Abstract
Background
Vorinostat, a histone deacetylase (HDAC) inhibitor, has shown radiosensitizing properties in preclinical studies. This open-label, single-arm trial evaluated the maximum tolerated dose (MTD; phase I) and efficacy (phase II) of vorinostat combined with standard chemoradiation in newly diagnosed glioblastoma.
Methods
Patients received oral vorinostat (300 or 400 mg/day) on days 1–5 weekly during temozolomide chemoradiation. Following a 4- to 6-week rest, patients received up to 12 cycles of standard adjuvant temozolomide and vorinostat (400 mg/day) on days 1–7 and 15–21 of each 28-day cycle. Association between vorinostat response signatures and progression-free survival (PFS) and overall survival (OS) was assessed based on RNA sequencing of baseline tumor tissue.
Results
Phase I and phase II enrolled 15 and 107 patients, respectively. The combination therapy MTD was vorinostat 300 mg/day and temozolomide 75 mg/m2/day. Dose-limiting toxicities were grade 4 neutropenia and thrombocytopenia and grade 3 aspartate aminotransferase elevation, hyperglycemia, fatigue, and wound dehiscence. The primary efficacy endpoint in the phase II cohort, OS rate at 15 months, was 55.1% (median OS 16.1 mo), and consequently, the study did not meet its efficacy objective. Most common treatment-related grade 3/4 toxicities in the phase II component were lymphopenia (32.7%), thrombocytopenia (28.0%), and neutropenia (21.5%). RNA expression profiling of baseline tumors (N = 76) demonstrated that vorinostat resistance (sig-79) and sensitivity (sig-139) signatures had a reverse and positive association with OS/PFS, respectively.
Conclusions
Vorinostat combined with standard chemoradiation had acceptable tolerability in newly diagnosed glioblastoma. Although the primary efficacy endpoint was not met, vorinostat sensitivity and resistance signatures could facilitate patient selection in future trials.
Keywords: glioblastoma, histone deacetylase inhibitors, phase I/II trial, temozolomide, vorinostat
Importance of the study
There is an urgent need for novel therapeutic approaches to improve outcomes for patients with glioblastoma. This trial evaluated the MTD and efficacy of vorinostat, an HDAC inhibitor with radiosensitizing properties, combined with standard chemoradiation, in patients with newly diagnosed glioblastoma. Vorinostat in combination with temozolomide and radiotherapy had acceptable tolerability, but the study did not meet its primary efficacy endpoint. However, findings based on RNA sequencing data of baseline tumor samples support an association between preclinically defined vorinostat response signatures and PFS and OS. Patients with high scores for the vorinostat resistance signature, sig-79, had shorter PFS and OS; conversely, patients with high scores on the vorinostat sensitivity signature, sig-139, had longer PFS and OS. Prospective validation of these findings could allow selection of glioblastoma patients who may derive benefit from HDAC inhibitors.
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults, with an incidence of approximately 3 per 100000 in the US.1,2 Patients diagnosed with this aggressive malignancy have a very poor prognosis, with recent clinical trials suggesting a median survival time of 15 to 17 months,3–6 despite use of multimodality treatment including surgery and temozolomide (TMZ)-based chemoradiation. Glioblastoma cells show inherent resistance to most cytotoxic drugs, and even active agents such as TMZ and nitrosoureas exhibit limited activity in this disease. The problem of resistance is compounded by the inability of many drugs to cross the blood–brain barrier, translating to limited efficacy.7,8 Consequently, an urgent need exists for novel therapeutic approaches to improve outcomes for patients with GBM. The methylation status of the gene promoter that encodes for the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) in the tumor is an independent prognostic factor for survival in GBM patients.3–5,9 Epigenetic silencing of the MGMT gene by promoter methylation is especially relevant in the context of TMZ therapy, with a greater survival benefit for TMZ plus radiotherapy (RT) observed in patients whose tumors contained a methylated MGMT promoter compared with RT alone.9
Other epigenetic mechanisms, such as acetylation and deacetylation of histones, are important in transcriptional regulation that occurs in eukaryotic cells. Histone deacetylases and histone acetyltransferases determine the acetylation status of histones and nonhistone proteins, thereby regulating transcription activation.10,11 Histone deacetylases (HDACs) act as transcriptional repressors through the deacetylation of nucleosomal histones, which promote chromatin compaction and silencing of various genes, including those implicated in the regulation of cell survival, proliferation, tumor cell differentiation, cell cycle arrest, and apoptosis.12 In addition, HDACs have nonhistone protein substrates, such as hormone receptors, chaperone proteins, and cytoskeleton proteins, that regulate cell proliferation and cell death.13 HDAC inhibitors selectively alter gene transcription in part by chromatin remodeling, thus changing in the structure of transcription factor complexes14 and lead into growth arrest, differentiation, and/or cell death of transformed cells. Altered gene expression and changes in nonhistone proteins caused by HDAC inhibitors play a key role in their antitumor activity. HDAC-induced transformed cell death results from both transcription-dependent and -independent mechanisms.15–18
Vorinostat (suberoylanilide hydroxamic acid) is a small-molecule inhibitor of most human class I and class II HDACs (inhibiting HDAC 1, 2, 3, 6, and 8) and binds directly to the catalytic domain of the enzyme. This allows the hydroxamic moiety to chelate the zinc ion located in the catalytic pockets of HDACs, thereby inhibiting deacetylation, leading to an accumulation of both hyperacetylated histones and transcription factors.19 Vorinostat represents a rational, targeted therapy in the treatment of GBM.20–22 Preclinical evidence shows that vorinostat has antitumor activity against malignant glioma cell lines in vitro and orthotopic glioma xenografts in vivo.20–22 In addition, vorinostat may serve as a potent sensitizer in RT and alkylating chemotherapeutic agents such as TMZ, with the potential to increase their cytotoxic activity23,24 in GBM. In addition, there is preclinical evidence of central nervous system penetration at concentrations that inhibit HDAC activity and cause accumulation of acetylated histones in the brain.25 A single-arm trial of vorinostat in recurrent GBM has shown good tolerability and met its primary efficacy endpoint.26
A North American Brain Tumor Consortium (NABTC) phase I dose-finding and dose-escalation trial of vorinostat in combination with TMZ in the adjuvant setting (ie, following completion of chemoradiation)27 established the maximum tolerated dose (MTD) of vorinostat at 400 mg daily when administered on days 1–7 and 15–21 of every 28-day cycle in combination with TMZ 150 mg/m2/day for 5 days during the first 28-day cycle, followed by TMZ dose escalation to 200 mg/m2/day on days 1–5 in subsequent 28-day cycles as per standard clinical practice. The current phase I/II trial (NCT00731731) was designed by the North Central Cancer Treatment Group (NCCTG; now part of the Alliance for Clinical Trials in Oncology) and the Adult Brain Tumor Consortium (ABTC) to address the hypothesis that addition of vorinostat to standard RT and concomitant and adjuvant TMZ in patients with newly diagnosed GBM and gliosarcoma would result in increased efficacy.
Materials and Methods
Study Design and Treatment
In phase I of this phase I/II study, a standard 3+3 cohort design28 was used to assess the safety of vorinostat in combination with RT and concomitant TMZ and to establish the MTD of the combination for use in the expansion cohort. For cycle 1 of treatment, patients received oral vorinostat 300 mg/day (dose level 0) or 400 mg/day (dose level 1) on days 1–5 weekly for 6 weeks beginning with the first dose of RT (total dose, 60 Gy) and oral TMZ 75 mg/m2/day administered on days 1–42. For both dose levels 0 and 1, following a 4- to 6-week rest period, patients received up to 12 further cycles of standard adjuvant TMZ (150 mg/m2 on days 1–5 in cycle 1 with escalation to 200 mg/m2 on days 1–5 in subsequent cycles, if well tolerated) in combination with 400 mg/day vorinostat on days 1–7 and days 15–21 of each 28-day cycle. Vorinostat dosing in the adjuvant setting was based on NABTC trial 04-03 findings.27 Determination of MTD was based on assessment of dose-limiting toxicities (DLTs) during the first 10 weeks of treatment, and was defined as the dose at which fewer than one-third of patients experienced a DLT to vorinostat. The MTD was defined as the dose level at which 0 of 3 or 1 of 6 patients experienced a DLT with the next higher dose having at least 2 of 3 or 2 of 6 patients who experienced a DLT. A DLT included any study drug–related event of grade 3 or 4 thrombocytopenia, grade 4 anemia, or grade 4 neutropenia lasting >7 days, grade 4 radiation-induced skin changes, or any nonhematologic grade ≥3 adverse event—excluding alopecia and venous thromboembolism—that occurred during treatment with vorinostat and TMZ.
In the phase II of the study, patients received oral vorinostat 300 mg/day with concomitant RT (total dose, 60 Gy) on days 1–5 weekly for 6 weeks in combination with oral TMZ 75 mg/m2 daily on days 1–42. Following a 4- to 6-week rest period, patients received up to 12 further cycles of standard adjuvant TMZ 150 mg/m2/day on days 1–5, increasing to 200 mg/m2/day on days 1–5 of subsequent cycles if well tolerated, in combination with vorinostat at a dose of 400 mg/day on days 1–7 and 15–21 of each 28-day cycle, as per NABTC trial 04-03.27 The primary objective was to determine the efficacy of vorinostat in combination with RT and TMZ by measuring overall survival (OS) at 15 months; other objectives were to determine progression-free survival (PFS) and to explore the relationship between baseline tumor gene expression signatures of vorinostat sensitivity/resistance and clinical outcomes.
The study received institutional review board approval; all patients provided written informed consent in accordance with federal and institutional guidelines.
Patient Eligibility
Patients were eligible who were age ≥18 years with histologically confirmed GBM, gliosarcoma, or other World Health Organization grade IV astrocytoma variants, as determined by pre-registration central pathology review. Patients’ disease had to be measurable or evaluable by gadolinium MRI or contrast CT scan. All patients were required to begin involved brain RT on the same day that treatment with vorinostat and TMZ commenced. In addition, eligible patients were required to have a KPS of ≥60 or Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; life expectancy ≥12 weeks; adequate hematologic, liver, and renal function; and ability to begin treatment ≥2 weeks to ≤5 weeks after surgery. Patients who met any of the following criteria were excluded: prior cytotoxic or noncytotoxic drug therapy, or experimental drug therapy for brain tumors; prior cranial RT; prior Gliadel wafers; use of valproic acid or another HDAC inhibitor ≤2 weeks prior to registration and during treatment; other active malignancy ≤3 years prior to registration; history of myocardial infarction or unstable angina ≤6 months prior to registration or congestive heart failure requiring use of ongoing maintenance therapy for life-threatening ventricular arrhythmias; New York Heart Association Class ≥II congestive heart failure; congenital long QT syndrome; prolonged QTc interval (>450 msec); or use of any Category I drugs associated with a risk of torsades de pointes ≤7 days prior to registration.
Study Objectives
The primary endpoint of the phase I component was to determine the MTD and safety of vorinostat in combination with RT and TMZ. In the phase II study, the primary endpoint was to determine percentage of OS at 15 months; secondary endpoints were to evaluate OS, PFS, and safety of the treatment regimen. Exploratory objectives were to evaluate the extent to which tumor molecular characteristics and gene expression signatures in baseline tumor samples correlate with outcomes.
Response Criteria
The study used criteria of the Response Assessment in Neuro-Oncology29 modified to require a second independent reviewer for assessment of evaluable (nonmeasurable) disease. Complete response was defined as complete disappearance of all measurable and evaluable disease, no new lesions, and no evidence of non-evaluable disease. Partial response was defined as ≥50% decrease from baseline in the sum of products of perpendicular diameters of all measurable lesions, no progression of evaluable disease, and no new lesions. Stable disease required a minimum 8-week duration (2 assessments of stable disease 8 wk apart). Progressive disease was defined as a 25% increase in the sum of products of all measurable lesions over smallest sum observed, or clear worsening of any evaluable disease, or appearance of any new lesion/site or clear worsening, or failure to return for evaluation due to death or deteriorating condition. In patients with evaluable disease, regression was defined as no new lesions and unequivocal reduction in extent of contrast compared with baseline enhancement or a decrease in mass effect as agreed upon independently by a primary physician and quality control physician.
Statistical Analysis
The primary efficacy endpoint of the phase II trial was improvement in OS at 15 months from 50% (associated with standard RT and TMZ therapy)6 to 63%. A Simon’s optimal design30 was employed to detect this improvement, based on a one-tailed alpha of 0.1 and 90% power. This design required 98 evaluable patients; a positive study required 56 or more successes (57.1%).
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
An NCCTG (Alliance) independent data and safety monitoring board was responsible for reviewing safety data for the phase II trial at least twice a year, based on reports provided by the NCCTG (Alliance) statistical center.
RNA Signature Analysis of Baseline Tissue
A protocol prespecified RNA sequencing analysis of baseline tumor tissue from patients treated at the phase II dose was performed by The Cancer Genome Atlas (TCGA) Genome Characterization Center at the University of North Carolina (UNC). The goals of this analysis were to determine mutation profiles and TCGA transcriptional subtypes and to validate 5 previously established expression signatures.31,32 Signature scores were determined for 4 vorinostat sensitivity signatures (sig-9, -77, -80, and -139, predominantly including genes involved in RNA and DNA metabolism; see Supplementary Tables S1–S4, respectively, for a detailed list of the genes and their functions for each signature) and 1 vorinostat resistance signature (sig-79, predominantly including genes involved in apoptosis, stress, and inflammatory response [Supplementary Table S5]). These signatures were developed by Rosetta Inpharmatics, using mRNA gene expression data from a panel of >200 tumor cell lines that were either sensitive to vorinostat (half-maximal inhibitory concentration [IC50] < 1 µM) or resistant to vorinostat (IC50 > 1 µM) in an Agilent platform. Different signatures (gene lists) were generated based on combinations of genes that were found to be statistically significantly associated with vorinostat IC50 in each panel. Genes for sig-80 were selected based on the median prediction of sensitivity/resistance across all 4 panels. Sig-77 has the top 5% of significant genes. Sig-139 was generated as a subset of sig-77 based on coexpression of genes on independent reference tumor profiling datasets.
Formalin-fixed, paraffin-embedded samples from study patients were obtained as 5 to 10 unstained sections (5 µm). Viable tumor was visualized on hematoxylin and eosin stained sections, which were used to guide tumor macrodissection on unstained slides. Total RNA was purified by the UNC Biospecimens Core Facility using a Roche High Pure RNA Paraffin Kit, and quality was assessed using an Agilent Bioanalyzer. Uniquely barcoded libraries were prepared in the UNC Genomics Core Facility from purified total RNA using an Illumina TruSeq Stranded Total RNA with Ribo-Zero Gold Kit. RNA sequencing was performed in the UNC High Throughput Sequencing Facility on an Illumina HiSeq 2500 using paired end reads and 48 cycles. Two individual tumor libraries were pooled for analysis on a single flow cell lane. Data were subjected to quality control as previously described.33 RNA reads were aligned to the hg19 genome assembly using MapSplice.34 Gene expression was quantified for the transcript models corresponding to TCGA GAF2.1, using RNA sequencing by expectation-maximization35 and normalized within sample to a fixed upper quartile. Gene-level data were restricted to genes expressed in at least 70% of samples. Data were log2 transformed and median centered across samples prior to further analysis.
The correlation between the 5 signature scores was determined using Spearman’s rank correlation analysis, ANOVA along with Tukey’s honest significant differences (HSD) tests were used to determine if the signature scores were related to TCGA transcriptional subtypes. Univariate differences for each signature score between the MGMT methylated and unmethylated patients were determined using unequal variance t-tests.
Univariate cutpoint analysis was used to determine the optimum cutpoint threshold for each signature for both OS and PFS: Patients were classified as either having a high- or low-signature score based on increments of 0.001 for all possible cutpoint thresholds between 0.25 and 0.85. At each of these cutpoint thresholds, the patient group with high-signature scores was compared with the group with low-signature scores via Kaplan–Meier survival analysis for both OS and PFS. For each signature, the cutpoint threshold with the minimum P-value was selected as the optimum cutpoint. In the case of ties, the cutpoint nearest to 0.50 was selected.
Multivariate Cox proportional hazards models were used to simultaneously evaluate the relationships of age, MGMT status, TCGA transcriptional subtypes, and signature scores with respect to both OS and PFS. The patients with complete data available with respect to age, MGMT status, and signature scores were included in recursive partitioning (RPART) to further evaluate relationships of those variables and determine the best partitioning of patients into groups which have different OS and PFS outcomes. RPART uses a sequential approach to determining cutpoints. The first best cutpoint was determined based on the minimum P-value. The patients were then grouped as equal to or above the cutpoint or below the cutpoint. Each of these high and low groups was then independently evaluated based on all variables for the next best cutpoint via the minimum P-value. This resulted in 3 groups which were then evaluated independently for the next minimum P-value. This analysis ended when no further significant P-values could be found. Thus results represent a series of univariate cutpoints; these RPART methods are highly exploratory and do not control for overfitting. Kaplan–Meier plots were used to depict the resulting OS and PFS for optimum partitions.
MGMT gene methylation assessment in baseline tumors was performed by LabCorp using methylation-specific quantitative PCR as previously described.9
Mutation analysis of isocitrate dehydrogenase (IDH) was performed by immunohistochemistry for the detection of R132H IDH1 mutation as previously described.36
Results
Patient Demographics
A total of 15 patients were enrolled in the phase I trial; 107 patients were enrolled in the phase II trial. Demographics and baseline characteristics of patients enrolled in both trials are shown in Table 1. Patients had a median age <60 years, with a slight preponderance of males. The majority of patients (96% in phase II) had ECOG performance status of 0 or 1; a higher percentage of patients in the phase II trial had undergone gross total resection (38%) compared with patients in the phase I trial (20%). Three of the 88 patients tested were positive for R132H IDH1 mutation by immunohistochemistry (3.4%), consistent with IDH1 mutation frequency in GBM.
Table 1.
Patient demographics and baseline characteristics
| Variable | Phase I Component | Phase II Component | ||
|---|---|---|---|---|
| Vorinostat Dose Level (mg/day) | ||||
| Overall N = 15 |
300 N = 12 |
400 N = 3 |
N = 107 | |
| Age, y | ||||
| Median (min, max) | 58 (43, 76) | 57 (43, 76) | 59 (58, 73) | 59 (20, 80) |
| Mean (SD) | 59.9 (10.6) | 59.0 (11.2) | 63.3 (8.4) | |
| Sex, n (%) | ||||
| Female | 7 (47) | 4 (33) | 3 (100) | 44 (41) |
| Male | 8 (53) | 8 (67) | 0 (0) | 63 (59) |
| Extent of primary resection, n (%) | ||||
| Biopsy | 5 (33) | 3 (25) | 2 (67) | 18 (17) |
| Subtotal resection | 7 (47) | 6 (50) | 1 (33) | 48 (45) |
| Gross total resection | 3 (20) | 3 (25) | 0 (0) | 40 (38) |
| Missing | 0 | 0 | 0 | 1 (0.93) |
| Corticosteroid therapy, n (%) at baseline | ||||
| Yes | 8 (53) | 5 (42) | 3 (100) | 76 (71) |
| No | 7 (47) | 7 (58) | 0 (0) | 31 (29) |
| ECOG performance status, n (%) | ||||
| 0 | 6 (40) | 6 (50) | 0 (0) | 51 (48) |
| 1 | 5 (33) | 5 (42) | 0 (0) | 51 (48) |
| 2 | 4 (27) | 1 (8) | 3 (100) | 5 (4) |
Safety and Efficacy
In the phase I trial, 12 patients were treated at dose level 0 (vorinostat 300 mg/day) with RT, and 3 patients were treated at dose level 1 (vorinostat 400 mg/day). At dose level 0, one of 6 patients experienced a DLT, grade 3 dyspnea. At dose level 1, DLTs were observed in each of the 3 patients: 1 patient experienced grade 4 neutropenia, thrombocytopenia, and grade 3 alanine aminotransferase elevation, blood glucose elevation, and fatigue; the second patient experienced grade 3 wound dehiscence; and the third patient experienced grade 3 fatigue. An additional 6 patients were subsequently enrolled to dose level 0 in an MTD expansion cohort. DLTs were observed in 2 of these 6 patients: 1 patient experienced grade 4 thrombocytopenia and grade 3 fatigue, and the other patient experienced grade 4 neutropenia and thrombocytopenia and grade 3 febrile neutropenia. In summary, of the 12 patients treated at dose level 0, a DLT was observed in 25% of patients (3 of 12 patients).
Based on these results, dose level 0 was determined to be the MTD for vorinostat in combination with RT/TMZ in newly diagnosed patients with GBM (300 mg/day, administered on days 1–5 each week during RT). This dose was used in the phase II component of the trial.
Safety data for the phase II trial are summarized in Table 2. Briefly, the most common grade 3 or 4 treatment-related adverse events were lymphopenia (32.7%), thrombocytopenia (28.0%), neutropenia (21.5%), leukopenia (19.6%), and fatigue (14.0%). There were 3 deaths on treatment, all of which were deemed unlikely to be related to treatment.
Table 2.
Common grade 3 or 4 treatment-related adverse events in the phase II trial
| Toxicity | Grade 3 n (%) |
Grade 4 n (%) |
|---|---|---|
| Lymphopenia | 30 (28.0) | 5 (4.7) |
| Thrombocytopenia | 15 (14.0) | 15 (14.0) |
| Neutropenia | 12 (11.2) | 11 (10.3) |
| Leukopenia | 12 (11.2) | 9 (8.4) |
| Fatigue | 15 (14.0) | 0 |
| Anorexia | 7 (6.5) | 0 |
| Anemia | 5 (4.7) | 2 (1.9) |
| Thrombosis | 3 (2.8) | 4 (3.7) |
| Alanine aminotransferase increased | 4 (3.7) | 1 (0.9) |
| Nausea | 3 (2.8) | 0 |
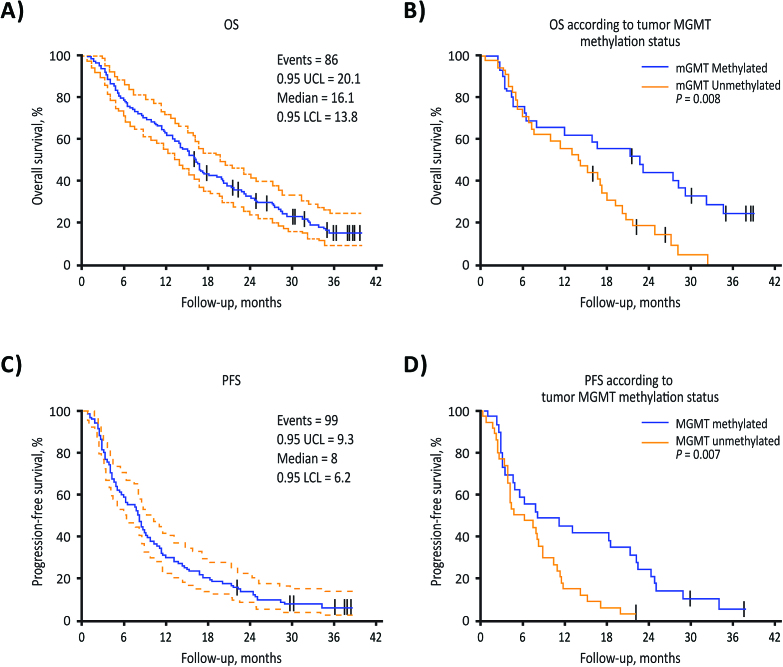
Survival and response data are summarized in Table 3 for the overall phase II cohort and for the subgroups of patients with MGMT methylated or unmethylated tumors. In the overall phase II cohort, 54 of the first 98 evaluable patients and 59 of all 107 evaluable patients were alive at 15 months, for an OS rate of 55.1% for both. Among 63 patients with MGMT methylation data available, 34 (54.0%) were alive at 15 months, including 18 of 29 (62.1%) patients with MGMT methylated tumors and 16 of 34 (47.1%) with unmethylated tumors (P = 0.312). Median OS was 16.1 months in the overall population (Table 3 and Fig. 1A). In patients with methylated tumors, median OS was 22.7 months compared with 14.1 months in patients with unmethylated tumors (hazard ratio [HR], 2.20 [1.21, 4.0]; P = 0.008; Table 3 and Fig. 1B). In the overall phase II population, median PFS was 8.0 months (Table 3 and Fig. 1C); the subpopulation of patients with MGMT methylated tumors had a median PFS of 8.1 months compared with 5.6 months in those with unmethylated tumors (HR, 2.17 [1.22, 3.87]; P = 0.007; Table 3 and Fig. 1D). The median patient follow-up was 16 months (range, 0.8–39.6 mo).
Table 3.
Survival, response, and duration outcome measures for the phase II trial with stratification according to tumor MGMT methylation status. Comparison of methylated versus unmethylated patients
|
All Patients
n = 107 |
MGMT Status (n = 63) | P-value | |||
|---|---|---|---|---|---|
| All Patients | Methylated | Unmethylated | |||
| OS at 15 mo | 59 (55.1%) | 34 (53.9%) | 18 (62.1%) | 16 (47.1%) | 0.312a |
| OS median (95% CI) |
16.1 mo (13.8, 20.1) |
16.7 mo (11.4, 21.6) |
22.7 mo (11.9, 34.5) |
14.1 mo (7.6, 19.2) |
HR, 2.20 (1.21, 4.0) P = 0.008b |
| PFS median (95% CI) |
8.0 mo (6.2, 9.3) |
7.6 mo (4.4, 11.0) |
8.1 mo (4.9, 22.3) |
5.6 mo (4.2, 9.0) |
HR, 2.17 (1.22, 3.87) P = 0.007b |
aFisher’s exact test for count data.
bLog-rank test.
Fig. 1.
Overall survival in the overall phase II cohort (A) and in patients with MGMT promoter methylated vs unmethylated tumors (B). Progression-free survival in the overall phase II cohort (C) and in patients with MGMT methylated vs unmethylated tumors (D). LCL, lower confidence limit; UCL, upper confidence limit.
RNA Signature Profile of Baseline Tissue
Previous studies have identified in vitro expression signatures from a broad panel of all cancer cell lines that predict in vitro sensitivity or resistance to vorinostat. Vorinostat-sensitive signatures were noted to be highly enriched RNA and DNA metabolism genes. Conversely, the resistance signature was composed of 79 genes and was enriched for genes related to apoptosis, stress, and inflammatory responses. Our study evaluated for the first time the prognostic potential of these signatures in a clinical trial context. We performed RNA sequencing analysis of baseline tumor tissue from study patients, and informative data were generated in 76 patients. Of these, MGMT status information was available in 59 patients. The vorinostat sensitivity signatures scores (sig-9, -77, -80, and -139) were highly positively correlated with each other, with the exception of sig-9 and sig-77 (P = 0.07). The vorinostat resistance signature (sig-79) was highly negatively correlated with 3 of the vorinostat-sensitive signatures: sig-77, -80, and -139 (P ≤ 0.0001).
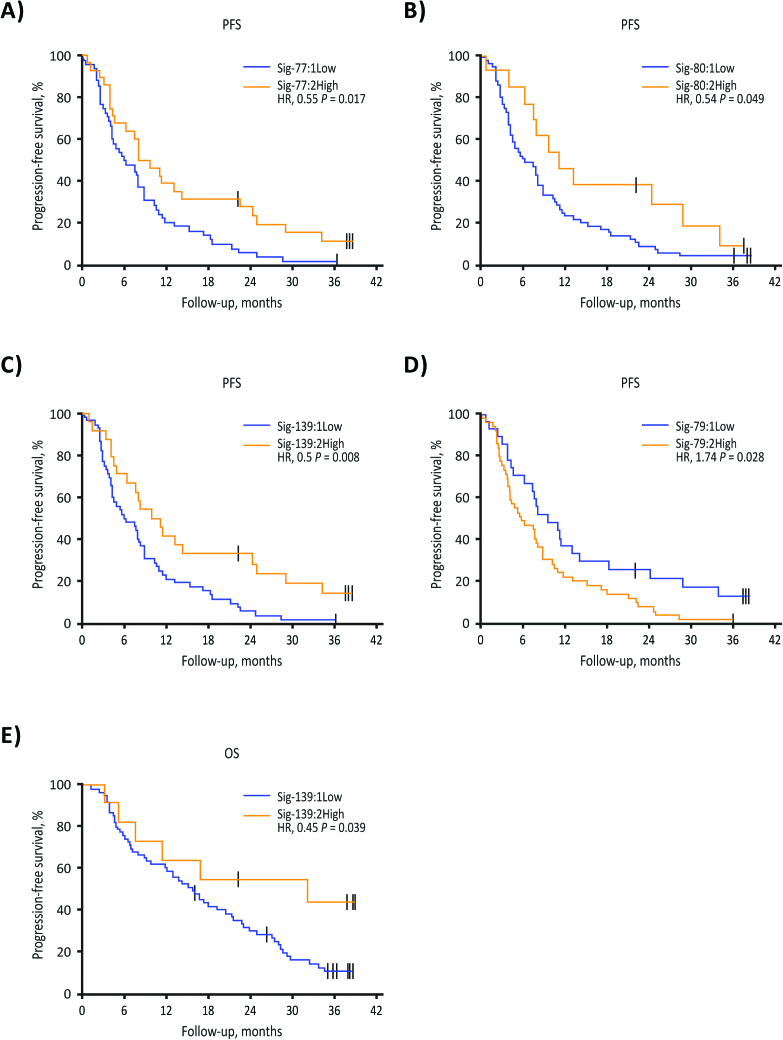
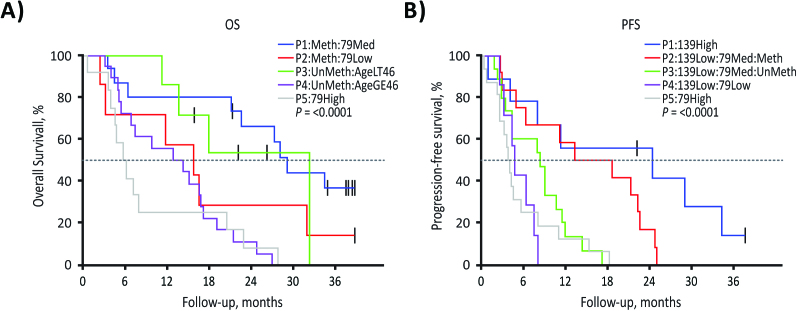
A statistically significant improvement for PFS was seen in patients with the higher scores for sig-77, -80, and -139, which all had at least 1 significant univariate cutpoint (HR, 0.55 [0.33, 0.91], 0.54 [0.28, 1.04], and 0.50 [0.29, 0.86], respectively; all P < 0.05; Fig. 2A-C,). Higher scores for sig-79 had a univariate cutpoint related to lower PFS (HR, 1.74 [1.05, 2.88], P = 0.028; Fig. 2D). The only signature that had a univariate cutpoint related to higher OS was sig-139 (HR, 0.45 [0.19, 1.04], P = 0.039; Fig. 2E). Optimum RPART cutpoint analysis for OS and PFS resulted in 5 groups of patients for each. Shorter OS and PFS were observed in patients with high sig-79 scores; shorter PFS was also observed in patients with low sig-139 scores, even if sig-79 scores were low. MGMT methylation status did not affect this negative association of sig-79 with PFS and OS, or the positive association of sig-139 with PFS (Fig. 3A, B).
Fig. 2.
RNA sequencing analysis of baseline tissue obtained from 76 patients in the phase II trial: PFS in patients with tumors expressing the vorinostat sensitivity (A, sig-77; B, sig-80; C, sig-139) and vorinostat resistance (D, sig-79) molecular signatures. Overall survival in patients with tumors expressing the vorinostat sensitivity molecular signature sig-139 (E).
Fig. 3.
Optimum RPART for OS and PFS subgroups using age, MGMT methylation status, and RNA sequencing analysis of baseline tissue obtained from 59 patients in the phase II trial: OS subgroups (A) and PFS subgroups (B). Overall survival subgroups: P1, patients with tumors that were MGMT methylated with medium values for sig-79; P2, patients with tumors that were MGMT methylated with low values for sig-79; P3, younger patients (age <46 y) with MGMT unmethylated tumors; P4, older patients (age ≥46 y) with MGMT unmethylated tumors; P5: patients with tumors with high values for sig-79. Progression-free survival subgroups: P1, patients with tumors with high values for sig-139; P2, patients with tumors that were MGMT methylated with low values for sig-139 and medium values for sig-79; P3, patients with tumors that were MGMT unmethylated with low values for sig-139 and medium values for sig-79; P4, patients with tumors with low values for sig-139 and low values for sig-79; P5, patients with tumors with high values for sig-79.
In a parallel exploratory analysis, the possible association of the vorinostat sensitivity/resistance signatures with TCGA subtypes was explored. Differences were observed in the signature scores for sig-139, sig-77, sig-80, and sig-79 between TCGA subtypes (all had an overall ANOVA P ≤ 0.001). No differences were observed for sig-9 between the subtypes from TCGA (ANOVA P = 0.35). For the sensitivity signatures sig-77 and sig-80, the average signature scores were lower in the mesenchymal subgroup compared with all other subgroups, and for sig-139 the average signature score in the mesenchymal subgroup was lower compared with the proneural and classical subgroups (all Tukey-HSD adjusted P-values ≤0.05). For the sensitivity signatures sig-139, sig-77, and sig-80 the average signature scores were higher in the proneural subgroup compared with all other subgroups (all Tukey-HSD adjusted P-values ≤0.05). For the resistance signature sig-79 the average signature scores were higher in the mesenchymal subgroup and lower in the proneural subgroup compared with all other subgroups (all Tukey-HSD adjusted P-values ≤0.05). Of note, in a recent analysis based on the AVAglio study population, patients with newly diagnosed GBM treated with standard-of-care RT/TMZ and whose tumors had the proneural signature fared worse compared with patients with mesenchymal signature tumors.37 Our findings suggest that proneural tumors may derive more benefit from incorporation of vorinostat to standard RT/TMZ approaches. Prospective validation of our findings would be important in order to characterize the predictive versus prognostic role of these signatures.
As it pertains to MGMT status, the 5 signatures were not correlated to MGMT status, with all unequal-variance t-tests showing P > 0.05.
Discussion
Glioblastoma is a highly aggressive tumor that is resistant to conventional antitumor treatments.38 Recent efforts to improve the survival of patients with newly diagnosed disease through the addition of novel agents to standard therapy, including bevacizumab,3,4,39 hydroxychloroquine,40 and cilengitide,41,42 have largely failed. The use of dose-dense TMZ similarly failed to improve OS beyond that expected with standard therapy, irrespective of tumor MGMT methylation status.5 Clinical testing of the HDAC inhibitor vorinostat represents a rational and targeted approach to the treatment of GBM. Histone deacetylase inhibitors are potent radiosensitizers43,44 and have been shown in preclinical studies to enhance the effects of other antitumor agents.45,46 Their multiple effects include activating apoptosis, inducing production of reactive oxygen species, and reducing the expression of proteins involved in DNA repair.38
Histone acetylation analysis and RNA expression profiling of patients with recurrent GBM who were treated with single-agent vorinostat in a previous phase II study found that vorinostat affects target pathways, with a resulting modest clinical benefit.47 Immunohistochemical analysis performed in paired baseline and post-vorinostat treatment samples in a separate surgical subgroup of 5 patients with recurrent GBM showed posttreatment increase in acetylation of histones H2B and H4 in 4 of 5 patients, and of histone H3 in 3 of 5 patients. Microarray RNA analysis in the same samples showed changes in genes regulated by vorinostat, such as upregulation of E-cadherin (P = 0.02), indicating that vorinostat at a dose of 200 mg orally twice a day for 14 days followed by a 7-day rest can affect target pathways in GBM.47 A subsequent study combining vorinostat with bortezomib in the same patient population was closed at the first interim efficacy analysis because none of the patients benefited from treatment.48 In patients with high-grade gliomas, the main effect of vorinostat when added to TMZ was hyperacetylation of histones H3 and H4 in peripheral mononuclear cells.27 The present Alliance/ABTC phase I/II study was designed to evaluate the addition of vorinostat to standard RT and concomitant TMZ in patients with newly diagnosed glioblastoma. The phase I component of the trial established the MTD of vorinostat as 300 mg/day when administered in combination with TMZ and RT on days 1–5 weekly during RT. Although vorinostat in combination with TMZ and RT had acceptable tolerability in these patients, the phase II trial did not meet its primary endpoint, with OS at 15 months of 55.1% in the entire cohort and median OS of 16.1 months, which is consistent with OS data from recent phase II and randomized phase III trial data.3–5,26,49 Data on MGMT methylation status were available in 63 of 107 patients (59%), primarily reflecting tissue availability in the context of tumor biopsy only or limited subtotal resection. Of note, however, both PFS (8.1 mo for MGMT methylated vs 5.6 mo in unmethylated patients) and OS data (22.7 mo for MGMT methylated vs 14.1 mo for MGMT unmethylated patients) are consistent with outcomes observed in the standard-of-care (RT/TMZ) arm of recent randomized trials.3–5,50 In contrast, a small single-arm phase II trial of 37 patients recently showed that the addition of the HDAC inhibitor valproic acid to concurrent RT and TMZ for the treatment of newly diagnosed GBM resulted in median OS of 29.6 months and 12-month survival of 86%, suggesting improved clinical outcomes compared with historical data. The small sample size represents a significant limitation of these data, however.51 Conversely, a recent analysis of survival association with valproic acid use at the start of chemoradiotherapy with TMZ performed in the pooled patient cohort (n = 1869) of 4 contemporary randomized clinical trials in patients with newly diagnosed GBM concluded that there was no justification for using valproic acid other than for seizure control.52
The findings based on the available RNA sequencing data of baseline tumor samples suggest an association between vorinostat response signatures and PFS and OS. Patients with high scores for the vorinostat resistance sig-79 experienced shorter PFS and OS; conversely, patients with high scores on the vorinostat sensitivity sig-139 experienced longer PFS and OS. These results suggest that these vorinostat signatures could prove useful in optimizing the selection of patients who may derive clinical benefit from the addition of vorinostat to standard chemoradiation therapy. Prospective trials are needed to validate these hypothesis-generating findings and conclusively evaluate the predictive versus possible prognostic role of these signatures. Furthermore, gene targets in the vorinostat resistance sig-79, for which targeted inhibitors exist, may merit future investigation as combination therapies capable of rendering tumor more sensitive to vorinostat or other HDAC inhibitors. Examples of such genes include FAS, NAMPT, and STAT, inhibitors of which have been tested as single agents in clinical trials.
In conclusion, the use of the HDAC inhibitor vorinostat in combination with standard RT/TMZ was associated with acceptable tolerability in patients with newly diagnosed GBM. With OS at 15 months of 55.1% and median OS of 16.1 months, the phase II study did not meet its primary efficacy endpoint. Additionally, RNA sequencing data from baseline tumor samples of 76 patients indicated that gene signatures could be useful in identifying subgroups of patients deriving benefit from vorinostat treatment in combination with standard chemoradiation in patients with newly diagnosed GBM. These data merit prospective validation in future trials.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the National Institutes of Health (U10 CA25224, U10 CA031946) to Alliance for Clinical Trials in Oncology and the Adult Brain Tumor Consortium. Support was also received by the Damon Runyon Cancer Research Foundation (CI-45-09 to C.R.M.).
Conflict of interest statement. P.Y.W. is a member of the speaker’s bureau for Merck Research Laboratories and serves as a consultant/advisory board member for Cavion LLC, Cortice Biosciences, Foundation Medicine Inc, Genentech/Roche Inc, Monteris Medical, Novartis Institute for Biomedical Research, and Vascular Biogenics Ltd. He has also received research support from Acerta Pharma, Agios Pharmaceuticals, AngioChem Inc, AstraZeneca PLC, Genentech/Roche Inc, GlaxoSmithKline PLC, Karyopharm Therapeutics Inc, Merck Research Laboratories, Novartis Institute for Biomedical Research, Oncoceutics Inc, Sanofi Aventis LLC, and Vascular Biogenics Ltd. A.L. is an employee and shareholder of Merck. M.N. is an employee of Merck. The remaining authors declare no potential conflicts of interest.
Supplementary Material
Acknowledgments
We thank Mark Vitucci, Katie Hoadley, Joel Parker, Piotr Mieczkowski, Amy Perou, Patricia Basta, Hendrik Dejong, and Yan Shi for assistance with RNA sequencing. The UNC Biospecimens, Genomics, and High Throughput Sequencing Core Facilities are supported by the National Cancer Institute (P30 CA016086).
Correlative analysis was supported by Merck & Co., Inc, Kenilworth, New Jersey, USA.
References
- 1. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Fulop J et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 4. Gilbert MR, Dignam JJ, Armstrong TS et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilbert MR, Wang M, Aldape KD et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 7. Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haar CP, Hebbar P, Wallace GC 4th et al. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37(6):1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hegi ME, Diserens AC, Gorlia T et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 10. Lehrmann H, Pritchard LL, Harel-Bellan A. Histone acetyltransferases and deacetylases in the control of cell proliferation and differentiation. Adv Cancer Res. 2002;86:41–65. [DOI] [PubMed] [Google Scholar]
- 11. Mai A, Massa S, Rotili D et al. Histone deacetylation in epigenetics: an attractive target for anticancer therapy. Med Res Rev. 2005;25(3):261–309. [DOI] [PubMed] [Google Scholar]
- 12. Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. [DOI] [PubMed] [Google Scholar]
- 13. Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–5552. [DOI] [PubMed] [Google Scholar]
- 14. Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101(5):1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. [DOI] [PubMed] [Google Scholar]
- 16. Marks PA, Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs. 2005;14(12):1497–1511. [DOI] [PubMed] [Google Scholar]
- 17. Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. [DOI] [PubMed] [Google Scholar]
- 18. Rosato RR, Grant S. Histone deacetylase inhibitors: insights into mechanisms of lethality. Expert Opin Ther Targets. 2005;9(4):809–824. [DOI] [PubMed] [Google Scholar]
- 19. Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. [DOI] [PubMed] [Google Scholar]
- 20. Eyüpoglu IY, Hahnen E, Buslei R et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93(4):992–999. [DOI] [PubMed] [Google Scholar]
- 21. Ugur HC, Ramakrishna N, Bello L et al. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J Neurooncol. 2007;83(3):267–275. [DOI] [PubMed] [Google Scholar]
- 22. Yin D, Ong JM, Hu J et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–1052. [DOI] [PubMed] [Google Scholar]
- 23. Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang SM, Harari PM. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005;62(1):223–229. [DOI] [PubMed] [Google Scholar]
- 24. Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63(21):7291–7300. [PubMed] [Google Scholar]
- 25. Hockly E, Richon VM, Woodman B et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100(4):2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galanis E, Wu W, Cloughesy T et al. Phase 2 trial design in neuro-oncology revisited: a report from the RANO group. Lancet Oncol. 2012;13(5):e196–e204. [DOI] [PubMed] [Google Scholar]
- 27. Lee EQ, Puduvalli VK, Reid JM et al. Phase I study of vorinostat in combination with temozolomide in patients with high-grade gliomas: North American Brain Tumor Consortium Study 04-03. Clin Cancer Res. 2012;18(21):6032–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–1147. [DOI] [PubMed] [Google Scholar]
- 29. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 30. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. [DOI] [PubMed] [Google Scholar]
- 31. Loboda A, Fantin VR, Randolph S et al. Vorinostat (suberoylanilide hydroxamic acid, SAHA) induces robust gene expression changes in peripheral blood mononuclear cells from patients with solid tumors, consistent with its effect in panels of human tumor cell lines. Paper presented at: AACR2007. [Google Scholar]
- 32. Loboda A, Fantin VR, Randolph S et al. Identification of informative gene expression signatures indicative of vorinostat (suberoylanilide hyroxamic acid, SAHA) exposure and clinical efficacy in patients with advanced cutaneous T-cell lymphoma. Paper presented at: AACR2007. [Google Scholar]
- 33. Brat DJ, Verhaak RG, Aldape KD et al. ; Cancer Genome Atlas Research Network Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang K, Singh D, Zeng Z et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38(18):e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leeper HE, Caron AA, Decker PA, Jenkins RB, Lachance DH, Giannini C. IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas. Oncotarget. 2015;6(30):30295–30305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandmann T, Bourgon R, Garcia J et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cornago M, Garcia-Alberich C, Blasco-Angulo N et al. Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe. Cell Death Dis. 2014;5:e1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Linde ME, Verhoeff JJ, Richel DJ et al. Bevacizumab in combination with radiotherapy and temozolomide for patients with newly diagnosed glioblastoma multiforme. Oncologist. 2015;20(2):107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenfeld MR, Ye X, Supko JG et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10(8):1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nabors LB, Fink KL, Mikkelsen T et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 2015;17(5):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stupp R, Hegi ME, Gorlia T et al. ; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 43. Pont LM, Naipal K, Kloezeman JJ et al. DNA damage response and anti-apoptotic proteins predict radiosensitization efficacy of HDAC inhibitors SAHA and LBH589 in patient-derived glioblastoma cells. Cancer Lett. 2015;356(2 Pt B):525–535. [DOI] [PubMed] [Google Scholar]
- 44. Shabason JE, Tofilon PJ, Camphausen K. Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic. J Cell Mol Med. 2011;15(12):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asklund T, Kvarnbrink S, Holmlund C et al. Synergistic killing of glioblastoma stem-like cells by bortezomib and HDAC inhibitors. Anticancer Res. 2012;32(7):2407–2413. [PubMed] [Google Scholar]
- 46. Booth L, Roberts JL, Conley A et al. HDAC inhibitors enhance the lethality of low dose salinomycin in parental and stem-like GBM cells. Cancer Biol Ther. 2014;15(3):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galanis E, Jaeckle KA, Maurer MJ et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Friday BB, Anderson SK, Buckner J et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro Oncol. 2012;14(2):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Domingo-Musibay E, Galanis E. What next for newly diagnosed glioblastoma?Future Oncol. 2015;11(24):3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stupp R, Taillibert S, Kanner AA et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 51. Krauze AV, Myrehaug SD, Chang MG et al. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2015;92(5):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Happold C, Gorlia T, Chinot O et al. Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J Clin Oncol. 2016;34(7):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.