This editorial refers to ‘Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury’ by L. Wang et al., pp. 805–821.
Myocardial injury is increased in aged hearts during ischaemia-reperfusion (IR) and accelerates the transition to post-infarct heart failure.1 Although many therapeutic interventions can effectively decrease cardiac injury in younger adult hearts, strategies to decrease cardiac injury in aged hearts are still limited.1 Therefore, understanding the mechanisms of injury in the aged heart during IR is critical to develop potential strategies to protect the high-risk aged heart.2
The impairment of substrate flexibility (balance between glucose usage and fatty acid oxidation) contributes to cardiac injury during IR as well as heart failure development.3 Limitation of glucose utilization and oxidation with concomitant increased fatty acid oxidation during reperfusion enhances cardiac injury.3 Dr Li et al. found that the substrate flexibility was markedly impaired in aged hearts following IR compared with young hearts.4 They further found that the decreased substrate flexibility was due in part to impairment of the AMPK (AMP-activated protein kinase) signalling pathway activation. Glucose uptake and utilization was decreased in parallel with enhanced fatty acid oxidation.4 AMPK contributes an essential role in the regulation of glucose and lipid metabolism. Activation of the AMPK leads to increased glucose uptake and enhances insulin sensitivity.5 Liver kinase B1 (LKB1) is an AMP-activated kinase that activates AMPK through phosphorylation of the alpha subunit of AMPK.6 The activity of LKB1 is regulated by SIRT1 (sirtuin 1), a member of the sirtuin (NAD-dependent deacetylase) family.7 Activation of SIRT1 leads to deacetylation and activation of LKB1 that increases AMPK phosphorylation and activity. Thus, SIRT1-dependent activation of LKB1 stimulates AMPK activity. Dr Li’s study clearly showed that the impaired AMPK activation in aged hearts was at least in part due to decreased SIRT1 activity.4 Increased expression of SIRT1 via adenoviral transfection rescued the attenuation of AMPK activation and reduced ischaemic injury, providing key mechanistic support. Interestingly, they also found that there was a feedback interaction between AMPK and SIRT1 activation. AMPK can act as an upstream enzyme to stimulate SIRT1 activity through modulation of intracellular NAD+ concentration.6 Sestrin 2 is another protein that forms a complex with AMPK and LKB1.8 Knockout of Sestrin 2 leads to decreased AMPK activation and enhanced ischaemic injury.9 Pharmacologic activation of AMPK or restoration of SIRT1 activity in aged hearts led to decreased cardiac injury during IR, indicating that restoration of the signalling defect in aged hearts is a promising strategy to decrease cardiac injury. The potential role of Sestrin 2 in modifying the AMPK-SIRT1 interaction will await further research (Figure 1).
Figure 1.
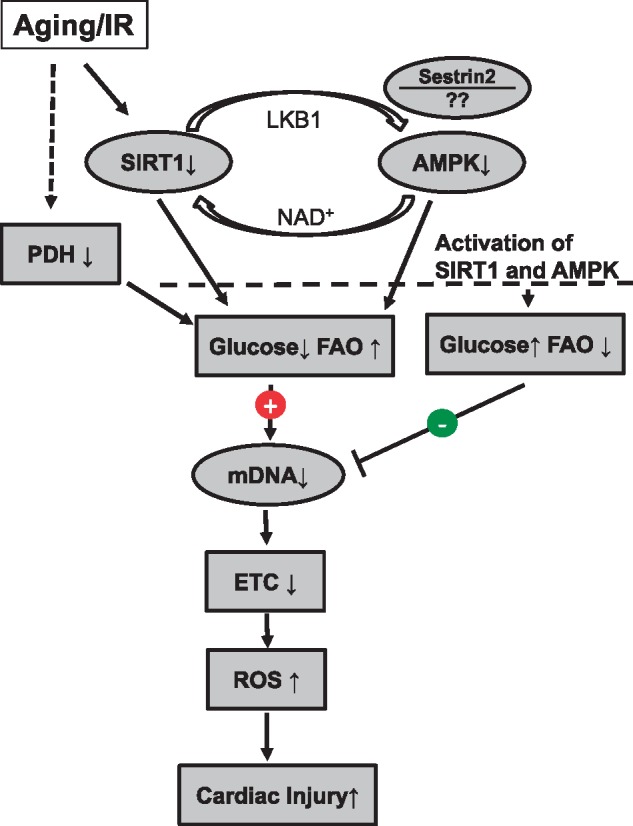
Activation of SIRT1 and AMPK decreases cardiac injury in aged hearts following IR. Aging and IR decreases SIRT1 activity that leads to decreased AMPK activity through acetylation and subsequent inhibition of LKB1. Deactivation of AMPK also affects SIRT1 activity due to altered NAD+ concentration. Sestrin 2 may also interact and affect AMPK activation. Deactivation of SIRT1 and AMPK leads to decreased glucose oxidation and increased fatty acid oxidation (FAO) that contribute to increased injury in aged hearts following IR. Aging also impairs mtDNA that increases ROS generation by inhibiting Complex III at mitochondrial ETC. IR leads to decreased PDH activity that also leads to altered metabolism in hearts with decreased glucose oxidation and increased FAO. Activation of SIRT1 and AMPK is a promising approach to decrease cardiac injury in aged hearts following IR.
Mitochondrial dysfunction contributes to increased cardiac injury in aged hearts.1 Aging impairs electron transport chain (ETC) activity in the interfibrillar population of mitochondria located between the fibrils (IFM). The Complex III activity is decreased in IFM isolated from the aged hearts. Complex III is one of the key sites to generate the reactive oxygen species (ROS).10 The defect of Complex III in aged hearts increases ROS generation that augments cardiac injury during IR. Dr Li et al. found evidence for increased ROS generation and deteriorated mitochondrial morphology after IR in aged hearts. Mitochondrial DNA (mtDNA) was further damaged in aged hearts by IR.4 Cytochrome b, a key catalytic subunit of Complex III, is encoded by mtDNA. Age-related defects in Cytochrome b lead to decreased Complex III activity in aged hearts.10 Thus, these results suggest that the aging-induced mtDNA damage contributes to the increased cardiac injury in aged hearts. It will be interesting to consider if activation of AMPK or SIRT1 can restore the Complex III defect present in aged hearts.
Pyruvate dehydrogenase (PDH) is a key enzyme to regulate glucose vs. fatty acid utilization. Inhibition of PDH leads to decreased glucose oxidation by diminishing the entry of acetyl-CoA into the tricarboxylic acid cycle. PDH activity is decreased in hearts following IR. PDH activity is affected by post-translational modification.11 Is impaired PDH activity involved in the decreased glucose oxidation in aged hearts? Is PDH more sensitive to IR damage in aged hearts? Does activation of AMPK or SIRT1 protect PDH activity in aged hearts following IR?
The key finding in this study is the clear crosstalk between decreased AMPK activity and the defect of SIRT1 in aged hearts. Metformin, a traditional AMPK activator, leads to decreased cardiac injury in hearts during IR.12,13 Activation of SIRT1 also decreases cardiac injury in aged hearts. These results are consistent with the notion raised in this study.
Interestingly, a recent study showed that metformin–leucine co-treatment can activate both AMPK and SIRT1.6 Addition of leucine to metformin increases the effect of metformin on insulin sensitivity and glycemic control in that both AMPK and SIRT1 contribute to the regulation of glucose and lipid metabolism. Metformin is the most commonly prescribed drug for the treatment of Type 2 diabetes. It is also known that metformin decreases cardiac injury during IR.12,13 Co-treatment of metformin–leucine improves insulin sensitivity and improves glucose metabolism.6 Does metformin–leucine co-treatment provide better protection in aged hearts following IR compared with individual AMPK activation or SIRT1 activation?
Clearly, this study which identifies the mechanisms of AMPK deficiency in aged hearts provides guidance to develop potential pharmacological approaches to decrease cardiac injury and subsequent heart failure development in aged hearts.
Funding
This work was supported by the Office of Research and Development, Medical Research Service Merit Review Award (2IO1BX001355-01A2), Department of Veterans Affairs (E.J.L.), NIH R21AG054975-01 (Q.C.), and the Pauley Heart Center, Virginia Commonwealth University (Q.C., E.J.L.).
Conflict of interest: none declared.
References
- 1. Lesnefsky EJ, Chen Q, Hoppel CL.. Mitochondrial metabolism in aging heart. Circ Res 2016;118:1593–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lesnefsky EJ, Lundergan CF, Hodgson JM, Nair R, Reiner JS, Greenhouse SW, Califf RM, Ross AM.. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-I angiographic experience. J Am Coll Cardiol 1996;28:331–337. [DOI] [PubMed] [Google Scholar]
- 3. Kolwicz SC Jr, Tian R.. Metabolic therapy at the crossroad: how to optimize myocardial substrate utilization? Trends Cardiovasc Med 2009;19:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Quan N, Sun W, Chen X, Cates C, Rousselle T, Zhou X, Zhao X, Li J.. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc Res 2018;114:805–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stein SC, Woods A, Jones NA, Davison MD, Carling D.. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 2000;345(Pt 3):437–443. [PMC free article] [PubMed] [Google Scholar]
- 6. Banerjee J, Bruckbauer A, Zemel MB.. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 2016;65:1679–1691. [DOI] [PubMed] [Google Scholar]
- 7. Brandes RP. Activating SIRT1: a new strategy to prevent atherosclerosis? Cardiovasc Res 2008;80:163–164. [DOI] [PubMed] [Google Scholar]
- 8. Morrison A, Chen L, Wang J, Zhang M, Yang H, Ma Y, Budanov A, Lee JH, Karin M, Li J.. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J 2015;29:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quan N, Wang L, Chen X, Luckett C, Cates C, Rousselle T, Zheng Y, Li J.. Sestrin2 prevents age-related intolerance to post myocardial infarction via AMPK/PGC-1alpha pathway. J Mol Cell Cardiol 2018;115:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moghaddas S, Hoppel CL, Lesnefsky EJ.. Aging defect at the Qo site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch Biochem Biophys 2003;414:59–66. [DOI] [PubMed] [Google Scholar]
- 11. Thompson J, Hu Y, Lesnefsky EJ, Chen Q.. Activation of mitochondrial calpain and increased cardiac injury: beyond AIF release. Am J Physiol 2016;310:H376–H384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM.. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther 2013;27:5–16. [DOI] [PubMed] [Google Scholar]
- 13. Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ.. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 2008;57:696–705. [DOI] [PubMed] [Google Scholar]


