significantly increased the participant’s risk for carrying blaCTX-M ESBL. Participants whose housemates had travelled abroad in the last year to the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) (120 participants, estimated prevalence 22.7%, 95% CI 14.2%–34.1%, P < 0.001), India (83 participants, estimated prevalence 24.9% 95% CI 14.5%–39.2%, P < 0.001) and the Middle East (43 participants, estimated prevalence 16.9%, 95% CI 7.5%–33.6%, P = 0.02) had a higher prevalence. In 1071 of 1234 participants, both the housemate and the main participant had travelled to the same country in the past 12 months.
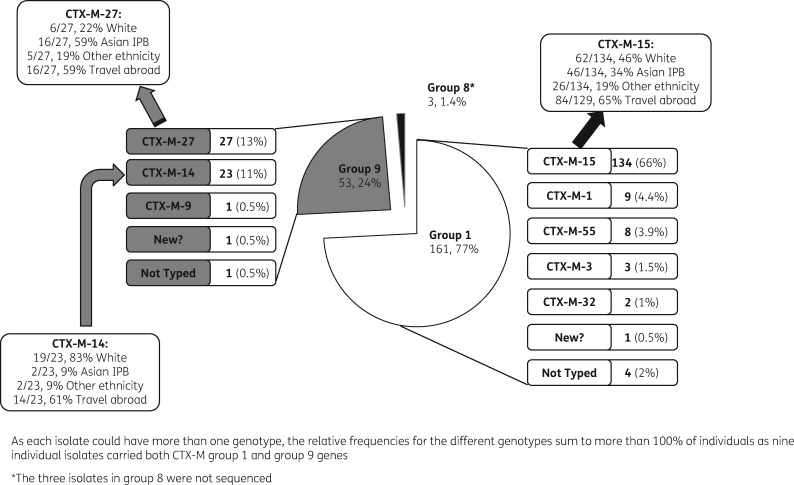
Relative frequency of CTX-M genotypes (Figure 2)
Figure 2.
Relative frequency of the blaCTX-M genotypes from 208 individuals, characterizations of these genotypes and risk factors for them.
Two hundred and eight participants were found to carry the CTX-M gene; 184 (88%) by direct culture (25% +, 26% ++, 37% +++) and a further 24 (12%) on enrichment; no single participant had more than one species of bacteria carrying CTX-M. Most isolates (199) were E.coli, 5 Klebsiella pneumoniae, 4 Enterobacter and 1 Citrobacter. All isolates could be allocated a CTX-M grouping. Seventy-seven percent of participants with CTX-M ESBLPE (161/208) were colonized with CTX-M group 1; 25% (53/208) were colonized with CTX-M group 9; 4% (9/208) were colonized with both group 1 and group 9; and 1.4% (3/208) were colonized with CTX-M group 8, 25 or 26. Four isolates from group 1 and one isolate from group 9 could not be sequenced. The most common genotype was blaCTX-M-15 (66%, 134/204) (Figure 2). Two other common genotypes were blaCTX-M-14 (11%, 23/207) and blaCTX-M-27 (13%, 27/207).
CTX-M genotypes and risk factors
Carriers of blaCTX-M-15 had a similar probability of being of white ethnicity compared with carriers of other genotypes (62/134, 46% of blaCTX-M-15 were white; 46/134, 34% Asian IPB; and 26/134, 19% other ethnicity) and 84/129 (65%) had travelled abroad in the last year. A carrier of blaCTX-M-27 had a higher chance of being of the Asian-IPB ethnic group (16/27, 59% Asian IPB; 6/27, 22% white; 5/27, 19% other ethnicity; 16/27, 59% travelled abroad) compared with carriers of other genotypes. A blaCTX-M-14 carrier had a higher chance of being of white ethnicity (19/23, 83% white; 2/23, 9% Asian IPB; 2/23, 9% other ethnicity; 14/23, 61% travelled abroad) compared with carriers of other genotypes. Carriers of the different genotypes CTX-M-15, -27 and -14 had a similar chance of having travelled abroad in the last year. Of the 199 E.coli, 87% (173/199) were ST131. ST131 was not significantly more common in CTX-M carriers who had spent time anywhere abroad in the last year, or in the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka), compared with those who had not (spent time anywhere abroad 87% ST131 versus not spent time abroad 79%, P = 0.17; spent time abroad in the Indian subcontinent 91% ST131 versus not spent time in the Indian subcontinent 82%, P = 0.17).
Risk factors for colonization with CTX-M ESBLPE
After adjusting for country of birth and region of origin (if born in the UK) we found no evidence for an independent association between CTX-M ESBLPE colonization and GP practice, age group, sex, overall antibiotic use in the past year or hospitalization in the past year (Table 2). Factors that remained significant after adjusting for country of birth and region of origin (if born in the UK) and were therefore considered for inclusion in the final model included: participant’s travel abroad, or diarrhoea or hospitalization abroad in the last year; use of ciprofloxacin or co-amoxiclav; being a domestic healthcare worker; and housemates’ travel abroad (overall and by country) in the last year. When added to the final multivariable model, there was no strong evidence that colonization was independently associated with either taking antibiotics in the last year (aOR 0.99, 95% CI 0.7–1.4, P = 0.95), hospitalization abroad in the last year (aOR 2.7, 95% CI 0.8–9.1, P = 0.11, 3/12 colonized), diarrhoea while abroad in the last year (aOR 1.1, 95% CI 0.6–1.9, P = 0.84, 16/137 colonized) or travel abroad by a participant’s housemate in the past year (aOR 1.4, P = 0.11, 103/1071 colonized). Use of ciprofloxacin remained a significant risk factor for ESBLPE-CTX-M in the final model (4/15 ESBLPE positive, aOR 3.2, P = 0.03), whereas co-amoxiclav was protective for presence of ESBLPE-CTX-M (0/48 ESBLPE positive, aOR 0, P = 0.006).
Being born in the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) was the most important identified risk factor for faecal colonization (aOR 5.4, 95% CI 3.0–9.7), and we estimate it accounted for 23.8% (95% CI 15.9%–30.9%) of people colonized in England in 2014 (Table 3). Being born in Afghanistan (aOR 46.0, 95% CI 9.6–218) or the Middle East (aOR 4.7, 95% CI 1.3–17.0) were factors strongly associated with colonization, but being relatively rare we estimate them to have accounted for relatively few people colonized [2.8% (95% CI 1.3%–4.3%) and 1.1% (95% CI −0.4% to 2.6%), respectively]. We found no evidence when tested in the final model that birth in or travel to other countries including Eastern Europe increased risk of colonization (travel to Eastern Europe aOR 0.8, P = 0.42; born in Eastern Europe aOR 0.5, P = 0.38). There was no evidence that being born in the UK with a UK region of origin was a risk factor for colonization (aOR 1.3, 95% CI 0.8–2.1, P = 0.24); but as there were so many participants in this group, we estimate it accounted for 9.9% (95% CI −7.1% to 24.1%) of people colonized. Being born in the UK with an IPB region of origin was strongly associated with colonization (aOR 3.8, 95% CI 1.5–9.2), and we estimate it accounted for 2.8% (95% CI 0.1%–5.4%) of people colonized. Being born in the UK with a Caribbean region of origin was almost as strongly associated with colonization as being born in the UK with an IPB region of origin (aOR 3.4, 95% CI 1.0–10.9), and we estimate it accounted for 1.4% (95% CI −0.6% to 3.3%) of people colonized.
Travel to South Asia (India, Pakistan, Bangladesh, Sri Lanka or Nepal) in the last year was strongly associated with colonization (aOR 2.9, 95% CI 1.8–4.8), and we estimate it accounted for 12.1% (95% CI 5.9%–17.8%) of people colonized. Travel to Africa, China, South or Central America, South East or Pacific Asia or Afghanistan in the last year also increased the risk of colonization (aOR 2.6, 95% CI 1.7–4.1), and we estimate it accounted for 9.9% (95% CI 4.3%–15.1%) of people colonized. Travel to other countries in the last year put participants at a small increased chance of colonization (aOR 1.3, 95% CI 0.9–1.8; P = 0.15) and, being relatively common, we estimate it accounted for 7.9% (95% CI −3.5% to 18.1%) of people colonized. Working as a domestic in the healthcare setting was strongly associated with colonization (aOR 6.2, 95% CI 1.3–31.0), but, being relatively rare, we estimate it to have accounted for just 1.1% (95% CI −0.2% to 2.3%) of people colonized. Collectively all risk factors in the final multivariable model explained 60.4% (95% CI 40.0%–73.8%) of cases.
Only 0.1% of participants (2/2430) were colonized with CPE; neither was born in the UK, and both had a history of travel to India in the past year.
Discussion
The 7.3% estimate for the prevalence of faecal colonization with CTX-M ESBLPE in adults living in England in 2014, and the high estimated prevalence in those born in South Asia (India, Pakistan, Bangladesh or Sri Lanka) and in those travelling to certain areas including South Asia, is of concern and has implications for empirical antimicrobial prescribing for suspected infections caused by Enterobacteriaceae and infection prevention and control within healthcare in England and beyond. The significantly higher estimated prevalence (15.7%) in the 52 participants born in the UK with an IPB region of origin, compared with those born in the UK with a UK region of origin (1459 participants, estimated prevalence 5.6%), is interesting; the higher estimated prevalence may be due to acquisition during repeated travel to their country of origin, or from visits by family and friends to or from their country of origin, during the last year or more than 1 year ago, or acquisition from relatives in the home.22
Previous studies in the UK
The only previous faecal colonization study of ESBLPE in the UK (in 2010) showed that Middle Eastern or South Asian (India, Pakistan, Bangladesh, Sri Lanka or Nepal) patients being investigated for gastrointestinal infections had a significantly higher prevalence than Europeans.11 Although UK studies estimating ESBLPE infection rates in hospitalized patients or patients with urine infections (UTIs) have suggested (similar to this present study) that rates of ESBLPE infection vary widely between different areas of the UK23,24 they have not investigated other risk factors.
Previous studies of a general population in Northern Europe
A 2011 postal study in urban Amsterdam estimated that the overall prevalence of ESBLPE faecal colonization was 8.6% and travel to Asia or Africa (aOR 2.1–2.2) in the last year increased the risk. Unlike the present or other studies, they found antibiotic use in the last year and travel to North America (aOR 2.7) were also risk factors.3 The Amsterdam study found, as in the present study, that country of origin was important, as having a mother born in Asia was a risk factor independent of foreign travel (aOR 2.4). In the present and Amsterdam studies, hospitalization abroad in the past year led to significantly higher faecal colonization and risk in the univariable analysis, but neither found hospitalization abroad significant on multivariable analysis.3 The Amsterdam study did not investigate travellers’ diarrhoea as a risk factor.3
A 2014/15 general population study in the rural Southern Netherlands estimated the prevalence of faecal colonization with ESBL/AmpC Enterobacteriaceae to be 4.5%. This is similar to our estimate for adults living in Shropshire in 2014, which is also rural with almost the entire population born in the UK and of UK origin. This study, like ours, found travel to Africa, Asia and Latin America (aOR 2.9), was a risk factor for carriage.25 Participants living in close proximity to mink farms (but not other farms) and keeping cows for a hobby in the last 5 years (aOR 3.56) had increased risk.25 Despite other studies showing that contact with broiler farms increased risk in the Netherlands,26 neither contact with animals, pets or eating meat (including chicken) increased the risk of ESBLPE colonization in our study. The risk factor associated with animals may only be evident when you closely examine particular livestock, which we did not do.
A systematic review of faecal colonization27 with Ambler class A ESBLPE in healthy individuals between 1992 and 2014 found that ESBLPE colonization had increased over time and was present worldwide in 2014. The pooled estimated prevalence was highest in the West Pacific (46%), followed by South East Asia and Africa (22%), the Eastern Mediterranean (15%) and Northern Europe (4%). Factors associated with a higher risk of colonization were international travel (RR 4.06) and antibiotic use in the previous year (RR 1.58), but this was a univariate analysis.27 The significant effect of antibiotic use in that review may not have remained on multivariable analysis or may be caused by relatively greater use of broader spectrum antibiotics (especially quinolones) in countries outside Northern Europe and in travellers. Interestingly, although overall antibiotic use was not a significant risk factor in our study, participants reporting ciprofloxacin use in the past year did have significantly increased estimated prevalence and this risk remained in the final multivariable model (aOR 3.2, P = 0.03). However, all four cases who reported taking ciprofloxacin and were CTX-M ESBLPE positive had other risk factors (all four participants were of Asian ethnicity, three were hospitalized in the past year and one travelled to Pakistan). In contrast, reported co-amoxiclav use in the last year appeared to be protective, as none of these 48 participants was positive for CTX-M ESBLPE (0%, P = 0.03). This is an important and interesting finding that needs further investigation. Although co-amoxiclav has limited clinical activity against CTX-M ESBLPE it is possible that there are high enough concentrations in the gut to eliminate faecal carriage. In contrast, ciprofloxacin would be expected to encourage colonization as almost all UK CTX-M ESBLPE are resistant.28 Only one of the previously published studies of ESBLPE colonization in the general population reported estimated prevalence by region of origin, and found birth in Africa, or parents born in Africa or Asia, to be an important risk factor.3
Previous studies of travellers
Like this study, studies of travellers from other European countries have identified travel outside Europe, and especially to the Middle East, Africa and South Asia, to be a risk for ESBLPE faecal colonization and acquisition;10,27,29–31 with travel to Southern India being the greatest risk.10 In several studies, travellers’ diarrhoea and the use of antimicrobials were independent risk factors for acquiring ESBLPE, which is not consistent with our own findings.10,27,29–31 However, another 2012 study of travellers from a Swiss travel clinic to South Asia found length of stay, visiting friends and relatives and eating ice-cream were risk factors for acquisition of ESBLPE E. coli; whereas travellers’ diarrhoea was not a risk factor.13 Visiting friends or relatives is probably a marker of being born in South Asia, which was a risk factor for colonization in our present study. In studies like this and ours, which included a larger proportion of travellers visiting relatives, travellers’ diarrhoea may not be such an important marker for faecal colonization, as these travellers’ gut microbiome may have already adapted to the South Asian diet and environmental flora. Although travel abroad by a housemate was associated with significantly higher carriage, this did not remain significant in the final model. Travel abroad by a housemate was closely correlated with spending time abroad by the participant themselves (1071/1234); so it is therefore not possible to distinguish between the effect of this variable and the effect of travel abroad by the person themselves.
Two longitudinal studies of travellers who were ESBLPE carriers on return from abroad report the prevalence of carriage 12 months after returning to be 11.3% and 2.2%, respectively, confirming that travel abroad more than 1 year ago could be an important factor to investigate in future studies.10,32 Our questions about travel abroad were limited to the last year; this omission could be important for travellers who travel repeatedly to areas with higher prevalence of CTX-M ESBLPE and poor hygiene or sanitation, for example participants born in the UK of IPB or Caribbean origin who may be more likely to repeatedly visit family and friends. This would help to explain the higher carriage and risk conferred by being UK-born but of IPB or Caribbean origin.
Unlike the 2016 systematic review we did not find that estimated prevalence of CTX-M ESBLPE was significantly higher in those born in or travelling to Eastern Europe (15/249 participants CTX-M ESBLPE positive, aOR 0.9, 95% CI 0.5–1.6, P = 0.77).27 This may be because sanitation facilities in these countries are better than on the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) so that acquisition in these countries is less common.
In our study the estimated prevalence of CTX-M ESBLPE among those working as domestics in healthcare was 37.5% (3/8 participants, 95% CI 8.5%–75.5%). The risk factor was found to be independently associated with an increased risk of colonization; and these eight participants were not similarly exposed to any other particular risk factor. Domestics in healthcare settings may be put at increased risk through their work cleaning toilet facilities; certainly studies suggest that environmental acquisition may be responsible for the spread of ESBLPE.13 Transmission of ESBLPE occurs between patients in healthcare settings and staff do not always adhere to infection control guidelines.33
As in other studies3,11,24,34 the dominant CTX-M ESBLPE genotype was blaCTX-M-15, making up 66% of carriers of CTX-M ESBLPE. Interestingly we saw very few carriers of blaCTX-M-1, typically associated with European farm animals,35,36 suggesting little acquisition from these sources. This is supported by our lack of evidence that either having a meat diet or working with animal livestock were risk factors for CTX-M ESBLPE colonization. A quarter of carriers in our study carried a genotype belonging to CTX-M group 9, similar to the previous UK study of diagnostic faeces samples in Birmingham.11blaCTX-M-27 made up half of the group 9 CTX-Ms in our study, but was not found in the Birmingham study; in contrast, we found only one blaCTX-M-9, whereas this constituted 74% of group 9 in the Birmingham study.11blaCTX-M-27, which is part of group 9, is a variant of blaCTX-M-14 that has been reported both from the Far East and from Europe, and in the UK has been reported at a low frequency from food animals, notably dairy cattle.37 In our study, carriers of blaCTX-M-27 were mainly from the Asian-IPB ethnic group (59%), whereas carriers of blaCTX-M-14 were mainly from the white ethnic group (83%); this variation by ethnicity warrants further investigation. In a Spanish study38, CTX-M-14-producing E. coli were mainly isolated from community UTIs; this was also found in a Welsh study, where 83% of the CTX-M-14 genotype were community acquired39 and they were more common in rural areas.40 CTX-M-14 E. coli may be indigenous in the UK community, possibly acquired from cattle.41 Consistent with other work, we found no group 2 isolates.1
The present study shows that the ST131 clone was the most prevalent among the isolated E. coli demonstrating that the spread of CTX-M-15 in the UK may be due to this clone. Our results indicate that being born in or travel to the Indian subcontinent (India, Pakistan, Bangladesh or Sri Lanka) was the biggest overall risk factor, but in the IPB countries the ST131 clone is uncommon.42,43 This may help us to further explore CTX-M ESBLPE originating in the UK.
As no data exist on the frequency of community colonization of CPE in the UK, we used our study to gain some insight into their prevalence. Our culture methods, whilst potentially missing some OXA-type carbapenemases, are recognized reference laboratory methods.20 Like other European studies, we found a low incidence of CPE (0.1%) in England in 2014;44,45 the two positive participants (one Asian woman with E.coli OXA-48 with CTX-M group 9, and one UK man with E. coli NDM-1 with CTX-M-15) had both travelled to South Asia (India, Pakistan, Bangladesh, Sri Lanka or Nepal). This represents a potential route for the introduction and future spread of CPE.
Strengths
This is the first study to estimate the prevalence of colonization with CTX-M ESBLPE in the UK general population. We invited a stratified random sample of individuals from the general population from GP patient lists rather than selecting patients who had given diagnostic faecal samples or returning travellers who are unrepresentative of the general population. An added value of this study is the specific focus on ethnicity and the oversampling to retrieve sufficient data in ethnic minority groups, which enabled us to achieve sufficient power to identify some ethnicities and some countries of birth as risk factors. The questionnaires that were returned were well completed and allowed us to investigate a broad range of potential independent risk factors for CTX-M ESBLPE colonization. Rather than use swabs, we used faecal samples that increase sensitivity of detection of ESBLPE by 10%17,46 and enrichment, which increased our sensitivity of isolating CTX-M ESBLPE by 12%.17
Limitations
We only sought CTX-M ESBLPE (and not ESBLPE carrying the other β-lactamase genes blaTEM,blaOXA and blaSHV) as CTX-Ms still constitute more than 90% of ESBLPE genotypes and cause more ESBLPE infections than any other type of ESBLPE worldwide.3,47,48 We have not examined the genetic context of blaCTX-M, which would have given an insight into its linkage to IS elements. However, this would be unlikely to impact on the control of ESBLPE transmission. For those exposed to a relatively rare risk factor, the sample size was small and the CI was wide.
We did not ask about the characteristics of the housemate who travelled or their relationship to the participant, and housemate travel did not remain a significant risk in the final multivariable model (P = 0.11). However, it was not our intention to study transmission. In future studies it would be interesting to confirm whether housemates usually travelled to the same countries with the participant or at a different time. Many cases of CTX-M faecal colonization may be unexplainable by any risk factor we could conceivably have collected data on; so, it is possible we have investigated all the important risk factors. However, it is also possible that some cases could be explained by a risk factor that we did not have sufficient power to detect or we did not ask about. Questions about travel abroad and about antibiotic use were limited to the last year, so this study was not able to investigate whether travel abroad more than a year earlier or antibiotic use more than a year earlier are risk factors for colonization. Furthermore we did not collect data on the use of proton-pump inhibitors, found to be a risk factor for faecal carriage in the Amsterdam 2011 community study (aOR 1.9)3 and the rural study.25 Since CTX-M ESBLPE are now widely established in the English general population we were not surprised to find that many cases were not directly attributable to travel abroad in the last year.
Most of our prevalence estimates come from a weighted analysis that weighted participants by ethnic group, age group and sex. Should our participants be unrepresentative in respect of any other variable then prevalence estimates for the general population could be biased. The risk of such a bias is compounded by the low response rate (4%) and variation in response rate in the different groups.
Implications
Faecal colonization with ESBLPE usually precedes an ESBLPE infection when it occurs7,8 so we believe that this study, showing CTX-M ESBLPE to be established in the general population of England and the prevalence of colonization to be considerably higher among some sections of the general population, has implications for empirical prescribing for all infections typically caused by Enterobacteriaceae, and that antimicrobial guidance should reflect our findings.49 The population that is most relevant to any conclusions about empirical antibiotic prescribing is the population of those who have infections likely caused by Enterobacteriaceae and are seen by clinicians. We haven’t studied this population but we believe that a high prevalence estimate for a section of the general population can be used to inform on the likely prevalence of those from the same section of the general population who seek treatment for infections likely caused by Enterobacteriaceae. When selecting empirical treatment for uncomplicated UTI we suggest that clinicians should consider the risk of ESBLPE, noting recent travel, country of birth and region of origin, especially South Asia (India, Pakistan, Bangladesh, Sri Lanka or Nepal). Nitrofurantoin, which has greater activity against ESBLPE, is preferable to trimethoprim as an empirical agent in most cases of acute uncomplicated UTI.49 Although nitrofurantoin will still be appropriate for most patients with acute uncomplicated UTI, in patients belonging to a section of the population that has a particularly high risk of ESBLPE carriage it may be preferable to obtain microbiology specimens before starting antibiotics. This might include those born in South Asia and in patients who have travelled to South Asia in the last year. Empirical antimicrobials prescribed for ‘sepsis’50 should always include an antimicrobial agent that treats ESBLPE.51
Previous studies of the length of faecal colonization with ESBLPE have been in patients who have attended a travel clinic and have acquired colonization abroad. There is wide variation in the estimates of the percentage still colonized, from 4.8%52 to 14.3%10 of travellers with faecal ESBLPE colonization at 6 months, and from 2.2%52 to 11.3%10 at 12 months. We need to understand the length of carriage and transmission in the general population, which may be different; this would be best investigated in a longitudinal study in a typical population. Prospective or case–control studies designed to look at the risk of UTI or future infections in those with ESBLPE faecal colonization are needed. To improve our understanding of evolving risk groups for ESBLPE infections, we suggest enhanced or periodic antimicrobial resistance surveillance should be extended to patients with uncomplicated infections (to reduce spectrum bias),6 and data collection should include ethnic group, age and sex of the patient. If feasible to do so, we also suggest collecting: country of birth, recent travel history, ethnic origins, occupation and use of antibiotics and antacids in the last year.
A study of healthcare domestics’ colonization with ESBLPE compared with other healthcare workers, and possibly patients, is needed as their numbers in our study were small, and there are large numbers of domestics working in healthcare with the potential to transfer ESBLPE. Additionally, domestics receive less training about infection prevention and control than other healthcare workers, as they have no direct role in hands-on patient care.
According to our results the prevalence of CPE in the general population is still very low, and therefore efforts to reduce UK healthcare transmission of CPE are worthwhile and could help to delay the inevitable expansion of these genes into the general population.
Supplementary Material
Acknowledgements
We thank all the individuals who returned stool samples and questionnaires; without you we could not have done this study. We wish to thank: Andre Charlett for all his help with the statistical interpretation and advice on the paper; Rahim Shabbir for building the Access database; Katherine Butler and Eileen Hamilton for administrative support; the GP practice staff; Primary Research network staff; ethics committees; Research Support Unit in Gloucester, especially Mark Walker; Elizabeth Coates, Head of research governance at PHE; communications teams at PHE and radio stations who advertised the project; and Mike Nelson.
Funding
The report is based on independent research commissioned and funded by the NIHR Policy Research Programme (Ref.041/0038S).
Transparency declarations
None to declare.
Author contributions
C. A. M. M. (Principal Investigator) led the writing of the grant application and protocol, was involved in the literature review, contributed to the design of the questionnaire, led the project steering group and led the writing of the manuscript. D. M. L. (Project Manager Mar–Jul 2014 and from Apr 2015) was involved in practice and patient recruitment, data collection and data management, was a steering group member and helped write the manuscript. L. X.-M. (Clinical Scientist) was a grant co-applicant and was involved in study design and laboratory supervision, supported laboratory data management, was a steering group member and contributed to the writing of the manuscript. D. N.-S. (Research Assistant) was involved in ethics application, practice and participant recruitment and data collection and entry, was a steering group member and commented on and agreed the final manuscript. K.-T. C. (Research Scientist) was involved in laboratory work, recording and data entry and agreed the final manuscript. T. N. (Statistician) was a grant co-applicant, was involved in study design, practice and participant selection, data management and data analysis, was a steering group member and contributed to the writing of and agreed the final manuscript. H. L. T. (Epidemiologist) was a grant co-applicant, was involved in study and questionnaire design and data interpretation, was a steering group member and commented on and agreed the final manuscript. M. T. was a grant co-applicant, was involved in study and questionnaire design, was a steering group member, was primary care lead, was involved in practice selection and commented on and agreed the final manuscript. A. A.-B. (Research Scientist) was involved in laboratory work and data cleaning and agreed the final manuscript. K. T. (Unit Administrator) was involved in participant recruitment and liaison and data collection and entry and agreed the final manuscript. S. Sh. (Research Scientist) was involved in laboratory work and data collection and agreed the final manuscript. S. M. (Research Scientist) was involved in laboratory work and data collection and agreed the final manuscript. S. Sm. was a grant co-applicant and was involved in study design, was a steering group member and commented on and agreed the final manuscript. L. C. (Project Manager Oct 2014–Mar 2015) was involved in organizing distribution of sample kits and data management and agreed the final manuscript. P. M. H. was a grant co-applicant and was involved in literature review, study design, questionnaire design, laboratory supervision and data interpretation, was a steering group member and contributed to the writing of and agreed the final manuscript.
Disclaimer
The views expressed in the publication are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health, ‘arms’ length bodies or other government departments.
Supplementary data
The questionnaire is available as Figure S1 at JAC Online.
References
- 1. Hawkey P, Jones A.. The changing epidemiology of resistance. J Antimicrob Chemother 2009; 64 Suppl 1: i3–10. [DOI] [PubMed] [Google Scholar]
- 2. Horner C, Fawley W, Morris K. et al. Escherichia coli bacteraemia: 2 years of prospective regional surveillance (2010-12). J Antimicrob Chemother 2014; 69: 91–100. [DOI] [PubMed] [Google Scholar]
- 3. Reuland EA, Al Naiemi N, Kaiser AM. et al. Prevalence and risk factors for carriage of ESBL-producing Enterobacteriaceae in Amsterdam. J Antimicrob Chemother 2016; 71: 1076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woerther PL, Burdet C, Chachaty E. et al. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 2013; 26: 744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ironmonger D, Edeghere O, Bains A. et al. Surveillance of antibiotic susceptibility of urinary tract pathogens for a population of 5.6 million over 4 years. J Antimicrob Chemother 2015; 70: 1744–50. [DOI] [PubMed] [Google Scholar]
- 6. PHE. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/575626/ESPAUR_Report_2016.pdf.
- 7. Harris AD, Perencevich EN, Johnson JK. et al. Patient-to-patient transmission is important in extended-spectrum β-lactamase–producing Klebsiella pneumoniae acquisition. Clin Infect Dis 2007; 45: 1347–50. [DOI] [PubMed] [Google Scholar]
- 8. Asir J, Nair S, Devi S. et al. Simultaneous gut colonisation and infection by ESBL-producing Escherichia coli in hospitalised patients. Australas Med J 2015; 8: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Epelboin L, Robert J, Tsyrina-Kouyoumdjian E. et al. High rate of multidrug-resistant Gram-negative bacilli carriage and infection in hospitalized returning travelers: a cross-sectional cohort study. J Travel Med 2015; 22: 292–9. [DOI] [PubMed] [Google Scholar]
- 10. Arcilla MS, van Hattem JM, Haverkate MR. et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. Lancet Infect Dis 2017; 17: 78–85. [DOI] [PubMed] [Google Scholar]
- 11. Wickramasinghe NH, Xu L, Eustace A. et al. High community faecal carriage rates of CTX-M ESBL-producing Escherichia coli in a specific population group in Birmingham, UK. J Antimicrob Chemother 2012; 67: 1108–13. [DOI] [PubMed] [Google Scholar]
- 12. von Wintersdorff CJH, Penders J, Stobberingh EE. et al. High rates of antimicrobial drug resistance gene acquisition after international travel, The Netherlands. Emerg Infect Dis 2014; 20: 649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuenzli E, Jaeger VK, Frei R. et al. High colonization rates of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in Swiss travellers to South Asia- a prospective observational multicentre cohort study looking at epidemiology, microbiology and risk factors. BMC Infect Dis 2014; 14: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freedman DO, Weld LH, Kozarsky PE. et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 2006; 354: 119–30. [DOI] [PubMed] [Google Scholar]
- 15. Steffen R, Baños A.. Travel epidemiology—a global perspective. Int J Antimicrob Agents 2003; 21: 89–95. [DOI] [PubMed] [Google Scholar]
- 16. Eurostat. Tourism Statistics http://ec.europa.eu/eurostat/statistics-explained/index.php/Tourism_statistics.
- 17. Jazmati N, Hein R, Hamprecht A.. Use of an enrichment broth improves detection of extended-spectrum-β-lactamase-producing Enterobacteriaceae in clinical stool samples. J Clin Microbiol 2016; 54: 467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willems E, Cartuyvels R, Magerman K. et al. Evaluation of 3 different agar media for rapid detection of extended-spectrum β-lactamase-producing Enterobacteriaceae from surveillance samples. Diagn Microbiol Infect Dis 2013; 76: 16–9. [DOI] [PubMed] [Google Scholar]
- 19. Xu L, Ensor V, Gossain S. et al. Rapid and simple detection of blaCTX-M genes by multiplex PCR assay. J Med Microbiol 2005; 54: 1183–7. [DOI] [PubMed] [Google Scholar]
- 20. Lolans K, Calvert K, Won S. et al. Direct ertapenem disk screening method for identification of KPC-producing Klebsiella pneumoniae and Escherichia coli in surveillance swab specimens. J Clin Microbiol 2010; 48: 836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Findlay J, Hopkins KL, Alvarez-Buylla A. et al. Characterization of carbapenemase-producing Enterobacteriaceae in the West Midlands region of England: 2007-14. J Antimicrob Chemother 2017; 72: 1054–62. [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez-Bano J, Lopez-Cerero L, Navarro MD. et al. Faecal carriage of extended-spectrum β-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother 2008; 62: 1142–9. [DOI] [PubMed] [Google Scholar]
- 23. Moore LS, Freeman R, Gilchrist MJ. et al. Homogeneity of antimicrobial policy, yet heterogeneity of antimicrobial resistance: antimicrobial non-susceptibility among 108717 clinical isolates from primary, secondary and tertiary care patients in London. J Antimicrob Chemother 2014; 69: 3409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horner CS, Abberley N, Denton M. et al. Surveillance of antibiotic susceptibility of Enterobacteriaceae isolated from urine samples collected from community patients in a large metropolitan area, 2010-2012. Epidemiol Infect 2014; 142: 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wielders CC, van Hoek AH, Hengeveld PD. et al. Extended-spectrum β-lactamase- and pAmpC-producing Enterobacteriaceae among the general population in a livestock-dense area. Clin Microbiol Infect 2017; 23: 120.e1–8. [DOI] [PubMed] [Google Scholar]
- 26. Huijbers PM, Graat EA, Haenen AP. et al. Extended-spectrum and AmpC β-lactamase-producing Escherichia coli in broilers and people living and/or working on broiler farms: prevalence, risk factors and molecular characteristics. J Antimicrob Chemother 2014; 69: 2669–75. [DOI] [PubMed] [Google Scholar]
- 27. Karanika S, Karantanos T, Arvanitis M. et al. Fecal colonization with extended-spectrum β-lactamase-producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis 2016; 63: 310–8. [DOI] [PubMed] [Google Scholar]
- 28. Xu L, Shabir S, Bodah T. et al. Regional survey of CTX-M-type extended-spectrum β-lactamases among Enterobacteriaceae reveals marked heterogeneity in the distribution of the ST131 clone. J Antimicrob Chemother 2011; 66: 505–11. [DOI] [PubMed] [Google Scholar]
- 29. Barreto Miranda I, Ignatius R, Pfuller R. et al. High carriage rate of ESBL-producing Enterobacteriaceae at presentation and follow-up among travellers with gastrointestinal complaints returning from India and Southeast Asia. J Travel Med 2016; 23: tav024.. [DOI] [PubMed] [Google Scholar]
- 30. Cummins C, Winter H, Cheng KK. et al. An assessment of the Nam Pehchan computer program for the identification of names of south Asian ethnic origin. J Public Health Med 1999; 21: 401–6. [DOI] [PubMed] [Google Scholar]
- 31. Kantele A, Laaveri T, Mero S. et al. Antimicrobials increase travelers’ risk of colonization by extended-spectrum β-lactamase-producing Enterobacteriaceae. Clin Infect Dis 2015; 60: 837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruppe E, Armand-Lefevre L, Estellat C. et al. Long-term persistence of multidrug-resistant Enterobacteriaceae after travel to the tropics: reply. Clin Infect Dis 2015; 61: 1767.. [DOI] [PubMed] [Google Scholar]
- 33. Andersson H, Lindholm C, Iversen A. et al. Prevalence of antibiotic-resistant bacteria in residents of nursing homes in a Swedish municipality: healthcare staff knowledge of and adherence to principles of basic infection prevention. Scand J Infect Dis 2012; 44: 641.. [DOI] [PubMed] [Google Scholar]
- 34. Ensor VM, Shahid M, Evans JT. et al. Occurrence, prevalence and genetic environment of CTX-M β-lactamases in Enterobacteriaceae from Indian hospitals. J Antimicrob Chemother 2006; 58: 1260–3. [DOI] [PubMed] [Google Scholar]
- 35. Petternel C, Galler H, Zarfel G. et al. Isolation and characterization of multidrug-resistant bacteria from minced meat in Austria. Food Microbiol 2014; 44: 41–6. [DOI] [PubMed] [Google Scholar]
- 36. Michael GB, Kaspar H, Siqueira AK. et al. Extended-spectrum β-lactamase (ESBL)-producing Escherichia coli isolates collected from diseased food-producing animals in the GERM-Vet monitoring program 2008-2014. Vet Microbiol 2017; 200: 142–50. [DOI] [PubMed] [Google Scholar]
- 37. Snow LC, Warner RG, Cheney T. et al. Risk factors associated with extended spectrum β-lactamase Escherichia coli (CTX-M) on dairy farms in North West England and North Wales. Prev Vet Med 2012; 106: 225–34. [DOI] [PubMed] [Google Scholar]
- 38. Valverde A, Canton R, Garcillan-Barcia MP. et al. Spread of blaCTX-M-14 is driven mainly by IncK plasmids disseminated among Escherichia coli phylogroups A, B1, and D in Spain. Antimicrob Agents Chemother 2009; 53: 5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tyrrell JM, Wootton M, Toleman MA. et al. Genetic & virulence profiling of ESBL-positive E. coli from nosocomial & veterinary sources. Vet Microbiol 2016; 186: 37–43. [DOI] [PubMed] [Google Scholar]
- 40. Wootton M, Tyrrell JM, Walsh TR. et al. Prevalence of CTX-M-14 type in Welsh hospitals. In: Abstracts of the Fiftieth Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, USA, 2010. Abstract C2-676. American Society for Microbiology, Washington, DC, USA.
- 41. Stokes MO, Cottell JL, Piddock LJ. et al. Detection and characterization of pCT-like plasmid vectors for blaCTX-M-14 in Escherichia coli isolates from humans, turkeys and cattle in England and Wales. J Antimicrob Chemother 2012; 67: 1639–44. [DOI] [PubMed] [Google Scholar]
- 42. Hussain A, Ranjan A, Nandanwar N. et al. Genotypic and phenotypic profiles of Escherichia coli isolates belonging to clinical sequence type 131 (ST131), clinical non-ST131, and fecal non-ST131 lineages from India. Antimicrob Agents Chemother 2014; 58: 7240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bevan ER, Jones AM, Hawkey PM.. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother 2017; 72: 2145–55. [DOI] [PubMed] [Google Scholar]
- 44. Gijon D, Curiao T, Baquero F. et al. Fecal carriage of carbapenemase-producing Enterobacteriaceae: a hidden reservoir in hospitalized and nonhospitalized patients. J Clin Microbiol 2012; 50: 1558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Hattem JM, Arcilla MS, Bootsma MC. et al. Prolonged carriage and potential onward transmission of carbapenemase-producing Enterobacteriaceae in Dutch travelers. Future Microbiol 2016; 11: 857–64. [DOI] [PubMed] [Google Scholar]
- 46. Lautenbach E, Harris AD, Perencevich EN. et al. Test characteristics of perirectal and rectal swab compared to stool sample for detection of fluoroquinolone-resistant Escherichia coli in the gastrointestinal tract. Antimicrob Agents Chemother 2005; 49: 798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hawkey PM. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect 2015; 89: 241–7. [DOI] [PubMed] [Google Scholar]
- 48. Canton R, Gonzalez-Alba JM, Galan JC.. CTX-M enzymes: origin and diffusion. Front Microbiol 2012; 3: 110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. PHE. Managing Common Infections: Guidance for Primary Care 2017. https://www.gov.uk/government/publications/managing-common-infections-guidance-for-primary-care.
- 50. National Institute for Health and Care Excellence. Sepsis: Recognition, Diagnosis and Early Management 2016. https://www.nice.org.uk/guidance/ng51/resources/sepsis-recognition-diagnosis-and-early-management-pdf-1837508256709. [PubMed]
- 51. Hawkey PM, Warren RE, Livermore DM. et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 2018; in press. [DOI] [PubMed] [Google Scholar]
- 52. Ruppe E, Armand-Lefevre L, Estellat C. et al. High rate of acquisition but short duration of carriage of multidrug-resistant Enterobacteriaceae after travel to the tropics. Clin Infect Dis 2015; 61: 593–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.