Abstract
Background
All-cause antibiotic prescribing affects bowel flora antimicrobial susceptibility, and may increase risk of urinary autoinoculation with antibiotic-resistant microbes. However, little is known about relative prevalence of, or risk factors for, antimicrobial resistance among potentially pathogenic microbes thought to be contaminating and infecting urine.
Methods
Secondary analysis of 824 children under 5 years of age consulting in primary care for an acute illness and their Escherichia coli isolates cultured at ≥103 cfu/mL from the Diagnosis of Urinary Tract infection in Young children (DUTY) study. Multivariable logistic regression investigating risk factors for resistance to amoxicillin, co-amoxiclav, cefalexin, ciprofloxacin, trimethoprim, nitrofurantoin and cefpodoxime in microbes meeting the laboratory criteria for urinary tract infection: ‘pathogens’ (>105 cfu/mL, n = 79) and ‘contaminants’ (103 to 105 cfu/mL, n = 745).
Results
Forty-three percent of E. coli were resistant to at least one tested antibiotic, with resistance highest to amoxicillin (49.37% pathogenic versus 37.32% contaminant, P = 0.04), trimethoprim (27.85% versus 16.52%, P = 0.01) and co-amoxiclav (16.46% versus 21.48%, P = 0.30). Multidrug resistance (to ≥3 antibiotic groups) was present in 17.07% of pathogens and 30.13% of contaminants (P = 0.04). No isolates were resistant to nitrofurantoin. Recent (0–3 months) exposure to antibiotics was associated with resistance in both pathogens (aOR: 1.10, 95% CI: 1.01–4.39) and contaminants (1.69, 1.09–2.67).
Conclusions
Prevalence of resistance (including multidrug) was high, but there was no consistent relationship between isolate pathogen/contamination status and resistance. Recent all-cause antibiotic prescribing increased the probability of antimicrobial resistance in both pathogenic and contaminating urinary E. coli in children in primary care.
Introduction
Bacterial resistance to antibiotics is one of the most challenging global health threats faced in modern medicine. It has been estimated that by 2050, 10 million lives per year will be at risk from antibiotic-resistant infections.1 In September 2016, 193 countries agreed to prioritize reducing antimicrobial resistance at the United Nations General Assembly following a worldwide campaign by the UK Government.2 This is particularly important in primary healthcare where over 75% of all healthcare antibiotics are prescribed,3 with our research repeatedly confirming the contribution primary care prescribing makes to antibiotic resistance.4–6
Urinary tract infections (UTIs) are among the most commonly encountered bacterial infections in primary care, and also represent the most common antibiotic-resistant infections in primary care,5,7 leading to repeat consultations and problems for clinicians in selecting appropriate treatments.8,9 Urinary bacteria are thought to originate from the faecal flora and colonize the urinary tract via autoinfection,10 highlighting the similarity between urinary and faecal bacteria, and the potential for transfer of resistant bacteria capable of causing infection. Escherichiacoli is the most commonly isolated bacterium from urine, often present at both lower and higher growth counts; therefore, it is reasonable to assume that bacteria commonly contaminating the urinary tract in healthy asymptomatic individuals are representative of the patients’ faecal flora and are the same organisms associated with UTI.
As key transmitters of infections within communities, children could also be both important recipients and onward transmitters of antimicrobial-resistant microbes and infections. We explored risk factors for carriage of antibiotic resistance in pre-school children’s pathogenic and contaminant urinary E. coli, using data previously collected from the Diagnosis of Urinary Tract infection in Young children (DUTY) study.11,12
Patients and methods
Participants and study design
We conducted a secondary analysis of DUTY study children’s urinary E. coli isolates. The DUTY study was a prospective cohort conducted between 2010 and 2013 and developed clinical algorithms to improve the diagnosis of UTIs in young, pre-school children (under 5 years of age).12,13 Study methods have been reported in full,11 but briefly children were eligible if aged under 5 years and presenting to primary care constitutionally unwell with any acute illness, or with acute urinary symptoms. Any child recruited to DUTY whose urine culture yielded a pure or mixed E. coli isolate at ≥103 cfu/mL was eligible for inclusion in our study. The laboratory UTI diagnostic criteria used in DUTY were pure or predominant growth (where urine contains more than one organism and growth of the second organism is 3 log10 lower than the predominant organism) of a single uropathogen at ≥105 cfu/mL;11 therefore, our cohort included both isolates which did meet and isolates which did not meet the diagnostic criteria for a UTI, and are referred to throughout as pathogens and contaminants, respectively. Contaminant E. coli does not necessarily imply that these isolates are not capable of causing UTI, but simply that they were present at low-growth counts which did not meet the UTI diagnostic criteria used in our cohort.
Ethics
Approval by amendment to original DUTY study (Ref: 09/H0102/64), approval on 26 February 2014 by NRES Committee South West - Central Bristol.
Urine collection and antimicrobial susceptibility testing
Urine samples were collected from children upon recruitment to the DUTY study at participating primary care practices, emergency departments or walk-in centres. The preferred method of urine collection was clean-catch; however, for those children still in nappies, nappy pads or bags were used.14
All culture and antimicrobial susceptibility testing was carried out at the Specialist Antimicrobial Chemotherapy Unit (SACU), Public Health Wales Microbiology Laboratory at Cardiff University Hospital, using standard methods.14 MICs were determined using agar dilution methods,15 against seven antibiotics commonly tested against urinary bacteria: amoxicillin, co-amoxiclav (Augmentin), cefalexin, ciprofloxacin, trimethoprim, nitrofurantoin and cefpodoxime. Cefpodoxime resistance can indicate the production of extended-spectrum β-lactamase enzymes within bacteria.16 Breakpoints were interpreted using EUCAST guidelines.17
Risk factors for bacterial resistance to antibiotics
Risk factor information was collected at DUTY recruitment using case report forms (CRFs) completed by parents. Risk factors to be investigated were identified a priori based on a consensus approach, and based on whether the data had been collected on the CRFs. The risk factor information we collected from each child’s CRF included primary care practice, age in months, gender, ethnicity, postcode, socioeconomic status and method of urine collection (clean-catch versus other). Socioeconomic status was reported according to the methods proposed by Payne and Abel in 2012, allowing Index of Multiple Deprivation (IMD) scores to be generated UK-wide.18
Previous exposure to antibiotics
In addition to the risk factor information available from each child’s DUTY CRF, we also collected risk factor information directly from the child’s primary care practice electronic clinical records relating to their previous antibiotic prescriptions in the 12 months prior to their DUTY recruitment date. Data collection took place between July 2014 and June 2015. This included a list of all antibiotics prescribed to the child in the 12 months prior to DUTY recruitment (or since birth if the child was under 1 year of age at the time of recruitment), and included specific details on: type of antibiotic prescribed, date of prescription, suspension, daily dose and total volume prescribed. We explored: (i) the relationship between any oral antibiotic prescribed in the 12 months prior to urine sampling and resistance to any antibiotic; (ii) the total number of oral antibiotic courses prescribed and resistance to any antibiotic; and (iii) the time of oral antibiotic prescriptions prior to urine sampling (0–3 months, 3–6 months, 6–9 months and 9–12 months) and resistance to any antibiotic.
Sample size
Our previous global systematic review investigating antibiotic resistance in UTI E. coli isolates indicated that in the ‘susceptible’ patient group 30% had prior exposure to antibiotics;5 hence, we used this as an assumption of exposure in the ‘susceptible’ group. Based on this systematic review an assumption of an absolute difference in exposure of 20% was deemed feasible, i.e. 0.1 or 0.5, corresponding to an OR of 0.26 or 2.34. Using these figures and assumptions, the study would yield at least 80% power to detect a minimum important difference of 20% at the 5% two-sided α level.
Data analysis
The primary outcome of interest was resistance to antibiotics, reported as a dichotomous (susceptible/resistant) outcome. The primary outcome measure for our risk factor analysis was an E. coli urinary isolate resistant to at least one antibiotic, versus E. coli susceptible to all antibiotics, for both pathogenic and contaminant E. coli. Multidrug resistance (MDR) was also reported, defined as non-susceptibility to at least one agent in three or more antimicrobial categories.19
Children’s demographic characteristics were compared with the DUTY study reference standard results to check the generalizability of the study findings. Previously, the children whose urine samples made up the DUTY reference standard culture results were compared with the UK 2011 Census data and found to be generalizable for children under 5 years of age in terms of gender and ethnicity.14
Statistical analysis
All patient characteristics were summarized descriptively, including all risk factors for resistance to ≥1 antibiotic. Age in months was reported as a continuous variable; data were transformed if skewed. All descriptive statistics were reported separately for total E. coli isolates, and for pathogenic and contaminant urinary E. coli. A univariate analysis was conducted to determine association between resistance (to ≥1 reported antibiotic versus susceptible to all reported antibiotics) and each risk factor investigated in pathogens and contaminants.
Multivariable logistic regression analysis was conducted to assess differences in those children with the investigated risk factor and those without. Separate regression models were developed for both pathogenic and contaminant urinary E. coli isolates and reported as such. Outcomes were expressed as an odds ratio comparing risk of carriage of a resistant E. coli in those children with and without each investigated risk factor. Both crude and adjusted regression models were produced and reported, the latter adjusting for risk factors identified a priori as described earlier, in order to reduce confounding.
Results
Participants and urinary isolates
Of the 5017 reference standard culture results available from the SACU at Cardiff University Hospital, 858 children had E. coli cultured from their urine samples at ≥103 cfu/mL as either a pure (one bacterial species grown) or mixed (multiple bacterial species grown) culture. Only one isolate per child was collected and included in the analysis. This was made up of 79 E. coli isolates which met the laboratory diagnostic criteria for a UTI of pure or predominant growth at >105 cfu/mL (pathogens); and 779 E. coli isolates cultured at 103–105 cfu/mL as either a mixed or pure culture (contaminants).
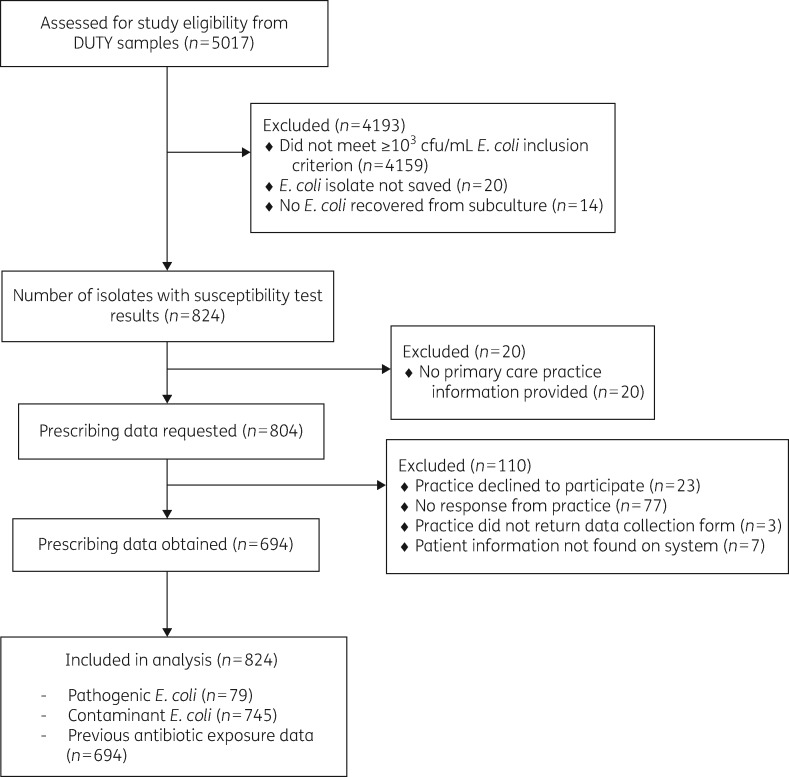
Of the 858 E. coli urinary isolates cultured at ≥103cfu/mL which either did (n = 79) or did not (n = 779) meet the diagnostic criteria for a UTI (Figure 1); 34 isolates (4.13%) did not have antimicrobial susceptibility results available. This left 824 isolates for the prevalence analysis and since we were unable to collect previous antibiotic prescription information for 110 children, 694 for the analysis of the role of previous antibiotic prescribing.
Figure 1.
Study inclusion flow chart.
Table 1 summarizes children’s characteristics for all included variables. For the 353 children whose urinary E. coli was resistant to at least one antibiotic, 75.45% of the cohort was recruited in England, 67.42% were female, 84.70% were white, and the mean age was 22 months. Deprivation was evenly distributed across all five rankings. Missing data for all variables other than previous antibiotic prescriptions was very low (2.00%); for previous antibiotic prescriptions, data could not be obtained for 15.82% of the 824 children. Pathogens were more frequently isolated from clean-catch urine specimens, whereas contaminants were more frequently isolated from either nappy pad or bag collection methods. This has been previously investigated and discussed elsewhere.13
Table 1.
Study characteristics for children with urinary E. coli resistant to at least one antibiotic
| Variable | All E. coli isolates (n = 353) | Pathogensa (n = 41) | Contaminantsb (n = 312) | P valuec |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, months (SD) | 22.17 (14.91) | 34.32 (15.38) | 20.58 (14.12) | <0.001 |
| Gender | ||||
| male | 32.58 | 12.20 | 35.26 | 0.003 |
| female | 67.42 | 87.80 | 64.74 | 0.003 |
| Ethnicity | ||||
| white | 84.70 | 85.37 | 84.62 | |
| non-white | 13.31 | 14.63 | 13.14 | |
| not given | 1.99 | 0 | 2.24 | 0.612 |
| Deprivation | ||||
| 1 (least deprived) | 21.25 | 17.07 | 21.79 | |
| 2 | 19.26 | 19.51 | 19.23 | |
| 3 | 19.83 | 29.27 | 18.59 | |
| 4 | 16.43 | 19.51 | 16.03 | |
| 5 (most deprived) | 23.23 | 14.64 | 24.36 | 0.378 |
| Urine culture and previous antibiotic exposure | ||||
| Method of urine collection | ||||
| clean-catch | 34.66 | 70.73 | 29.59 | |
| otherd | 65.01 | 29.27 | 70.19 | |
| not reported | 0.33 | 0 | 0.22 | <0.001 |
| Any antibiotic prescription in 12 months prior to urine sample?e | ||||
| yes | 39.66 | 43.90 | 39.10 | |
| no | 43.91 | 43.90 | 39.10 | |
| missing | 16.43 | 43.90 | 43.91 | 0.744 |
| Number of antibiotic courses prescribed in 12 months prior to urine sample?e | ||||
| 0 | 52.50 | 50.01 | 52.88 | |
| 1 | 25.78 | 25.03 | 25.96 | |
| 2 | 12.46 | 13.86 | 12.40 | |
| 3 | 3.43 | 2.78 | 3.53 | |
| ≥4 | 5.83 | 8.32 | 5.23 | |
| missing | 16.40 | 12.22 | 17.04 | 0.959 |
| Time of antibiotic prescription (prior to sampling)e,f | ||||
| never prescribed | 52.51 | 50.00 | 52.91 | |
| 0–3 months | 18.74 | 17.12 | 18.87 | |
| 3–6 months | 14.42 | 12.19 | 14.72 | |
| 6–9 months | 11.88 | 14.63 | 11.51 | |
| 9–12 months | 12.25 | 26.84 | 10.36 | |
| missing | 16.43 | 12.20 | 17.02 | 0.282 |
Values are percentages unless otherwise indicated.
Includes all E. coli isolates cultured at ≥105 cfu/mL, as either pure or the predominant growth (where urine contains more than one organism and growth of the second organism is 3 log10 lower than the predominant organism).
Includes all E. coli isolates cultured between 103 and 105 cfu/mL as pure or mixed growth.
All P values reported from univariate analyses or t-test (for age) comparing pathogens and contaminants; missing data were not included.
Includes nappy pad (n = 221) and bag (n = 9) urine collection.
Total number of children with previous antibiotic prescription data collected was 694.
Data may not add up to 100%, as children prescribed multiple antibiotic courses within different time periods will appear more than once, thus this represents the proportion of the total number of courses of antibiotics prescribed within each time period prior to urine sampling.
Generalizability of study participants
To assess generalizability, we compared our cohort to the original DUTY population for whom reference standard urine results were available (n = 5107); these data were representative of the 2011 UK census for children under 5 years.11 Our study had an over-representation of younger age groups and of females (see Figure S1, available as Supplementary data at JAC Online).
Resistance of urinary E. coli to antibiotics
Table 2 shows the prevalence of resistance to antibiotics in children’s urinary E. coli isolates. Resistance to amoxicillin was observed most frequently (in 49.37% of pathogens and 37.32% of contaminants). Trimethoprim resistance was higher in pathogens at 27.85%, with co-amoxiclav resistance higher in contaminants at 21.48%. No isolates were resistant to nitrofurantoin. No pathogens were cefpodoxime resistant.
Table 2.
Prevalence of urinary E. colia resistant to antibiotics in pathogens and contaminants
| Antibiotic | Pathogens (%) (n = 79)a | Contaminants (%) (n = 745)b | P valuec |
|---|---|---|---|
| Amoxicillin | 49.37 | 37.32 | 0.04 |
| Trimethoprim | 27.85 | 16.52 | 0.01 |
| Co-amoxiclav | 16.46 | 21.48 | 0.30 |
| Ciprofloxacin | 3.80 | 3.62 | 0.84 |
| Cefalexin | 1.27 | 4.03 | 0.22 |
| Cefpodoxime | 0 | 4.83 | 0.05 |
| Nitrofurantoin | 0 | 0 | – |
Includes all E. coli isolates cultured at ≥105 cfu/mL, as either pure or the predominant growth (where urine contains more than one organism and growth of the second organism is 3 log10 lower than the predominant organism).
Includes all E. coli isolates cultured between 103 and 105 cfu/mL as pure or mixed growth.
P values obtained from χ2 analysis.
MDR
Of the 353 urinary E. coli resistant to at least one antibiotic, made up of 41 pathogens and 312 contaminants, 28.05% (n = 99) were MDR. The proportion of MDR in pathogens was 17.07%, compared with 30.13% in contaminants (P value 0.04).
Previous exposure to antibiotics
Previous antibiotic prescribing information was collected for 694 children; 303 children (43.66%) had received at least one antibiotic during the 12 month time period (see Table 3). Prescribing data for 130 children could not be collected, either due to lack of engagement from primary care practices or missing information on the child’s DUTY CRF. There were no key differences between children whose prescribing data were and were not collected. Amoxicillin was the most common antibiotic prescribed in our cohort for the 12 months prior to urine sampling.
Table 3.
Number and type of antibiotics prescribed to study children in the 12 months prior to urine sampling, grouped by pathogens and contaminants
| Antibiotic | Number of children who received ≥1 antibiotic course during previous 12 months | Proportion of children who received ≥1 antibiotic course during previous 12 monthsa | Total number of prescriptions during previous 12 months in study cohort | Proportion of total prescriptions in study cohort during previous 12 monthsb |
|---|---|---|---|---|
| Pathogensc(prescribed ≥1 antibiotic = 27; total prescriptions = 62) | ||||
| Oral antibiotics | ||||
| amoxicillin | 12 | 44.44 | 24 | 38.71 |
| flucloxacillin | 6 | 22.22 | 6 | 9.68 |
| trimethoprim | 5 | 18.52 | 13 | 20.97 |
| erythromycin | 4 | 14.81 | 7 | 11.29 |
| co-amoxiclav | 3 | 11.11 | 4 | 6.45 |
| penicillin | 2 | 7.41 | 2 | 3.23 |
| cefalexin | 1 | 3.70 | 1 | 1.61 |
| clarithromycin | 0 | 0 | 0 | 0 |
| azithromycin | 0 | 0 | 0 | 0 |
| cefixime | 0 | 0 | 0 | 0 |
| metronidazole | 0 | 0 | 0 | 0 |
| nitrofurantoin | 0 | 0 | 0 | 0 |
| cefaclor | 0 | 0 | 0 | 0 |
| cefuroxime | 0 | 0 | 0 | 0 |
| No antibiotics | ||||
| unexposed | 35 | – | – | – |
| Unknown | ||||
| missing data | 17 | – | – | – |
| Contaminantsd(prescribed ≥1 antibiotic = 276; total prescriptions = 568) | ||||
| Oral antibiotics | ||||
| amoxicillin | 184 | 66.66 | 284 | 50.00 |
| flucloxacillin | 25 | 9.06 | 29 | 5.11 |
| trimethoprim | 18 | 6.52 | 23 | 4.05 |
| erythromycin | 30 | 10.87 | 32 | 5.63 |
| co-amoxiclav | 20 | 7.25 | 25 | 4.40 |
| penicillin | 26 | 9.42 | 36 | 6.34 |
| cefalexin | 11 | 3.99 | 14 | 2.46 |
| clarithromycin | 8 | 2.90 | 9 | 1.58 |
| azithromycin | 2 | 0.72 | 2 | 0.35 |
| cefixime | 2 | 0.72 | 2 | 0.35 |
| metronidazole | 1 | 0.36 | 3 | 0.53 |
| nitrofurantoin | 1 | 0.36 | 2 | 0.35 |
| cefaclor | 1 | 0.36 | 1 | 0.18 |
| cefuroxime | 1 | 0.36 | 1 | 0.18 |
| No antibiotics | ||||
| unexposed | 356 | – | – | – |
| Unknown | ||||
| missing data | 113 | – | – | – |
Proportion of the total number of children (and their corresponding E. coli isolates) from either the pathogen or contaminant group where antibiotic prescription information was collected and at least one antibiotic was prescribed in the 12 months prior to urine sampling.
Grouped by pathogens and contaminants: total number of prescriptions in the pathogen group was 62; total number of prescriptions in the contaminants group was 568.
Includes all E. coli isolates cultured at ≥105 cfu/mL, as either pure or predominant growth (where urine contains more than one organism and growth of the second organism is 3 log10 lower than the predominant organism).
Includes all E. coli isolates cultured between 103 and 105 cfu/mL as pure or mixed growth.
Of those children for whom previous antibiotic prescription information was known, 56.34% had not been prescribed any antibiotics within the 12 months prior to urine sampling. The proportion of children previously exposed to antibiotics in the ‘susceptible’ group was 54.25%, versus 45.75% previously exposed to antibiotics in the ‘resistant’ group.
Risk factors for acquisition of resistant urinary E. coli
Of all the patient demographic variables considered, there was a suggestion that older children may be more likely to carry a resistant pathogenic E. coli compared with younger children [adjusted OR (aOR): 1.85, 95% CI: 1.02–3.53, P value 0.04]. We also found that children living in the most deprived areas were more likely to carry resistant contaminant E. coli, compared with children living in the least deprived areas (aOR: 1.80, 95% CI: 1.12–2.89, P value 0.01). All crude and adjusted results for pathogens and contaminants are presented in Table 4.
Table 4.
Crude and adjusted odds ratios for investigated risk factors associated with urinary E. coli resistance to any reported antibiotic, by UTI diagnostic status
| OR (95% CI) |
||||||
|---|---|---|---|---|---|---|
| pathogensa (n = 79) |
contaminantsb (n = 745) |
|||||
| Variable | crude | adjusted | P value | crude | adjusted | P valuec |
| Demographics | ||||||
| Mean age, monthsd | 1.33 (1.07–1.77) | 1.85 (1.02–3.53) | 0.04 | 1.06 (0.97–1.15) | 1.08 (0.94–1.24) | 0.16 |
| Gender | ||||||
| female | 2.23 (0.67–7.40) | 2.42 (0.46–12.84) | 0.30 | 0.88 (0.65–1.19) | 0.86 (0.60–1.23) | 0.40 |
| male | REF | REF | REF | REF | ||
| Ethnicity | ||||||
| white | 1.02 (0.88–1.14) | 1.07 (0.91–1.11) | 0.11 | 1.03 (1.01–1.16) | 0.98 (0.90–1.17) | 0.15 |
| non-white | REF | REF | REF | REF | ||
| Deprivation | ||||||
| 1 (least deprived) | REF | REF | REF | REF | ||
| 2 | 0.71 (0.16–3.23) | 0.55 (0.08–3.65) | 0.54 | 1.22 (0.77–1.91) | 1.40 (0.85–2.29) | 0.18 |
| 3 | 0.86 (0.21–3.55) | 1.19 (0.17–8.11) | 0.86 | 1.01 (0.64–1.56) | 0.98 (0.61–1.61) | 0.86 |
| 4 | 0.57 (0.13–2.50) | 0.25 (0.04–1.78) | 0.16 | 1.05 (0.66–1.68) | 0.86 (0.50–1.49) | 0.59 |
| 5 (most deprived) | 0.86 (0.16–4.47) | 0.81 (0.10–6.75) | 0.85 | 1.60 (1.04–2.49) | 1.80 (1.12–2.89) | 0.01 |
| Urine collection and previous antibiotic exposure | ||||||
| Method of urine collection | ||||||
| clean-catch | 1.65 (0.64–4.22) | 1.47 (0.07–3.33) | 0.45 | 1.06 (0.77–1.46) | 0.85 (0.52–1.38) | 0.52 |
| other | REF | REF | REF | REF | ||
| Any antibiotic prescription in 12 months prior to urine sampling? | ||||||
| yes | 1.89 (0.67–5.34) | 1.42 (0.43–4.71) | 0.56 | 1.27 (0.92–1.74) | 1.20 (0.85–1.68) | 0.29 |
| no | REF | REF | REF | REF | ||
| Number of antibiotic courses prescribed in 12 months prior to urine sampling | ||||||
| 0 | REF | REF | REF | REF | ||
| 1 | 2.83 (0.65–12.26) | 3.37 (0.55–20.57) | 0.19 | 1.36 (0.92–2.01) | 1.29 (0.86–1.94) | 0.21 |
| 2 | 1.57 (0.33–7.62) | 0.61 (0.10–3.61) | 0.59 | 1.42 (0.84–2.39) | 1.30 (0.76–2.23) | 0.34 |
| 3 | 0.47 (0.04–5.67) | 0.76 (0.04–13.17) | 0.85 | 0.96 (0.41–2.25) | 0.94 (0.39–2.26) | 0.89 |
| ≥4 | 2.83 (0.27–29.96) | 2.20 (0.12–39.98) | 0.59 | 0.93 (0.47–1.86) | 0.86 (0.43–1.77) | 0.70 |
| Time of antibiotic prescription (prior to urine sampling) | ||||||
| never prescribed | REF | REF | REF | REF | ||
| 0–3 months | 1.06 (1.01–5.69) | 1.10 (1.01–4.39) | 0.04 | 1.55 (1.09–2.62) | 1.69 (1.09–2.67) | 0.01 |
| 3–6 months | 0.47 (0.04–6.53) | 0.24 (0.03–5.66) | 0.38 | 1.60 (0.92–2.79) | 1.53 (0.91–2.71) | 0.14 |
| 6–9 months | 0.45 (0.04–6.82) | 1.03 (0.34–31.24) | 0.89 | 1.39 (0.80–2.43) | 1.25 (0.70–2.23) | 0.46 |
| 9–12 months | 2.08 (0.60–7.24) | 1.10 (0.24–4.35) | 0.75 | 0.88 (0.54–1.40) | 0.75 (0.45–1.26) | 0.28 |
REF, reference group.
Includes all E. coli isolates cultured at ≥105 cfu/mL, as either a pure or predominant growth (where urine contains more than one organism and growth of the second organism is 3 log10 lower than the predominant organism).
Includes all E. coli isolates cultured between 103 and 105 cfu/mL as pure or mixed growth.
P values reported from adjusted regression model.
From young to old, i.e. an OR >1 indicates older children at increased risk; an OR <1 indicates older children at reduced risk.
Regarding previous exposure to antibiotics, there was an association between recent oral antibiotic exposure (within 0–3 months) and resistance in both pathogenic (aOR: 1.10, 95% CI: 1.01–4.39, P value 0.04) and contaminant (aOR: 1.69, 95% CI: 1.09–2.67, P value 0.01) E. coli urinary isolates.
Amoxicillin prescribing and resistance
Of all the antibiotics prescribed to children in our cohort, amoxicillin was the most frequent, with 308 prescriptions given to 195 children within the 12 months prior to their recruitment to the DUTY study. There was an association between being prescribed amoxicillin in the 3 months prior to urine sampling and resistance to amoxicillin in contaminant E. coli (adjusted OR: 1.23 CI: 1.01–2.84, P value 0.04); no association was found for pathogenic E. coli.
Discussion
Prevalence of single and MDR in pathogenic and contaminant urinary E. coli was high against several commonly prescribed primary care antibiotics, namely amoxicillin, co-amoxiclav and trimethoprim, and lower for less commonly prescribed antibiotics including ciprofloxacin and cefalexin. No isolates were resistant to nitrofurantoin. An association was observed between recent (0–3 months) exposure to primary care antibiotics and resistance in both pathogenic and contaminant E. coli cultured from children’s urine.
Strengths and limitations
To our knowledge, this is the first study to explore prevalence of, and risk factors for, resistance in urinary E. coli from children with acute illness, irrespective of UTI diagnosis. All urine samples were obtained from incident cases which was often an unknown in previous studies. The inclusion of contaminant E. coli isolates allows for the investigation into carriage of resistance in children’s urinary bacteria, as they are likely very similar to opportunistic pathogenic E. coli isolates which met the UTI diagnostic criteria, and to E. coli residing in the faecal flora, an important reservoir for the transfer of resistance genes. This study therefore contributes to one of the overarching aims of the UK Five Year Antimicrobial Resistance Strategy—to improve the knowledge and understanding of antimicrobial resistance.20
This study also has some limitations. Firstly, estimates used to produce a sample size for this cohort were inaccurate with a difference in exposure of 8% rather than 20% reported in previous studies.5 Our sample size was based on our previous systematic review exploring the global prevalence of resistance and associations with previous antibiotic prescribing in children;5 and similar to those reported in adults previously; therefore, our study was underpowered given the small difference in exposure we observed.4,21 Secondly, our cohort included only 79 UTI-positive E. coli isolates compared with 745 contaminant isolates. However, all organisms likely originate from the children’s own faecal flora.10 This made it difficult to explore the likelihood of developing a UTI if their E. coli were resistant to at least one antibiotic. However, the main objective of our study was to explore risk factors for carriage of E. coli within the urinary tract in an otherwise healthy population of children; therefore, inclusion of contaminant urinary bacteria was important.
Results in the context of existing research
Prevalence of resistance in urinary E. coli
Prevalence of MDR in urinary E. coli was higher in contaminants than in pathogens. Whilst the reasons for this are largely unknown, as we have little previous data to compare our findings to, this may be a result of circulating MDR E. coli clonal types. This was previously reported in the USA, where a single MDR clonal type was responsible for almost half of all community-acquired UTIs in three geographically diverse communities.22,23
Another key finding was the pan-susceptibility to nitrofurantoin in all E. coli isolates tested. Previous research has similarly suggested that resistance to nitrofurantoin in children’s E. coli is low;5,24 therefore, our findings provide additional evidence to support the continued availability of nitrofurantoin as an effective first-line treatment choice for uncomplicated UTI. Recent NICE guidance now recommends nitrofurantoin be used over trimethoprim as a first-line treatment for uncomplicated UTIs in adults,25 but the same recommendation has not yet been made for children; likely due to the limited resistance data which is made available specifically for children in the UK.
Risk factors for acquisition of resistant pathogenic and contaminant urinary E. coli
Previous exposure to antibiotics
There was an association between the child being prescribed an antibiotic in the 3 months prior to urine sampling and carriage of bacterial resistance, evident in both pathogenic and contaminant E. coli isolates. This association was also observed for amoxicillin prescribing and amoxicillin-resistant pathogenic E. coli. These findings were comparable to our previous systematic reviews suggesting stronger associations with more recent antibiotic exposure and resistance in children’s E. coli UTI and faecal isolates.5,6 Another previous study also found a relationship between exposure to antibiotics and resistance in common community-acquired infections including UTI, where resistance was also associated with poorer clinical outcomes for patients.26 We were unable to compare our data directly to previous studies, as the majority of current research focuses on pathogenic bacteria only.
Policy, clinical and research implications
The findings in this study suggest high-level resistance to some of the most commonly prescribed antibiotics in primary care for children. Overall prevalence of resistance was highest against amoxicillin, trimethoprim and co-amoxiclav in both pathogenic and contaminant E. coli, all of which are recommended first-line treatments for UTI in children. In line with recommendations from infectious disease societies across the USA and Europe of a 20% resistance threshold for first-line UTI treatment,27 it may be that these antibiotics, particularly amoxicillin, should no longer be recommended for use as a first-line treatment for UTI in the UK. The interplay between organisms that cause infections and those which simply colonize is poorly investigated, but becoming of increasing interest due to rising antimicrobial resistance rates. It is important to better understand whether isolates gain resistance when present as an infecting organism, under antimicrobial challenge, or before infection starts when it is present as a colonizer. It may be that colonizing antimicrobial-resistant bacteria act as a reservoir for future hard-to-treat, antimicrobial-resistant infections.
We found evidence of an association between recent antibiotic prescribing and resistance in this cohort of children. Frequent challenge with antibiotics can disrupt normal balance of the microbial flora within the urinary and gastrointestinal tract, which can lead to increased risk of bacterial overgrowth and infection.28 Clinicians should consider the impact and necessity of further antibiotic treatment before prescribing.
Further research should focus on more than just pathogenic bacteria, but also on understanding the connection between resistance in pathogenic and non-pathogenic bacteria. If we were able to predict resistance rates from both pathogenic and non-pathogenic bacteria (which are also capable of causing infection) and update prescribing guidelines to reflect this, the cycle of primary care antibiotic prescribing and development of bacterial resistance as a result could potentially be interrupted.
Conclusions
Prevalence of resistance in children’s pathogenic and contaminant urinary E. coli was high for several commonly prescribed primary care antibiotics, namely amoxicillin, co-amoxiclav and trimethoprim; no isolates were resistant to nitrofurantoin. High-level resistance could render such antibiotics ineffective as first-line treatments, and as a result require prescribing guidelines to be updated to reflect local resistance patterns.
Antibiotic prescribing within 3 months prior to urine sampling in children consulting to primary care with an acute illness was associated with antimicrobial resistant E. coli in both pathogenic and contaminant isolates. Recent antibiotic exposure exerts selective pressure on both pathogenic and non-pathogenic bacteria which can alter the gut flora and subsequently the urinary tract. This can create an environment where resistant bacteria are able to thrive and persist. Future research must prioritize understanding resistance in non-pathogenic bacteria, which could allow prescribing guidelines to be updated before it affected patient therapeutic outcomes.
Supplementary Material
Acknowledgements
Jennifer Richards (Medical Technical Officer at SACU, University Hospital of Wales, Cardiff) assisted with antimicrobial susceptibility testing of all isolates.
Funding
A. B. was supported to conduct this study through a doctoral fellowship from the National Institute for Health Research School for Primary Care Research (NIHR-SPCR). C. C. is supported by a NIHR Career Development fellowship (CDF-2016-09-015). A. D. H. is supported by a NIHR Research Professorship (NIHR RP-R2-12-012). The original DUTY study was funded by the NIHR Health Technology Assessment programme. The funder had no role in: the study design; data collection, data analysis and interpretation; in the writing of the report; or in the decision to submit the article for publication.
Transparency declarations
None to declare.
Author contributions
A. D. H. and C. C. conceived and secured funding for the study. A. D. H. and C. C. B. conceived the original DUTY study and acted as Principal Investigators. A. B., A. D. H., C. C. B. and C. C. refined the research questions and developed a statistical analysis plan. A. B. collected data from primary care practices. A. B. performed all statistical analyses. M. W. was responsible for all laboratory testing and made substantial contributions to the overall presentation and interpretation of results. A. B., C. C. and A. D. H. drafted first sections of the text. All authors contributed to, reviewed and approved the final draft. All authors received access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. A. B. is guarantor for the study. A. B. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any departures from the study as planned have been explained.
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, the NHS or the Department of Health.
Supplementary data
Figure S1 appears as Supplementary data at JAC Online.
References
- 1. O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations http://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
- 2. UK Department of Health. UK Secures Historic UN Declaration on Antimicrobial Resistance https://www.gov.uk/government/news/uk-secures-historic-un-declaration-on-antimicrobial-resistance.
- 3. Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) 2010–2014 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/477962/ESPAUR_Report_2015.pdf.
- 4. Costelloe C, Metcalfe C, Lovering A. et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340: C2096.. [DOI] [PubMed] [Google Scholar]
- 5. Bryce A, Hay AD, Lane IF. et al. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ 2016; 352: i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryce A, Costelloe C, Hawcroft C. et al. Faecal carriage of antibiotic resistant Escherichia coli in asymptomatic children and associations with primary care antibiotic prescribing: a systematic review and meta-analysis. BMC Infect Dis 2016; 16: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chin TL, MacGowan AP, Bowker KE. et al. Prevalence of antibiotic resistance in Escherichia coli isolated from urine samples routinely referred by general practitioners in a large urban centre in South-west England. J Antimicrob Chemother 2015; 70: 2167–9. [DOI] [PubMed] [Google Scholar]
- 8. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon 2003; 49: 53–70. [DOI] [PubMed] [Google Scholar]
- 9. Petersen I, Hayward AC.. Antibacterial prescribing in primary care. J Antimicrob Chemother 2007; 60: i43–7. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto S, Tsukamoto T, Terai A. et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol 1997; 157: 1127–9. [PubMed] [Google Scholar]
- 11. Hay AD, Birnie K, Busby J. et al. The Diagnosis of Urinary Tract infection in Young children (DUTY): a diagnostic prospective observational study to derive and validate a clinical algorithm for the diagnosis of urinary tract infection in children presenting to primary care with an acute illness. Health Technol Assess 2016; 20: 1–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hay AD, Sterne JA, Hood K. et al. Improving the diagnosis and treatment of urinary tract infection in young children in primary care: results from the DUTY prospective diagnostic cohort study. Ann Fam Med 2016; 14: 325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butler CC, Sterne JA, Lawton M. et al. Nappy pad urine samples for investigation and treatment of UTI in young children: the ‘DUTY’ prospective diagnostic cohort study. Br J Gen Pract 2016; 66: e516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Downing H, Thomas-Jones E, Gal M. et al. The Diagnosis of Urinary Tract infection in Young children (DUTY): protocol for a diagnostic and prospective observational study to derive and validate a clinical algorithm for the diagnosis of UTI in children presenting to primary care with an acute illness. BMC Infect Dis 2012; 12: 158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 2001; 48: 5–16. [DOI] [PubMed] [Google Scholar]
- 16. Rawat D, Nair D.. Extended-spectrum β-lactamases in Gram-negative bacteria. J Glob Infect Dis 2010; 2: 263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. EUCAST. EUCAST: Clinical Breakpoints 2015 http://www.eucast.org/clinical_breakpoints/.
- 18. Payne RA, Abel GA.. UK indices of multiple deprivation—a way to make comparisons across constituent countries easier. Health Stat Q 2012; 53: 1–16. [Google Scholar]
- 19. Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant and pandrug resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2011; 18: 268–81. [DOI] [PubMed] [Google Scholar]
- 20. Davies SC, Gibbens N.. UK 5 Year Antimicrobial Resistance Strategy 2013–2018 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf.
- 21. Hay AD, Thomas M, Montgomery A. et al. The relationship between primary care antibiotic prescribing and bacterial resistance in adults in the community: a controlled observational study using individual patient data. J Antimicrob Chemother 2005; 56: 146–53. [DOI] [PubMed] [Google Scholar]
- 22. Manges AR, Johnson JR, Foxman B. et al. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med 2001; 345: 1007–13. [DOI] [PubMed] [Google Scholar]
- 23. Smith SP, Manges AR, Riley LW.. Temporal changes in the prevalence of community-acquired antimicrobial-resistant urinary tract infection affected by Escherichia coli clonal group composition. Clin Infect Dis 2008; 46: 689–95. [DOI] [PubMed] [Google Scholar]
- 24. Sanchez GV, Baird AM, Karlowsky JA. et al. Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother 2014; 69: 3259–62. [DOI] [PubMed] [Google Scholar]
- 25. National Institute for Health and Care Excellence. Three-Day Courses of Antibiotics for Uncomplicated Urinary Tract Infection https://www.nice.org.uk/advice/ktt10/chapter/evidence-context.
- 26. Van Hecke O, Wang K, Lee JJ. et al. The implications of antibiotic resistance for patients’ recovery from common infections in the community: a systematic review and meta-analysis. Clin Infect Dis 2017; 65: 371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta K, Hooton TM, Naber KG. et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in woman: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 28. Willing BP, Russell SL, Finlay BB.. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 2011; 9: 233–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.