Abstract
Objectives
To determine whether local trainer-led TARGET antibiotic interactive workshops improve antibiotic dispensing in general practice.
Methods
Using a McNulty–Zelen-design randomized controlled trial within three regions of England, 152 general practices were stratified by clinical commissioning group, antibiotic dispensing rate and practice patient list size, then randomly allocated to intervention (offered TARGET workshop that incorporated a presentation, reflection on antibiotic data, promotion of patient and general practice (GP) staff resources, clinical scenarios and action planning, 73 practices) or control (usual practice, 79 practices). The primary outcome measure was total oral antibiotic items dispensed/1000 patients for the year after the workshop (or pseudo-workshop date for controls), adjusted for the previous year’s dispensing.
Results
Thirty-six (51%) intervention practices (166 GPs, 51 nurses and 101 other staff) accepted a TARGET workshop invitation. In the ITT analysis total antibiotic dispensing was 2.7% lower in intervention practices (95% CI −5.5% to 1%, P = 0.06) compared with controls. Dispensing in intervention practices was 4.4% lower for amoxicillin/ampicillin (95% CI 0.6%–8%, P = 0.02); 5.6% lower for trimethoprim (95% CI 0.7%–10.2%, P = 0.03); and a non-significant 7.1% higher for nitrofurantoin (95% CI −0.03 to 15%, P = 0.06). The Complier Average Causal Effect (CACE) analysis, which estimates impact in those that comply with assigned intervention, indicated 6.1% (95% CI 0.2%–11.7%, P = 0.04) lower total antibiotic dispensing in intervention practices and 11% (95% CI 1.6%–19.5%, P = 0.02) lower trimethoprim dispensing.
Conclusions
This study within usual service provision found that TARGET antibiotic workshops can help improve antibiotic use, and therefore should be considered as part of any national antimicrobial stewardship initiatives. Additional local facilitation will be needed to encourage all general practices to participate.
Introduction
Antibiotic prescribing can most effectively be improved long term through multifaceted interventions.1 These interventions work well if they include training for professionals that increases their capability and motivation to prescribe well, combined with feedback about their antibiotic prescribing, and readily accessible tools to use during the consultation. This may include tools to improve information sharing with the patient,2–4 symptom scores and clinical prediction rules,5 or near-patient tests.3,6 Most evaluations of these interventions have been undertaken in general practice within research networks, with clinical researchers who both recruit participants and provide the intervention delivery. Practices consent to take part and therefore self-select.6 Participants who decline to take part (and therefore may be less inclined to improve their antibiotic prescribing) are not included in these studies. Furthermore, previous evaluations have usually included a subset of patients with specific symptoms, for example: acute cough,7 sore throat5 or children with acute cough.8 This focus on one syndrome in an intervention may facilitate an improvement in clinicians’ antibiotic prescribing behaviour during the study period, especially if clinicians are reimbursed for recruiting patients or for participating in research studies. These evaluations may not reflect the reality of everyday practice for a primary care clinician, who has limited time, and is being asked to improve their prescribing for all infections.9,10 Only one study exclusively in UK general practice has evaluated the effect of a multifaceted intervention on total antibiotic dispensing over a whole year and when individual patient recruitment was not required,2 but the ‘Stemming the Tide of Antibiotic Resistance’ (STAR) educational programme was also undertaken within a research network.
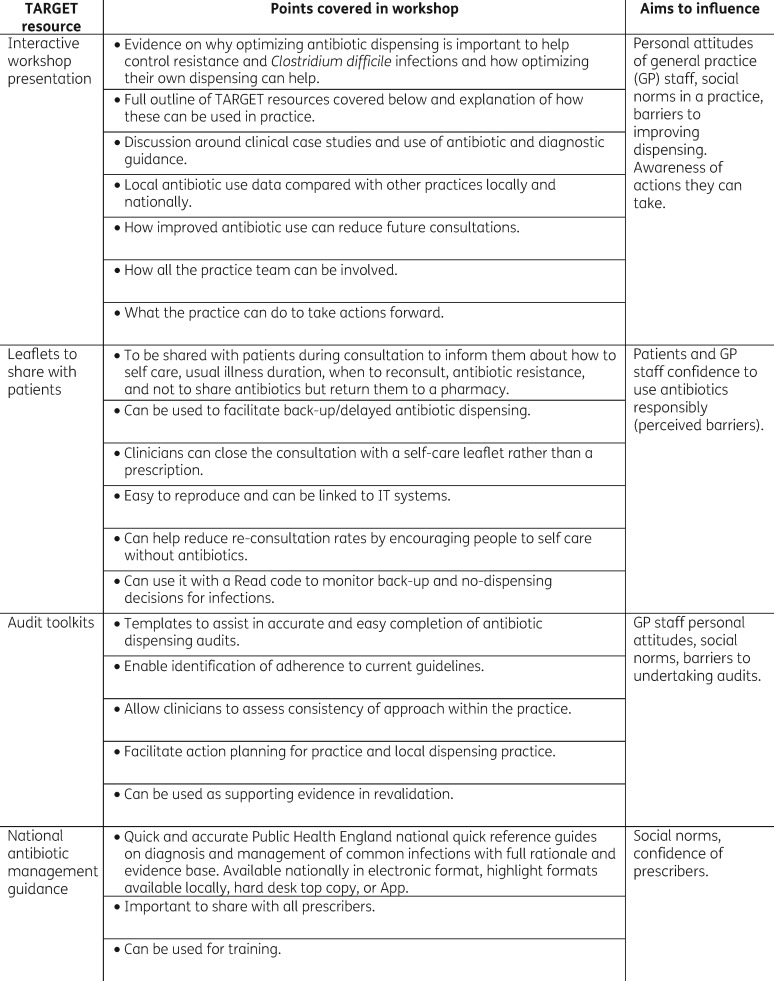
The ‘Treat Antibiotics Responsibly, Guidance, Education, Tools’ (TARGET) antibiotics toolkit,11 developed by PHE with the Royal College of General Practitioners (RCGP) and other professional societies, and hosted on the RCGP web site, aims to influence prescribers’ and patients’ personal attitudes, social norms and perceived barriers to responsible antibiotic prescribing. The toolkit is evidence based and includes many of the principles proven to be effective in previous successful interventions.3,5,12 It includes an interactive workshop13 that incorporates participant reflection on their own antimicrobial stewardship (AMS) activities using a self-assessment questionnaire14 and the practice’s own antibiotic dispensing data [non-anonymized compared with other practices in the clinical commissioning group (CCG) and nationally], the importance of antimicrobial resistance (AMR) and AMS strategies, and information on using and accessing other resources including: free leaflets to share with patients,15 resources for clinical and waiting areas, audit toolkits,16 national antibiotic management guidance,17 other online training resources13 and a facilitator’s manual.18
We aimed to determine the effect of the TARGET 1 h outreach workshop facilitated by existing UK NHS healthcare staff with promotion of TARGET web site resources, on general practice (GP) antibiotic dispensing within routine NHS service provision.
Methods
Setting
In 2013 we selected four primary care CCGs across three rural and urban regions of England that were not involved in implementing similar AMS interventions or research. CCGs are state funded and commission primary medical care from general practices. All the UK population is registered with, or has access to, a general medical practice that they consult for primary care.
Design
We used a McNulty–Zelen19 randomized controlled trial design. This means that the research subjects (GP medical practices) were not aware that they had been randomly assigned to intervention or control or that they were taking part in a trial. Consent was given on their behalf by a senior clinician in the CCG, who was requested not to inform local GPs about the study. Workshop facilitators were informed that they were participating in an evaluation of the TARGET workshops, but were not involved in the randomization of practices. As we were using the McNulty–Zelen design, to keep the trial unknown to participants, the trial was not registered.
Sample size
We undertook a standard sample size calculation based on the prescribing in one average prescribing CCG in England between 2010 and 2012; to detect a 10% reduction in the average dispensing rate for GP practices [from 1.0 item per specific therapeutic group age-sex related prescribing unit (STAR PU) to 0.9 STAR PU] with 90% power at the 5% significance level, two equal-sized groups of 35 practices were required. To allow for lack of clustering, anticipated 50% uptake of workshops, and any practices merging or closing, we planned to recruit at least 150 practices.
Ethics
The NHS Research Ethics Committee waived participant consent for this trial, since obtaining consent would invalidate the results and create an administrative burden on practice participants.
This trial was not registered as it assigned healthcare providers, rather than patients, to intervention and comparison/control groups, and examined the effect of education only on the providers’ behaviour. When the study started we were advised that trial registration was unnecessary, and threatened unblinding the McNulty–Zelen design.
We did obtain Cardiff University ethical approval (SMREC 13/58), and local R&D approvals in each CCG to proceed (Gloucestershire: 13/025/PXT, West Sussex ID: 1562/NOCI/2013, Swindon: 2013/068; Oldham provided a confirmation letter).
Intervention allocation
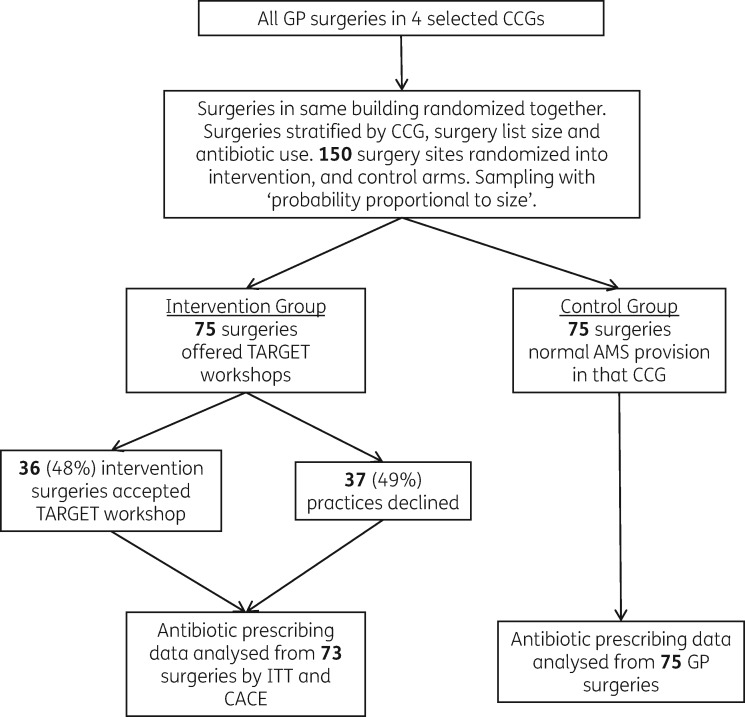
We stratified GP medical practices within each CCG by the number of patients on their list (list size) and total antibiotic dispensing/1000 patients. CCGs were asked how many workshops they had staff capacity to undertake within their usual service delivery, which provided the basis for the number of study practices randomized in each CCG. Multiple practices within the same building were grouped. Practices were excluded if they had previously received a TARGET workshop, or merged with another GP practice. In total, 152 general practices were randomly (using computer-generated pseudo-random numbers) allocated into intervention (73, offered a TARGET workshop) or control (79, usual AMS provision by CCG). The units for randomization and analysis were GP practices rather than individual prescribers because all practice staff are involved in patient care, and all practice staff were invited to the workshop to influence subjective norms.
Participants in workshops
All intervention practices were invited to participate in a practice-based face-to-face workshop by a letter from CCG AMS leads. The letter stated that:
PHE is currently providing outreach workshops with GP surgery staff to showcase the materials available on the Royal College of General Practitioner’s TARGET web site to help improve antibiotic prescribing. The Workshop should last no longer than one hour and will cover: how TARGET can help influence antibiotic prescribing and AMR; improving education of patients about antibiotics through the TARGET web site; how to implement the TARGET web site materials into your practice, using the self-assessment checklist; the resources from the web site, including leaflets, posters, patient information, audit tools; developing a surgery prescribing antibiotic and audit plan.
All practice staff were invited (to influence subjective norms and help with action planning), but were able to accept or refuse any part of the workshop and TARGET materials at any time. Non-responders were followed up by email and/or telephone call.
The TARGET workshop
The workshop (Figure 1) and TARGET resources aimed to influence antibiotic prescribing behaviour in prescribers based on the theory of planned behaviour (TPB).20–22 TPB proposes that whether a person intends to do something (in this case prescribing antibiotics appropriately) is influenced by whether the person is in favour of doing it (‘personal attitude’); how much the person feels social pressure to do it (‘subjective norm’); and whether the person feels in control of the action in question (‘perceived behavioural control, including confidence to prescribe appropriately’).23
Figure 1.
TARGET antibiotics workshop and toolkit content.
The workshop was designed to be delivered to all members of the primary healthcare team. Workshops were delivered by trained health professionals already involved locally in AMS (GP, microbiologist or medicines manager). Local facilitators received 1 h of face-to-face or Skype training on the principles of the TARGET toolkit and workshop, and a toolkit pack with a video of the workshop presentation, together with TARGET materials and workshop planning materials. The importance of presenting the practice antibiotic data compared with others locally and nationally, and discussion was stressed. Facilitators usually spent up to 2 h familiarizing themselves with the presentation, TARGET materials and local prescribing data.
Workshop participants were first asked to complete the TARGET AMS self-assessment checklist.14 Central to the 1 h workshop was the TARGET PowerPoint presentation, which aimed to stress the advantages to staff and patients of AMS, as well as the evidence around benefits for or against antibiotics for common community infections using national PHE antibiotic and NICE guidance and clinical scenarios. Facilitators showed and discussed participants’ own practice antibiotic dispensing data including total and broad-spectrum antibiotics and patient-facing materials on the TARGET web site24 (in particular the TARGET ‘Treat Your Infection’ leaflet).24 Facilitators stressed the importance of a practice-wide approach, and the importance of improving prescribing for respiratory tract infections (RTIs), increasing the use of nitrofurantoin for lower urinary tract infection (UTI), and reducing unnecessary broad-spectrum antimicrobials especially co-amoxiclav. Facilitators showed participants, and encouraged future use of, the TARGET audit templates and online clinical courses, and asked participants to plan future actions. Practice staff were reminded that their local medicine managers would be following their antibiotic prescribing, and that antibiotic prescribing was being monitored nationally.
The TARGET RCGP web site hosting AMS materials for primary care staff and their patients was launched in November 2012. During the study period, all practices in England were encouraged to reduce their antibiotic prescribing through an annual national campaign aligning with European Antibiotic Awareness Day. In April 2015, 9 months after the workshops were completed, the national quality premium (QP) encouraging CCGs to increase their AMS activities with general practices was launched. This QP incentivized CCGs by £0.40 per patient to reach a 1% reduction in total antibiotic prescription items/STAR PU, and a 10% reduction in broad-spectrum antibiotics (cephalosporins, quinolones, co-amoxiclav) or to stay below the England median value of 11.3%. A small number of practices within each CCG were sent a personal letter from the Chief Medical Officer of England informing them that they were in the top 10% of prescribing practices, and encouraging them to reduce their prescribing (this was added to the regression model below). No other specific AMS activities were held in any of the CCGs involved.
Data collection
Data on antibiotic use in England are collected electronically from pharmacies when each antibiotic prescription is dispensed; each prescription dispensed is referred to as an ‘item’. These GP practices’ oral antibiotic dispensing data and the size of the GP practice were obtained from the Centre of Infectious Disease Surveillance and Control, Information Management and Technology department for 32 months from January 2013 to August 2015 inclusive, including both numbers of items dispensed each month and practice list size for each quarter. The potential effect modifier of Index of Multiple Deprivation was collected from the Public Health Profiles.25
Primary outcome
This was: total oral antibiotics dispensed (per 1000 practice patients, excluding anti-tuberculosis and minocycline) within intervention practices compared with controls in the year following the intervention, taking into account dispensing in the previous year.
Secondary outcomes
These were: workshop uptake, dispensing of antibiotics typically prescribed for RTIs [amoxicillin, clarithromycin, tetracyclines excluding minocycline and limecycline (as these are only prescribed as long courses for acne in the UK), and phenoxymethylpenicillin], UTIs (nitrofurantoin, trimethoprim and pivmecillinam) and broad-spectrum antibiotics (co-amoxiclav, quinolones and cephalosporins).
Data analysis
We undertook descriptive data analysis of GP practice demographic data, workshop practice attendance and trends in dispensing data for each GP practice over the period before and during the study. Mixed-effects negative binomial time-series regression models were used to provide estimates of the intervention effect. As workshops were delivered over 7 months (January–August 2014) and prescribing has seasonal patterns, to ensure that the months of outcome data for the control practices mirrored precisely the months after the workshops were delivered, the practice dispensing rate in 2013 was used by the statistician (A. C.), using Stata 1326 to undertake post hoc matching of intervention practices accepting the workshop and control practices. A ‘change point’ was assigned to intervention practices that declined the workshop, and to control practices. The empirical cumulative distribution function (CDF) of the 2013 dispensing rate for each of the three study groups (intervention-accepted workshops, intervention-declined workshops and controls) was constructed and the date of the workshop assigned to percentiles within that distribution. For example, the practice with the lowest dispensing rate had a workshop in February 2014, and those control and intervention practices that declined workshops whose rate fell below the 2.5th percentile were also allocated a change point of February 2014. Within the mixed-effects models, GP was included as a random intercept.
As planned in the protocol, we undertook an ITT analysis, including all intervention practices (whether they had a workshop or not), using baseline dispensing rate in the 12 months prior to the workshops, ‘change point’ and CCG, sequential month (and sequential month squared), calendar month and other AMS interventions as explanatory variables in the regression model. The natural logarithm of the practice list size was included as an offset. In addition, to overcome the effects of confounding due to non-compliance (refusing the intervention) we used a Complier-Average Causal Effect (CACE) analysis using an instrument variable approach with randomization as an instrumental variable for workshop received.27 A Poisson regression model with an endogenous regressor using a two-step generalized method of moments estimator was employed. This provides an estimate of the study effect in compliant practices, compared with the theoretical compliant practices in the control arm. All analyses were performed using Stata 13.26
Results
Thirty-six of the 73 intervention practices (49%) received a workshop between January and July 2014 (Figure 2). Baseline total antibiotic dispensing in 2013 was similar in control and intervention (accepted or declined workshops) practices. Workshops were attended by 166 GPs, 51 nurses, and 101 other staff including receptionists, healthcare assistants and practice managers (median for each practice 5–6, range 1–16). On average, each workshop consisted of 4 GPs (mode 5, range 1–10), 3 nurses (mode 1, range 1–7) and 2 other staff (mode 1, range 1–9).
Figure 2.
Study flow chart.
The absolute number and rate of total antibiotic items dispensed in the year after the workshop compared with the year before was lower in the intervention practices compared with controls (Table 1). Dispensing of phenoxymethylpenicillin, amoxicillin/ampicillin, co-amoxiclav, oral cephalosporins, macrolides and trimethoprim was lower in the intervention practices accepting a workshop compared with those who declined, while dispensing of oral nitrofurantoin and pivmecillinam was higher.
Table 1.
Oral antibiotic items dispensed and rate per 100 patients per year (excluding anti-TB and minocycline) for 1 year before and 1 year post workshop, aggregated across all practices by study group
| Intervention practices offered 1 h workshop |
|||||||
|---|---|---|---|---|---|---|---|
| Antibiotic groupings | Time | Control practices |
declined |
accepted |
|||
| items | ratea | items | ratea | items | ratea | ||
| Total antibiotics | |||||||
| pre | 368 940 | 61.44 | 180 270 | 61.42 | 164 126 | 61.84 | |
| post | 372 427 | 62.45 | 182 946 | 62.75 | 160 946 | 59.82 | |
| Respiratory tract infection antibiotics | |||||||
| phenoxymethylpenicillin | pre | 20 276 | 3.376 | 9447 | 3.219 | 8990 | 3.387 |
| post | 20 162 | 3.381 | 9650 | 3.310 | 8928 | 3.318 | |
| amoxicillin and ampicillin | pre | 100 913 | 16.804 | 48 615 | 16.564 | 44 125 | 16.626 |
| post | 103 992 | 17.437 | 50 324 | 17.260 | 42 774 | 15.898 | |
| all tetracyclines excluding minocycline/lymecycline | pre | 30 429 | 5.067 | 13 390 | 4.562 | 13 802 | 5.201 |
| post | 28 979 | 4.859 | 13 033 | 4.470 | 14 463 | 5.376 | |
| all macrolides | pre | 48 022 | 7.997 | 25 139 | 8.565 | 22 182 | 8.358 |
| post | 50 750 | 8.509 | 25 330 | 8.687 | 21 216 | 7.885 | |
| Urinary tract infection antibiotics | |||||||
| trimethoprim | pre | 45 951 | 7.652 | 23 106 | 7.873 | 20 280 | 7.642 |
| post | 44 022 | 7.381 | 22 940 | 7.868 | 19 853 | 7.379 | |
| nitrofurantoin | pre | 23 502 | 3.914 | 11 861 | 4.041 | 11 866 | 4.471 |
| post | 23 164 | 3.884 | 11 487 | 3.940 | 12 907 | 4.797 | |
| pivmecillinam/mecillinam | pre | 529 | 0.442 | 232 | 0.389 | 83 | 0.124 |
| post | 24 | 0.021 | 19 | 0.032 | 644 | 0.968 | |
| Broad-spectrum antibiotics | |||||||
| co-amoxiclav | pre | 26 917 | 4.482 | 13 803 | 4.703 | 11 463 | 4.319 |
| post | 28 784 | 4.826 | 14 780 | 5.069 | 9 237 | 3.433 | |
| all cephalosporins | pre | 9917 | 1.651 | 4545 | 1.549 | 4111 | 1.549 |
| post | 10 727 | 1.799 | 4921 | 1.688 | 3630 | 1.349 | |
| all quinolones | pre | 9983 | 1.662 | 5603 | 1.909 | 4703 | 1.772 |
| post | 10 224 | 1.714 | 5563 | 1.908 | 4706 | 1.749 | |
| all broad spectrum | pre | 46 817 | 7.796 | 23 951 | 8.160 | 20 277 | 7.640 |
| post | 49 735 | 8.339 | 25 264 | 8.665 | 17 573 | 6.531 | |
All decreased rates in bold. Values in bold are statistically significant (P ≤ 0.05).
Rate per 100 patients per year.
Intention-to-treat analyses
In the ITT analyses (Table 2) there was non-significantly lower total antibiotic dispensing in the intervention practices allocated to receive the workshop compared with controls, which almost attained significance [−2.7%, dispensing rate ratio (DRR) 0.973, 95% CI 0.945–1.001, P = 0.06]. Amoxicillin/ampicillin dispensing was significantly lower (−4.4%, DRR 0.956, 95% CI 0.920–0.994, P = 0.02), trimethoprim dispensing was significantly lower, but almost attained significance (−5.6%, DRR 0.944, 95% CI 0.898–0.993, P = 0.03) and dispensing of nitrofurantoin was non-significantly higher (+7.1%, DRR 1.071, 95% CI 0.997–1.150, P = 0.06) in the intervention practices compared with control practices. There was no significant change in overall usual ‘UTI-specific’ antibiotic use (trimethoprim, plus nitrofurantoin plus pivmecillinam).
Table 2.
Estimated DRR comparing intervention practices with controls from ITT and the CACE analyses
| Oral antibiotics (excluding anti-TB and minocycline) | Analysis |
|||
|---|---|---|---|---|
| ITT |
CACE |
|||
| DRR (95% CI) | P | DRR (95% CI) | P | |
| Total antibiotics | 0.973 (0.945–1.001) | 0.06 | 0.939 (0.883–0.998) | 0.04 |
| Usual respiratory tract infection antibiotics | ||||
| phenoxymethylpenicillin | 0.971 (0.908–1.038) | 0.39 | 0.928 (0.811–1.063) | 0.28 |
| amoxicillin/ampicillin | 0.956 (0.920–0.994) | 0.02 | 0.924 (0.839–1.017) | 0.11 |
| All tetracyclines | 0.987 (0.921–1.058) | 0.71 | 0.925 (0.809–1.058) | 0.26 |
| All macrolides | 1.007 (0.956–1.062) | 0.79 | 1.005 (0.889–1.136) | 0.93 |
| Usual urinary tract infection antibiotics | ||||
| trimethoprim | 0.944 (0.898–0.993) | 0.03 | 0.890 (0.805–0.984) | 0.02 |
| nitrofurantoin | 1.071 (0.997–1.150) | 0.06 | 1.116 (0.964–1.293) | 0.14 |
| pivmecillinam | 1.611 (0.852–3.046) | 0.14 | a | |
| all UTI only: trimethoprim, nitrofurantoin and pivmecillinam | 0.988 (0.947–1.031) | 0.58 | 0.964 (0.893–1.041) | 0.35 |
| Broad-spectrum antibiotics | ||||
| all: co-amoxiclav, quinolones and cephalosporins | 0.986 (0.927–1.048) | 0.65 | 0.967 (0.847–1.104) | 0.61 |
| co-amoxiclav only | 0.969 (0.891–1.054) | 0.46 | 0.945 (0.778–1.148) | 0.57 |
| quinolones only | 1.037 (0.946–1.136) | 0.44 | 1.043 (0.849–1.281) | 0.69 |
| cephalosporins only | 1.003 (0.871–1.155) | 0.97 | 0.976 (0.726–1.312) | 0.87 |
Values in bold are statistically significant (P < 0.05).
Unable to converge to a solution.
CACE analysis
In these analyses, total antibiotic dispensing and trimethoprim dispensing were significantly lower (total 6.1%, 95% CI 0.2%–11.7%, P = 0.04; trimethoprim 11%, 95% CI 1.6%–19.5%, P = 0.02), respectively, in intervention practices compared with control practices (Table 2). However, for both amoxicillin/ampicillin and nitrofurantoin, the estimates from this analysis were larger in magnitude [7.6% lower (95% CI −16.1% to +17%, P = 0.11)] and 11.6% higher (95% CI −3.6% to +29.3%, P = 0.14, respectively) but less precise (with larger P values).
In the ITT and CACE analyses, phenoxymethylpenicillin, co-amoxiclav, oral cephalosporin and quinolone dispensing were all lower in the intervention practices, while pivmecillinam dispensing from these practices was higher. However, none of these changes was significant. Macrolide use remained unchanged.
Discussion
We found that face-to-face TARGET AMS workshops, including reflection on antibiotic prescribing, guidance, clinical scenarios, strategies to improve prescribing, and demonstration of TARGET patient-facing resources, audits and educational resources, improved antibiotic dispensing for infections in general practices outside a research setting. However, in 2014 before any QP was introduced in England, around half of NHS general practices turned down the offer of a workshop.
Strengths
The McNulty–Zelen design minimizes the risk of research processes influencing outcome. It is particularly useful for evaluating education interventions, and estimates of effect are more likely to reflect what would happen if the intervention were to be introduced in non-trial conditions. It minimizes many of the biases discussed in a 2014 systematic review of educational interventions to improve antibiotic prescribing.28 It is difficult to determine the exact impact of ‘knowing you are in a study’ (the Hawthorne effect) without repeating the randomized controlled trial within research practices, and with full awareness and consent of all GP staff taking part.
Contamination between intervention and control groups was minimized by randomizing all practices in the same location into the same arm. Other cross-contamination would reflect usual events in everyday practice. There was some evidence that GPs knew other practices were getting educated, as three practices not in the study requested the intervention. We used local staff to give the workshops, so there was no reason for staff to think they were in a trial; no GP staff participating in focus groups or interviews to explore their views of the intervention reported that they thought they were part of a trial.29 The TARGET workshop and toolkit are based on a behavioural approach,28 and address prescribing for all common infections rather than just one condition, which was highlighted as a weakness of studies in the 2014 systematic review.28 Another systematic review found UTIs were under-represented;30 the TARGET workshop includes UTI cases and encourages more appropriate prescribing for UTIs, including advice to use nitrofurantoin for acute uncomplicated UTIs in line with national guidance,31 and in line with the 2017/18 QP incentive.10 Only half of the intervention practices took up the offer of workshops in 2014, but this is probably a true reflection of the difficulties that CCG facilitators face in the context of stretched general practices with many conflicting priorities. Changes to the organization of general practice or incentives from CCGs to practices encouraged by the QP could make the workshops more attractive.10 Furthermore the effect of the workshops may be greater in the presence of the QP, as practices will know that their prescribing is being closely monitored. Practice randomization was stratified by antibiotic use in the year prior to the intervention, and all practices were involved, not just high-prescribing practices. The effect may be greater if directed only at practices with higher than average prescribing.32,33 To match usual service provision, intervention practices were able to opt in or out of the offer of a workshop. We controlled for key potential confounders induced by the non-response (practice list size, baseline dispensing, CCG, seasonal variations and Chief Medical Officer letter to practices) and found no evidence of important differences between randomized groups.
Limitations
Although practices that declined workshops had similar pre-intervention total antibiotic dispensing to those that accepted workshops, they had on average higher broad-spectrum antibiotic dispensing. This supports the use of the CACE analysis, instead of a per-protocol analysis, to allow for selection bias due to differences in intervention practices that declined and attended workshops in their attitude to AMS. The study was powered to determine an effect on all-cause antibiotic dispensing; the sample size would have had to be much larger to determine the effect of the intervention on individual antibiotic classes. We did not attempt to directly collect data on appropriateness of antibiotic use for different infections, or clinical outcomes including adverse events, as this would require practice searches that would be time consuming and lead to a loss of concealment. Furthermore, assessment of the appropriateness of prescribing is difficult as clinicians tend to legitimize an antibiotic prescription with a diagnosis that requires an antibiotic.34–36 There was some evidence of more appropriate use of antibiotics for probable UTI, in line with PHE antibiotic guidance,17 and there was some evidence of a decrease in trimethoprim and increase in nitrofurantoin dispensing, though only the reduction in trimethoprim was statistically significant. A 10% reduction in total antibiotic dispensing (used in our sample size calculation) was probably over-ambitious; the QP that was introduced in England in 2015 only sought a 1% reduction in total antibiotic use.37 The 2017/18 QP is seeking a 10% reduction in trimethoprim items dispensed for patients aged ≥70 years, and a 10% reduction in the ratio of trimethoprim to nitrofurantoin dispensing in primary care (baseline 2015/16).10 Both of these reductions were achieved in our study before the QP was in place. Unintended consequences of reducing antibiotic use, such as increased complications from common infections, will need to be monitored by health providers. In England, Escherichiacoli bacteraemias are being specifically monitored prospectively from 2017.10,38
As this was a pragmatic study, workshops were arranged at the convenience of practices, and undertaken mostly after peak winter prescribing when staff were under less pressure. The workshop effect may have been greater if undertaken before the winter peak prescribing. The workshops were completed 9 months before the start of the English QP (which incentivized CCGs to reduce total antibiotic prescribing by 1% and broad-spectrum antibiotics by 10%). When this QP intervention, starting in April 2015, was added to the model, it had no effect on outcomes. We could only study the effect of the offer of the TARGET workshop on normal service provision, as all the TARGET resources are available free to any users on the web site. Although we performed a qualitative evaluation on a subset of the intervention practices (details to be published separately), we could not ask all practices to collect prospective information on what TARGET resources they had used. We have not presented the cost effectiveness of this approach, but it used freely available PHE/RCGP resources, the usual staff involved in AMS, and the usual practice continuing professional development sessions. The opportunity costs, ie the costs of taking staff away from their other work for training themselves, and the costs of travel and practice workshops, will need to be balanced locally against the amount an area is willing to pay to achieve reductions in antibiotic use and more appropriate prescribing, which in turn may help to control AMR.
Comparison with other studies
The general public have some awareness that most coughs, sore throats, colds and flu get better without antibiotic treatment, but when it comes to themselves they are concerned about the possible severity and duration of their own illness, and consequently about one-fifth will seek advice at a GP practice.39 Not surprisingly, therefore, a 2016 review of interventions to change prescribing in primary care indicated that although media campaigns are more effective than medical professionals at disseminating information about antibiotics, health professionals are more effective at actually changing behaviours.40 Thus, not surprisingly, multifaceted interventions involving health professionals as well as patients and community education have consistently produced moderate changes in prescribing behaviours, in comparison with single interventions aimed at only one of these components, which are often unsuccessful.1 The TARGET workshop gives health professionals the impetus and intentions to change their behaviour, and then gives them the patient-facing materials and dispensing data to facilitate this intentional change. Workshops alone without feedback about antibiotic prescribing do not give prescribers the information they need regarding their prescribing, and how they could improve it compared with their peers locally, nationally or in Europe. Consequently, they may be happy to continue their current prescribing, unaware where they lie compared with others.41 Involving the whole practice staff rather than just interested individuals or ‘champions’ is therefore important so that an action plan for antibiotic prescribing can be established that involves all staff.41
Audit and feedback are often used as a strategy to improve professional practice on their own or as a component of multifaceted interventions (as in TARGET workshops); this is based on the belief that prescribers will modify their practice when given performance feedback showing that their clinical practice is inconsistent with a desirable target,32 thus influencing their personal attitudes and social norms within a given setting. Although guidance and data provision form the bedrock of AMS, they may not change behaviour when used in isolation.42 Systematic reviews show that audit and feedback can lead to small but potentially important improvements in professional practice, but are more effective if the baseline performance is poor,33 if the source is a supervisor or colleague, and if they are provided more than once,43 and are delivered in both written and verbal formats, with explicit targets and an action plan.32 The TARGET workshops fulfilled most of these criteria, but we did not insist on repeated feedback of antibiotic use within the study. The TARGET workshop did encourage ongoing antibiotic audits in the practices, but we have not collected data on how many practices performed these. Feedback from commissioners and TARGET facilitators suggested that making time at the TARGET workshop to do even more specific action planning and set targets for dispensing, and pledges to do audits, may facilitate more behaviour change (data presented separately).29 TARGET training resources stress the extreme importance of action planning and setting targets in the workshop.
Our TARGET workshops within routine practice had effects on total antibiotic dispensing similar to those of the STAR programme (4.2% difference between intervention and controls, which was evaluated within research practices). STAR had a phased engagement and implementation with a practice-based seminar reflecting on practice dispensing and local resistance data, followed by online educational materials and practising consulting skills in routine care through web-based exchange of learning.2 Our results indicate that if the correct behavioural components are in place, a longer programme may not be needed. Further follow-up of the study practices would determine if the effect lasted longer than 1 year, but this has become more difficult as additional QPs that would influence outcomes are now in place across England.
A time-series analysis of a similar health board intervention in Tayside showed that a multifaceted intervention led to a 33.5% reduction in prescribing the 4Cs broad-spectrum antibiotics (cephalosporins, ciprofloxacin, co-amoxiclav and clindamycin), when specific targets and actions for individual practices were set.44 Although there was a reduction in co-amoxiclav use this was not significant, as this was a secondary outcome and the sample size was too small. Interestingly quinolone use increased slightly but this was not statistically significant; therefore trainers will need to focus on this antibiotic in future workshops if the practice prescribes high levels of this antibiotic. NHS England has now set dispensing indicators for English practices using the QP. Combined with the QP,10 the TARGET resources should lead to greater changes in dispensing.
The patient-focused TARGET leaflets are an important behavioural component of the resources, as they give the patient information about self-care, likely illness duration and when to reconsult. They also provide the prescriber with something to give to the patient when they leave (thus legitimizing their visit) and provide the clinician with the opportunity to use a back-up/delayed prescription.4,15 A systematic review has shown that back-up/delayed antibiotic dispensing is an effective strategy to reduce immediate antibiotic dispensing.45 Although GP staff in 2014 in routine practice across England reported that delayed antibiotic dispensing was useful, they reported that a lack of an agreed strategy within a practice, perceived damage to the patient/doctor relationship, lack of training on the strategy, and lack of feedback on dispensing data to inform if their action had made a difference reduced its use.46 The TARGET workshop and resources could address most of these barriers if complemented with focused action planning around the back-up dispensing and how to implement it using the TARGET leaflet during consultations. There is also a TARGET audit template that includes an assessment for delayed/back-up dispensing in common RTIs and UTI.
Implications
This study indicates that the TARGET workshop with its freely available resources can help improve antibiotic dispensing in primary care. We suggest that commissioners and prescribers access the free workshop and TARGET resources, and promote these to their practices and prescribers with action planning and dispensing feedback in line with any local performance indicators. Further work is needed to study the effect of TARGET workshops long term on antibiotic use and resistance using the antibiotic dispensing, patient and routine laboratory susceptibility data that are now available. Ongoing evaluation of the TARGET antibiotics workshop intervention, ideally using routine prescribing data and monitoring of unexpected consequences, should be incorporated into any scale-up that is planned by primary care commissioners.
Acknowledgements
Part of the data were presented at a General Practice Research on Infections Network meeting (Session 5, Abstract 4), and also at the European Congress of Clinical Microbiology and Infectious Diseases, 2016 (Abstract 3638).
We thank all the GP staff, CCG staff and others involved in this study who helped it succeed, including Harpal Dhillon who was involved in initial discussions, Micaela Gal, CCGs (Coastal West Sussex, Swindon, Gloucestershire and Oldham). Those involved in setting up and teaching in each CCG: Paul Wilson and Sue Carter; Susan Dawson, Anita Sharma, Tariq Sharf, Nigel Dunkerley; medicine managers, those who provided data, those involved in local approvals especially in Gloucester: Mark Walker, Marie-Jet Bekkers, Graham Tanner (the patient representative), Alison West, Ivor Cartmill, Adam Simon, Thomas Daines, Paul Clarke, Jen Whibley, Julie Sadler, Gloria Omisakin and Sarah Clarke; and those who helped to develop and trial the TARGET resources.
Funding
This study is independent research funded by Public Health England.
Transparency declarations
None to declare.
The corresponding author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Arnold SR, Straus SE.. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005; issue 4: CD003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butler CC, Simpson SA, Dunstan F. et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ 2012; 344: d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cals JW, Butler CC, Hopstaken RM. et al. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009; 338: b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Francis NA, Butler CC, Hood K. et al. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial. BMJ 2009; 339: b2885.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Little P, Hobbs FD, Moore M. et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013; 347: f5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llor C, Cots JM, Hernández S. et al. Effectiveness of two types of intervention on antibiotic prescribing in respiratory tract infections in primary care in Spain. Happy Audit Study. Aten Primaria 2014; 46: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cals JW, Ament AJ, Hood K. et al. C-Reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. J Eval Clin Pract 2011; 17: 1059–69. [DOI] [PubMed] [Google Scholar]
- 8. Hay AD, Redmond NM, Turnbull S. et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016; 4: 902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Department of Health. UK 5 Year Antimicrobial Resistance Strategy 2013 to 2018 https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018.
- 10. NHS England. Quality Premium Guidance for 2016/17 2016. https://www.england.nhs.uk/resources/resources-for-ccgs/ccg-out-tool/ccg-ois/qual-prem/.
- 11. Public Health England. The TARGET Antibiotics Toolkit http://www.rcgp.org.uk/clinical-and-research/toolkits/target-antibiotics-toolkit.aspx.
- 12. McNulty C, Francis N.. Optimizing antibiotic prescribing in primary care settings in the UK: findings of a BSAC multidisciplinary workshop 2009. J Antimicrob Chemother 2010; 65: 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Public Health England. Training Resources: The TARGET Antibiotics Toolkit http://www.rcgp.org.uk/clinical-and-research/toolkits/∼/link.aspx?_id=2FC34B3CA5B446F19CB795B37AFF5083&_z=z.
- 14. Public Health England. Self Assessment Checklist: The TARGET Antibiotics Toolkit http://www.rcgp.org.uk/clinical-and-research/toolkits/∼/link.aspx?_id=E1FC849D426E420E941FB9CF8E47D508&_z=z.
- 15. Bunten AK, Hawking MKD, McNulty CAM.. Patient information can improve appropriate antibiotic prescribing. Nursing in Practice 2015; 82: 55–7. [Google Scholar]
- 16. Public Health England. Audit Toolkits and Action Planning: The TARGET Antibiotics Toolkit http://www.rcgp.org.uk/clinical-and-research/toolkits/∼/link.aspx?_id=4725F0AA89A349E991425E510F7D6371&_z=z.
- 17. Public Health England. National Antibiotic Management Guidance: The TARGET Antibiotics Toolkit https://www.gov.uk/government/publications/managing-common-infections-guidance-for-primary-care.
- 18. Public Health England. Resources for Commissioners: The TARGET Antibiotics Toolkit http://www.rcgp.org.uk/clinical-and-research/toolkits/∼/link.aspx?_id=F6F9D0EA63B243F4A0ACFCD7390E79CF&_z=z.
- 19. McNulty CA, Hogan AH, Ricketts EJ. et al. Increasing chlamydia screening tests in general practice: a modified Zelen prospective cluster randomised controlled trial evaluating a complex intervention based on the theory of planned behaviour. Sex Transm Infect 2014; 90: 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ajzen I. The theory of planned behaviour. Organ Behav Hum Decis Process 1991; 50: 179–211. [Google Scholar]
- 21. Ajzen I. Perceived behavioral control self-efficiacy locus of control and theory of planned behavior. J Appl Soc Pyschol 2002; 32: 665–83. [Google Scholar]
- 22. Godin G, Kok G.. The theory of planned behavior: a review of its applications to health-related behaviors. Am J Health Promot 1996; 11: 87–98. [DOI] [PubMed] [Google Scholar]
- 23. McNulty CA. European Antibiotic Awareness Day 2012: general practitioners encouraged to TARGET antibiotics through guidance, education and tools. J Antimicrob Chemother 2012; 67: 2543–6. [DOI] [PubMed] [Google Scholar]
- 24. Public Health England. Leaflets to Share with Patients: The TARGET Antibiotics Toolkit http://www.rcgp.org.uk/clinical-and-research/toolkits/∼/link.aspx?_id=9FCF9DA4B4A045519593320478DFD9E7&_z=z.
- 25. Public Health England. Public Health Profiles http://fingertips.phe.org.uk/.
- 26. StataCorp. Stata Statistical Software: Release 13. College Station, TX, USA: StataCorp LP, 2013. [Google Scholar]
- 27. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2000; 29: 722–9. [DOI] [PubMed] [Google Scholar]
- 28. Roque F, Herdeiro MT, Soares S. et al. Educational interventions to improve prescription and dispensing of antibiotics: a systematic review. BMC Public Health 2014; 14: 1276.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones LF, Hawking MKD, Owens R. et al. An evaluation of the TARGET (Treat Antibiotics Responsibly; Guidance, Education, Tools) Antibiotics Toolkit to improve antimicrobial stewardship in primary care—is it fit for purpose? Fam Pract 2017; doi: 10.1093/fampra/cmx131. [DOI] [PMC free article] [PubMed]
- 30. Drekonja DM, Filice GA, Greer N. et al. Antimicrobial stewardship in outpatient settings: a systematic review. Infect Control Hosp Epidemiol 2015; 36: 142–52. [DOI] [PubMed] [Google Scholar]
- 31. Public Health England. Management of Infection Guidance for Primary Care for Consultation and Local Adaptation 2017. https://www.gov.uk/government/consultations/managing-common-infections-guidance-for-primary-care.
- 32. Ivers N, Jamtvedt G, Flottorp S. et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012; issue 6: CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hallsworth M, Chadborn T, Sallis A. et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016; 387: 1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andre M, Odenholt I, Schwan A. et al. Upper respiratory tract infections in general practice: diagnosis, antibiotic prescribing, duration of symptoms and use of diagnostic tests. Scand J Infect Dis 2002; 34: 880–6. [DOI] [PubMed] [Google Scholar]
- 35. van Duijn HJ, Kuyvenhoven MM, Tiebosch HM. et al. Diagnostic labelling as determinant of antibiotic prescribing for acute respiratory tract episodes in general practice. BMC Fam Pract 2007; 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gulliford M, Latinovic R, Charlton J. et al. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health (Oxf) 2009; 31: 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. NHS England. Quality Premium 2015/16: Guidance for CCGs 2015. https://www.england.nhs.uk/wp-content/uploads/2013/12/qual-prem-guid.pdf.
- 38. Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2017. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/656611/ESPAUR_report_2017.pdf.
- 39. McNulty CAM, Nichols T, French DP. et al. Expectations for consultations and antibiotics for respiratory tract infection in primary care: the RTI clinical iceberg. Br J Gen Pract 2013; 63: e429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Public Health England, Department of Health. Behaviour Change and Antibiotic Prescribing in Healthcare Settings Literature Review and Behavioural Analysis 2015. https://www.gov.uk/government/publications/antibiotic-prescribing-and-behaviour-change-in-healthcare-settings.
- 41. Ackerman SL, Gonzales R, Stahl MS. et al. One size does not fit all: evaluating an intervention to reduce antibiotic prescribing for acute bronchitis. BMC Health Serv Res 2013; 13: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hemkens LG, Saccilotto R, Reyes SL. et al. Personalized prescription feedback using routinely collected data to reduce antibiotic use in primary care. JAMA Intern Med 2017; 177: 176–83. [DOI] [PubMed] [Google Scholar]
- 43. Meeker D, Linder JA, Fox CR. et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices a randomized clinical trial. JAMA 2016; 315: 562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez-Santiago V, Marwick CA, Patton A. et al. Time series analysis of the impact of an intervention in Tayside, Scotland to reduce primary care broad-spectrum antimicrobial use. J Antimicrob Chemother 2015; 70: 2397–404. [DOI] [PubMed] [Google Scholar]
- 45. Spurling GK, Del Mar CB, Dooley L. et al. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev 2017; issue 9: CD004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ryves R, Eyles C, Moore M. et al. Understanding the delayed prescribing of antibiotics for respiratory tract infection in primary care: a qualitative analysis. BMJ Open 2016; 6: e011882. [DOI] [PMC free article] [PubMed] [Google Scholar]