Abstract
The use of contrast enhancement within the brain on CT or MRI has been the gold standard for diagnosis and therapeutic response assessment in malignant gliomas for decades. The use of contrast enhancing tumor size, however, remains controversial as a tool for accurately diagnosing and assessing treatment efficacy in malignant gliomas, particularly in the current, quickly evolving therapeutic landscape. The current article consolidates overwhelming evidence from hundreds of studies in the field of neuro-oncology, providing the necessary evidence base and specific contexts of use for consideration of contrast enhancing tumor size as an appropriate surrogate biomarker for disease burden and as a tool for measuring treatment response in malignant glioma, including glioblastoma.
Keywords: biomarker, contrast enhancement, GBM, glioblastoma, qualification
A major barrier in the qualification of imaging biomarkers for use in diagnosis and predicting outcomes in clinical trials remains the lack of evidentiary standards to support the specific contexts of use for these biomarkers. The detailed challenges associated with these barriers were discussed at an in-depth workshop sponsored by Dr Anna Barker (Arizona State University) and the National Biomarker Development Alliance, in collaboration with FDA leadership and experts in the field of imaging and biomarker development. The current article describes the comprehensive historic evidence from the field of neuro-oncology, providing the necessary evidence base and specific contexts of use for consideration of contrast enhancing tumor size as a surrogate imaging biomarker for disease burden and as a tool for measuring treatment response in malignant glioma, including glioblastoma (GBM), using “Framework for Defining Evidentiary Criteria for Biomarker Qualification,” which describes a general framework for proposed evidentiary criteria for biomarker qualification and their specific contexts of use (https://fnih.org/sites/default/files/final/pdf/Evidentiary%20Criteria%20Framework%20Final%20Version%20Oct%2020%202016.pdf).
A Brief History of Brain Tumor Imaging Technology
In 1884, Rickman J. Godlee and Dr A. Hughes Bennett performed the first recognized resection of a primary, intracranial glioma.1 This surgery occurred more than 10 years prior to the discovery of any technology that could non-invasively identify tumors within the brain of living humans. In 1895, the X-ray was discovered by Wilhelm Roentgen, which for the first time provided the means of visualizing masses within the brain. In the early twentieth century, X-rays were used extensively to visualize and localize brain tumors,2 although the lack of contrast between normal and malignant brain tissue significantly limited the use of X-ray for diagnosis of gliomas.3 By 1950–1960, the use of cerebral angiography (iodine contrast combined with X-ray films) was the standard for differential diagnosis and serial follow-up evaluations4 of brain tumors, becoming routine in both academic institutions as well as small community hospitals.5 In 1959, the use of cerebral angiography was declared the imaging method of choice over all other techniques (eg, pneumoencephalography, ventriculograms) for suspected brain tumors.6
It was not until the advent of computed tomography (CT) by Sir Godfrey Hounsfield in 1971 that cerebral angiography was replaced with CT as the modality of choice for clinical diagnosis and monitoring of brain tumors. In 1980, a seminal study from the National Cancer Institute declared contrast enhanced CT the new clinical standard for brain tumor diagnosis and clinical monitoring after it was declared to be the best accurate diagnostic test in over 1000 patients with brain tumors,7 as was also suggested by other studies.8,9 Thus began the era of contrast enhanced CT as the new clinical standard for brain tumor diagnosis and clinical monitoring.
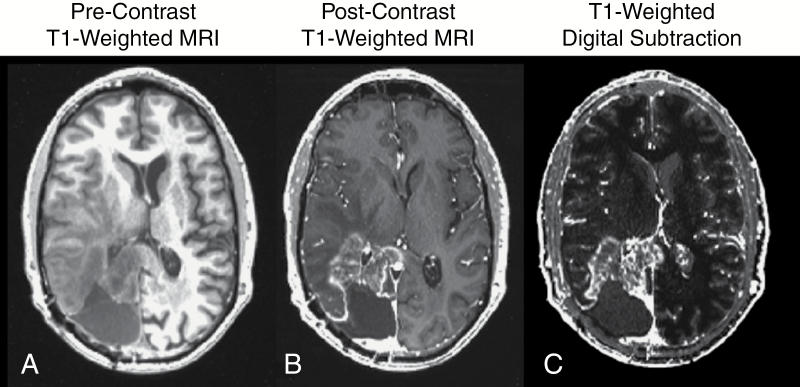
Although contrast enhanced CT was the standard for brain tumor imaging, a new technology was on the horizon that was set to revolutionize the field of medical imaging: magnetic resonance imaging (MRI). In 1971, Raymond Damadian first reported that nuclear magnetic resonance characteristics were different between normal and tumor tissues.10 In 1973, Paul C. Lauterbur created the first MR images in the mouse,11 and by 1977 Peter Mansfield created the first MR images in the human.12 In 1984, the first use of gadopentetate dimeglumine (Gd-DTPA) as a contrast agent for detection of primary intra-axial tumors was documented in Europe,13 followed by subsequent studies in 1985 substantiating these findings.14,15 By the late 1980s to early 1990s several studies demonstrated the ability for gadolinium chelates to be used to improve tumor diagnosis and identify areas for biopsy,16–18 with several studies demonstrating that contrast enhanced MRI shows similar lesion measurements when compared directly with contrast enhanced CT.16,19 Since the early 1990s, T1-weighted MRI used with the addition of contrast agents that shorten T1 relaxation time constants has been the gold standard for brain tumor detection, diagnosis, clinical monitoring, and response assessment for new therapies in clinical trials (Figure 1).
Fig. 1.
(A) Precontrast T1-weighted MR image; (B) postcontrast T1-weighted MR image; and (C) contrast enhanced T1-weighted digital subtraction maps in a patient with recurrent glioblastoma.
Contrast Enhancement as a Surrogate of Disease in Malignant Glioma
A seminal study by Butler et al20 in 1978 was the first to document the association between contrast enhancement on postcontrast CT and corresponding histological features of malignancy (cellularity, pleomorphism, vascularity, and necrosis) in anaplastic astrocytoma. This was followed by a series of similar studies with improved in vivo21–24 and postmortem stereotactic localization,25 demonstrating similar association between pathology and contrast enhancement (Table 1).
Table 1.
Contrast enhancement on CT or MRI as a surrogate of disease in malignant glioma*
| Biomarker | Incremental Context of Use | Incremental Evidentiary Standards for Regulatory Qualification | ||||
|---|---|---|---|---|---|---|
| Risk-Benefit | Evidentiary Standards | |||||
| Purpose | Drug Development Circumstance | Decision/Action | Benefit | Risk | ||
| Prognosis | ||||||
| Initial/Residual Contrast- Enhancing Tumor Size (with or without digital image subtraction) |
Prognosis (pretreatment) | Risk stratification (homogenize risk) enables drug development and use in more clearly defined patient groups (risk stratification) | Establish as “required/ necessary” test/ measurement | Reduced bias associated with large and small enhancing lesions, which tend to have dramatically different prognoses, independent of therapy. Better enable drug development by homogenizing risk. |
Not accounting for enhancing tumor size prior to therapy may result in premature “failure” of new therapies due to risk bias associated with initial tumor size. Larger sample sizes needed to observe a treatment effect for a drug in the phase III setting. |
Surgical studies demonstrating contrast- enhancing tumor contains the highest tumor cell density and proliferation rate, along with other histologic measures of malignancy.16,18,20–31 Prospective single and/or multicenter data associating initial enhancing tumor size or residual enhancing tumor size after resection with time to event outcome (TTP, PFS, objective response rate, OS).32–77,79–91,129,191–194 Prospective phase I–III trials associating initial tumor size, residual enhancing tumor size, or extent of resection with time to event outcome (TTP, PFS, objective response rate, OS).61,92–99,166 |
*Descriptions of the context of use (prognosis), how it will be used in drug development (risk stratification), balance of potential benefits and risks, as well as the evidentiary standards available to support use of contrast enhancement as a surrogate of disease in malignant glioma, per recommendations by the FDA and NIH.
In 1987, a series of studies published by Kelly et al26,27 acquired 195 serial biopsies from various locations within volumes defined by CT and MRI, noting that contrast enhancement most often corresponded to highest density of tumor tissue. Studies by Burger et al,28 Earnest et al,16 Dean et al,18 and others subsequently29–31 have since confirmed that areas of contrast enhancement on MRI or CT consistently contain the highest density of tumor cells along with the most aggressive histological features in malignant glioma.
Prognostic Significance of Tumor Size: Single and/or Multicenter Data
A multitude of single and/or multicenter studies have confirmed that contrast enhancing tumor size (volume or bidirectional measurements) is a significant prognostic factor contributing to overall survival (OS) in GBM, including pretreatment tumor size, postsurgical residual tumor size, and extent of surgical resection. One of the first studies examining the extent of surgical resection was from Reeves et al32 in 1979, who examined the prognostic significance of tumor size on contrast enhanced CT, age, radiation dose, and performance status. This study noted that tumor size as measured by contrast enhancement plus surrounding edema was not prognostic; however, results suggested small tumors (<300 mm2) trended toward longer OS compared with large tumors (>300 mm2). A study by Andreou et al33 in 1983 examined CT scans in 115 patients in the Cooperative Brain Tumor Study and found that postoperative residual tumor burden was inversely related to OS. In 1987, a study by Ammirati et al34 demonstrated that gross total resection, or resection without any remaining contrast enhancement, is directly associated with longer survival and better quality of life compared with subtotal resection.
A large study in 1988 performed by the Brain Tumor Cooperative Group identified postsurgical residual tumor size as a significant prognostic factor for malignant gliomas, independent of other prognostic variables, including age, tumor grade, or neurological status.35 A study by Vecht et al36 from the Netherlands confirmed this observation. By 1993, a large study by Curran et al37 had examined 1578 malignant gliomas from 3 Radiation Therapy Oncology Group (RTOG) trials from 1974 to 1989. Using a partition analysis consisting of a variety of potential prognostic variables, authors noted that extent of resection divided patients by almost 4 months difference in OS. Also in 1993, a study by Devaux et al38 demonstrated a significant survival advantage in 263 malignant glioma patients with resection compared with biopsy, even when both groups were given radiation therapy. A study in 1994 by Albert et al39 showed that approximately 80% of tumor recurrences emerged from enhancing tumor remnants revealed after postoperative MRI and that patients with residual, postoperative enhancing tumor were at a 6.6× higher risk of death compared with patients without residual enhancing tumor. In 2003, the Glioma Outcomes Project collected data from 788 patients accrued from multiple sites over a 4-year period (1997–2001), providing Class II evidence to support that resection, compared with biopsy, was a strong prognostic factor for newly diagnosed malignant gliomas.40
In 2009, McGirt et al41 published a large study of 949 cases with MR images that underwent surgical procedures at Johns Hopkins University between 1996 and 2006, again noting that extent of resection was associated with improved survival independent of age, World Health Organization (WHO) grade, disability, or subsequent treatments. In 2010, a large French epidemiology study by Bauchet et al42 involving 952 patients clearly determined extent of resection to be a significant prognostic factor in patients with newly diagnosed GBM. RTOG investigators again used partitioning analysis in 2011 to reexamine prognostic factors associated with GBM, again noting that extent of resection is an important factor in determining OS in patients with GBM.43 Additionally, another study in 2011 indicated that extent of resection was prognostic and showed that subtotal resection as low as 78% still had a survival benefit.44 This is similar to a 2014 study by Chaichana et al,45 who found a survival benefit for GBM obtaining more than a 70% extent of resection and a 2014 study by Oppenlander et al,46 who observed an improvement in survival for GBM patients with an extent of resection greater than 80%.
In 2013, investigators from the National Cancer Institute Clinical Center again confirmed the relationship between tumor size and location on prognosis in 92 patients with GBM treated with surgery followed by radiation and temozolomide, noting that patients with the smallest tumor size had a significantly longer OS compared with patients having the largest tumors.47 Additionally, a study by Gutman et al48 confirmed that contrast enhancing tumor volume was strongly associated with poor survival when examining 75 patients with GBM from The Cancer Genome Atlas. Also in 2013, Zinn et al49 conducted possibly the largest study evaluating extent of resection to date, evaluating a total of 21783 patients from 1973–2007 from the population-based Surveillance, Epidemiology, and End Results (SEER) registry. Results from this study again confirmed that gross total resection had a significantly longer median survival compared with subtotal resection. This observation was subsequently confirmed in 2015 by Pan et al50 using the SEER registry to examine 14675 GBM patients from 2000–2009, again noting a significant survival advantage in patients with gross total surgical resection. Additionally, a large 2015 Chinese study in 816 patients by Qin et al51 examined a wide range of potential prognostic variables and again confirmed that extent of surgical resection was a strong, independent predictor of OS.
Together, these single and multicenter studies, along with an extensive list of noteworthy studies that could not be adequately described in detail within the current document,52–91 provide overwhelming evidence that contrast enhancing tumor size—including baseline enhancing tumor size prior to therapy, residual tumor size after surgical resection, and extent of surgical resection—is a significant prognostic factor for malignant glioma, including GBM.
Prognostic Significance of Tumor Size: Prospective Phase I–III Trials
In addition to single and/or multicenter studies, a few prospective phase I–III clinical trials have also demonstrated that baseline tumor size, extent of resection, and residual tumor volume are significant prognostic factors for survival in malignant glioma, including GBM. In 1994, Rostomily et al92 determined that a larger extent of resection and smaller postoperative tumor volumes were associated with prolonged progression-free survival (PFS) in a phase II study of multi-agent chemotherapy in recurrent malignant gliomas. Similarly, a phase II trial of pre-irradiation carboplatin and etoposide along with accelerated hyperfractionated radiotherapy showed that extent of resection prior to therapy was a significant independent predictor of survival.93
In 2003, Vuorinen et al94 performed a prospective, randomized trial directly comparing prognosis in malignant glioma patients who received either stereotactic biopsy or open craniotomy and resection of the tumor. Results confirmed findings in previous studies, indicating that surgical resection provides approximately a 2.8× greater benefit in survival compared with biopsy alone. In 2005, Hauch et al95 performed a meta-analysis summarizing findings from 220 publications containing results from phase II and III trials in high-grade gliomas, totaling 17213 cases mostly treated with tumor resection, radiation therapy, and chemotherapies, including nitrosoureas. Results from this study suggested that extent of tumor resection had a significant positive impact on median OS when combining data from multiple studies. In 2008, Stummer et al61 provided Level 2b evidence that the extent of surgical resection of enhancing tumor is a significant predictor of survival in patients with GBM by examining 243 GBM patients enrolled in a study of 5-aminolevulinic acid (5-ALA), a parallel, randomized, balanced, group-sequential, 2-armed, controlled multicenter phase III study of 5-ALA fluorescence-guided resection versus conventional microsurgery.
In 2009, Stupp et al96 published results from a randomized phase III trial by the European Organisation for Research and Treatment of Cancer and the National Cancer Institute of Canada examining the effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone in a total of 573 patients with newly diagnosed GBM. Results of the study clearly indicated that extent of resection was a significant, independent predictor in both treatment arms. In 2015, Suchorska et al97 reported results from the well-characterized Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma (DIRECTOR) study (ClinicalTrials.gov #NCT00941460), noting that a complete resection of contrast enhancing tumor on MRI versus residual enhancing tumor at recurrence was associated with a significantly improved OS (median OS = 11.5 mo vs 6.7 mo).
Bevacizumab and anti-angiogenic agents
In 2014, Ellingson et al98 demonstrated that pretreatment enhancing tumor volume was a significant prognostic factor associated with shorter PFS and OS in recurrent GBM treated with bevacizumab with and without adjuvant irinotecan by examining 160 patients in the BRAIN trial, an open-label, multicenter, randomized, noncomparative phase II trial performed to assess the effectiveness of bevacizumab with or without irinotecan. In 2015, Ellingson et al99 confirmed these findings and demonstrated that pretreatment enhancing tumor volume was a significant prognostic factor for survival in recurrent GBM treated with bevacizumab and chemotherapy by examining 123 recurrent GBM patients in ACRIN 6677/RTOG 0625, a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent GBM.
In summary, there is a general consensus that extent of resection is one of the most important prognostic factors for GBM, largely based on the multitude of single and multicenter studies outlined previously. A recent study by Ellingson et al100 that included 497 patients from 4 different data sources, including 2 single-center sites and 2 multicenter phase II trials, solidified this point by showing that baseline contrast enhancing tumor volume was a significant predictor of OS in temozolomide, lomustine, bevacizumab (with or without irinotecan), and cabozantinib. It is important to point out, however, that most clinical trials with >2 treatment arms do not directly account for baseline tumor size or extent of resection despite overwhelming evidence that this is important. Instead, most studies balance the proportion of patients with different extents of resection between the various treatment arms.
Change in Contrast Enhancing Lesion Size as a Surrogate for Treatment Efficacy in Malignant Glioma
Change in contrast enhancing tumor size (MRI or CT) has been used to inform clinical decision making in malignant gliomas for more than 50 years and remains the gold standard for treatment efficacy in GBM (Table 2). Until relatively recently, response was determined by the criteria proposed by Macdonald et al in 1990, 101 which significantly improved upon earlier methods of response assessment, including the Levin criteria102 and the WHO oncology response criteria,103 by accounting for corticosteroid use and changes in neurological status. These “Macdonald criteria” used changes in the measurement of contrast enhancing tumor burden to determine treatment efficacy, similar to the previous criterion and similar to the Response Evaluation Criteria in Solid Tumors (RECIST).104
Table 2.
Change in contrast enhancing lesion size as a surrogate for treatment efficacy in malignant glioma*
| Biomarker | Incremental Context of Use | Incremental Evidentiary Standards for Regulatory Qualification | ||||
|---|---|---|---|---|---|---|
| Risk-Benefit | Evidentiary Standards | |||||
| Purpose | Drug Development Circumstance | Decision/Action | Benefit | Risk | ||
| Control/Relieve/Eliminate | ||||||
| Change in Contrast- Enhancing Tumor Size (with or without digital image subtraction) |
Response indicator (posttreatment prognosis) | Use as posttreatment measure of a favorable effect of a drug. | Inform decision to move to “continue a therapy” in a patient or inform the decision to move to the next phase of development | Absence of favorable effect informs the decision to change therapy, limiting patient exposure to ineffective therapy. Reduced drug development and trial costs as total trial durations needed to observe a treatment effect are reduced. Ability to isolate individual therapeutic effects in the recurrent setting (whereas OS can be complicated by combinations of various therapies). |
Effective therapies that significantly modulate vascular permeability may result in premature “failure” or “acceptance” of new therapies (eg, anti-angiogenic or immunotherapies). Favorable effect may not reflect “clinical benefit” in terms of neurologic symptoms or quality of life. |
Change in contrast enhancing tumor size (on MRI or CT) has been used to inform clinical decision making in malignant gliomas for >50 years and is the gold standard for response assessment.101–103,106–109 Prospective single and/or multicenter studies using change in contrast enhancing tumor size as a surrogate endpoint for treatment efficacy or associating change in enhancing tumor size with time to event outcome (TTP, PFS, ORR, QOL, PRO, neurocognitive function, and/or OS).89,118–123,127–132,147,195,196 Phase I–III trials in recurrent malignant gliomas using change in contrast enhancing tumor size as a surrogate endpoint for treatment efficacy (ie, PFS, TTP, or ORR), or have shown an association between radiographic response and outcome (QOL, PRO, neurocognitive function, PFS, TTP, ORR, or OS).92,93,98,129,130,133–146, 148–153,155,156,158,160–165, 167–189,196–204 |
Abbreviations: ORR, objective response rate; QOL, quality of life; PRO, patient reported outcome.
*Descriptions of the context of use (response indicator), how it will be used in drug development (measure of efficacy), balance of potential benefits and risks, as well as the evidentiary standards available to support use of change in contrast enhancement as a surrogate of posttreatment prognosis or outcome in malignant glioma.
For nearly 20 years the Macdonald criteria and general use of contrast enhancement as a surrogate of tumor burden were used for the evaluation of new therapies, including use for the accelerated approval of temozolomide in recurrent anaplastic astrocytoma and identification of the chemotherapy responsiveness of anaplastic oligodendroglioma, which was subsequently confirmed in OS benefit in phase III trials.105 In 2010, the Response Assessment in Neuro-Oncology (RANO) criteria were developed106 to comprehensively reform the Macdonald criteria by using the evolving principles outlined in previous work.107–109 At the core of the RANO criteria remains the use of changes in contrast enhancing tumor burden as a biomarker for treatment efficacy; however, the RANO criteria also added language for the evaluation of non-enhancing tumor progression, better definitions of measurable and nonmeasurable disease, definitions of progression for patients being considered for enrollment into clinical trials, recommendations to address pseudoprogression and pseudoresponse, the requirement of confirmatory scans for response, and recommendations for dealing with patients with equivocal imaging changes. Use of the RANO criteria is the current standard for response assessment in GBM clinical trials, and the details and practical implementation strategies are well documented.110–116
Change in Contrast Enhancing Tumor Size as a Surrogate for Treatment Efficacy: Single and/or Multicenter Data
As early as 1979, a study by McCullough et al117 examined 2 children with GBM undergoing chemotherapy and demonstrated that volumetric measurements of enhancing tumor revealed good correlations between radiographic and anatomic data obtained at autopsy and suggested for the first time that volumetric measurements using contrast enhanced CT may be a practical method for monitoring results of radiotherapy and chemotherapy in GBM. In 1988, a study by the Brain Tumor Cooperative Group involving 510 patients revealed that change in contrast enhancing tumor size was prognostic.35 Also in 1988, a study by Kumar et al118 reported the correlation between radiographic response and survival in 38 GBM patients treated with intraoperative endocurietherapy using cobalt-60.
In 1990, a single-center study by Finlay et al119 reported radiographic response of histologically confirmed malignant astrocytoma treated with high-dose bis-cloroethylnitrosourea (BCNU) followed by bone marrow infusion, noting that 4 of 10 patients showed early complete radiographic response, or complete shrinkage of contrast enhancing tumor, which resulted in remission for more than 290 days. This study even noted that early stability of enhancing tumor size resulted in sustained disease control for more than 13 months and that total response rate for this therapy was nearly 60%. In 1993, Couldwell et al120 reported that a minority of patients with recurrent malignant gliomas showed radiographic response to high-dose tamoxifen, which corresponded to long-term clinical benefit, whereas patients showing rapid early progression died within 3–6 months from start of therapy. A single-center study by Jakacki et al89 examining dose-intensive, time-compressed probarbazine, CCNU, procarbazine/lomustine/vincristine (PCV), and peripheral blood stem cell support with concurrent radiation in newly diagnosed high-grade gliomas reported early radiographic response via a decrease on contrast enhancing tumor in only 3 of 12 patients, which corresponded to a decrease in clinical symptoms. Additionally, 4 patients showed early radiographic progression coinciding with rapid clinical deterioration.
In 2004, See et al121 performed a retrospective analysis of recurrent GBM patients treated with 13-cis-retinoic acid, noting low radiographic response rates and longer PFS in patients with partial or minor radiographic response via change in contrast enhancement. In 2009, a single-center study by Patel et al122 investigated the efficacy of performing salvage re-irradiation for recurrent GBM using radiosurgery. Results indicated that radiographic response via contrast enhancement was observed in nearly 40% of patients, and these patients had a significant survival advantage compared with nonsurvivors (15.8 vs 7.3 mo). In 2011, a single-center study by Gladwish et al123 involving 30 patients with GBM demonstrated that the magnitude of early imaging response in patients treated with standard radiochemotherapy was predictive of outcome, particularly when observing early decreases in tumor size.
Bevacizumab and anti-angiogenic agents
The use of bevacizumab and other anti-angiogenic agents has been shown to alter vascular permeability, often resulting in large radiographic responses as measured using contrast enhancement on postcontrast T1-weighted images. However, this decrease in contrast enhancement often is associated with a real change in tumor biology and, since bevacizumab targets blood vasculature by neutralizing vascular endothelial growth factor (VEGF), suggests increased biological activity in these tumors. Further, there is evidence from single-center studies as well as multicenter trials showing that radiographic response to anti-angiogenic agents results in improved clinical symptoms, increased PFS, and often increased OS.
In 2006, Pope et al124 first noticed the high number of patients exhibiting a decrease in contrast enhancing tumor treated with bevacizumab. Investigators noted that patients who did not exhibit stability or radiographic response experienced rapid tumor growth on postcontrast MRI resulting in death. In 2008, a single-center study by Bokstein et al125 examined treatment response of bevacizumab and irinotecan for recurrent high-grade gliomas, noting 2 of 19 patients having a complete response and 7 of 19 patients exhibiting a partial radiographic response via a decrease in contrast enhancement. The authors note that patients with early radiographic response, partial or complete, had a substantially higher PFS and OS. A single-center retrospective study involving bevacizumab in recurrent malignant gliomas by Norden et al126 in 2008 further outlined challenges associated with using early changes in contrast enhancement as a surrogate for drug efficacy. However, authors note that progression is difficult to evaluate using contrast enhancement alone, as progression on bevacizumab was shown to result in an increase in T2 hyperintense, infiltrative tumor. The authors do show only a modest radiographic response to bevacizumab, with only a 2.3% complete response in these patients. In 2009, a single-center study of bevacizumab plus irinotecan reported by Zuniga et al127 again demonstrated dramatic radiographic response rates largely attributed to the decrease in vascular permeability accompanying anti-VEGF therapy.
These dramatic radiographic responses via contrast enhancing tumor did, however, correspond with improved clinical outcome compared with historical controls, including a longer PFS. A retrospective evaluation of salvage therapy using single agent bevacizumab in recurrent GBM reported by Chamberlain et al128 showed no patients with complete shrinkage of contrast enhancing tumor, 42% of patients demonstrating partial response on the first scan, and 58% of patients demonstrating contrast enhancing tumor growth. Interestingly, the data show that patients who had a partial radiographic response via a decrease in contrast enhancement had significantly longer OS from the start of bevacizumab compared with patients who did not respond (median OS = 12 vs 5.5 mo). A 2013 study by Huang et al129 examining volumetric change in contrast enhancing tumor in 91 recurrent GBM patients treated with bevacizumab found that patients exhibiting a large decrease on contrast enhancing tumor had significantly longer PFS and OS.
This evidence, combined with evidence from a wealth of additional single-center studies that were not described in detail,130–132 suggests that the change in contrast enhancing tumor size is useful for determining early drug efficacy in malignant glioma, including GBM.
Change in Contrast-Enhancing Tumor Size as a Surrogate for Treatment Efficacy: Phase I–III Clinical Trials
Change in contrast enhancing tumor size has historically been used to measure response to new therapies in phase I–III clinical trials for more than 30 years. In 1986, Greenberg et al133 utilized change in contrast enhancement on CT to assess treatment response in a phase I–II evaluation of intra-arterial diaziquone for recurrent malignant astrocytomas, noting that 2 of 20 patients experienced partial radiographic responses, 4 of 20 patients showed stabilization of disease over a period of a few months, and 1 patient had tumor shrinkage. In 1994, Rostomily et al92 evaluated 51 patients with recurrent malignant gliomas in a phase II trial of multidrug therapy (6-thioguanine, dibromodulcitol, procarbazine, etc.), noting that approximately 57% of patients had an objective radiographic response, or at least partial shrinking of contrast enhancing tumor, or stabilization of enhancing tumor, which translated to a significantly higher PFS (38 wk vs 7 wk) and OS (79 wk vs 18 wk).
A study reported by Macdonald et al134 in 1996 explored the use of topotecan in a phase II trial in patients with recurrent malignant glioma. Results showed that 2 of 31 patients had either a complete or a partial radiographic response with respect to contrast enhancing tumor, whereas 21 of 31 patients had stable disease. Investigators used the lack of radiographic response as defined by change in contrast enhancing tumor to conclude that topotecan has only modest activity in recurrent malignant glioma. In a single-arm phase I–II multicenter trial of BCNU-fluosol and oxygen inhalation in recurrent malignant glioma, Hochberg et al135 reported longer PFS and OS in patients with radiographic response or stabilization compared with patients showing early failure.
In 1997, Fine et al136 reported results from a phase I trial of a new recombinant human beta-interferon (BG9015) in patients with recurrent gliomas. Results demonstrated a strong association between radiographic response using change in contrast enhancing tumor and BG9015 serum levels along with survival, again supporting the use of change in contrast enhancing tumor as a surrogate of early treatment efficacy. In a 1997 phase II multicenter study by Fetell et al137 exploring the efficacy of pre-irradiation paclitaxel in GBM, investigators noted that none of the 15 patients showed radiographic response as determined by change in contrast enhancing tumor size, suggesting to the investigators that paclitaxel has only minimal activity in GBM. In 1998, Chang et al138 described results from a phase II trial of high dose oral tamoxifen and subcutaneous interferon alpha-2a in recurrent gliomas, for which radiographic response was used as the primary endpoint. Despite extreme toxicity issues associated with the treatment resulting in early study closure, the majority (12 of 16) of evaluable patients showed early progressive disease indicative of treatment failure 6 weeks after therapy, suggesting to the authors that this treatment strategy is not effective in recurrent gliomas.
A 1999 phase II study of pre-irradiation carboplatin and etoposide and accelerated hyperfractionated radiation therapy in patients with high-grade astrocytomas by Jeremic et al93 again used radiographic response using change contrast enhancing tumor size on postcontrast CT as a surrogate for drug efficacy. Results of this study reported that no patients had complete shrinkage of enhancing tumor, and the lack of radiographic evidence of treatment effect resulted in study failure as reported by the investigators. In 1999, Yung et al139 reported results from a phase II trial of temozolomide in anaplastic gliomas at first relapse, again using radiographic response as a measure of drug efficacy. This study reported that radiographic response, as evaluated by a decrease in enhancing tumor volume, was associated with benefits to health-related quality of life. In line with this finding, a 1999 report by Hess et al140 summarized the relationship between radiographic response via a change in contrast enhancement and outcomes in 375 patients previously enrolled in 8 consecutive phase II chemotherapy trials. The investigators conclude that when response was treated as a covariate in a multivariate Cox proportional hazards regression model, contrast enhancing tumor shrinkage was associated with a significantly lower treatment failure rate.
In support of these observations, a 2000 report on a phase II trial of thalidomide in recurrent high-grade gliomas by Fine et al141 reported 4 of 36 patients had at least a partial radiographic response as determined by a change in contrast enhancement, while 12 had stable disease. Again in support of the use of change in contrast enhancing tumor size as a surrogate of treatment efficacy, median time to tumor progression (TTP) for nonresponders was approximately 8 weeks compared with 15 and 33 for stable and responding patients, respectively. Additionally, patients exhibiting radiographic response via a decrease in contrast enhancing tumor had a median OS of 74 weeks, whereas patients with stable or progressing disease had a survival of 30 and 22 weeks, respectively. In 2000, Yung et al142 reported results from a phase II study of temozolomide versus procarbazine in recurrent GBM, noting that radiographic response was higher in patients receiving temozolomide, which also corresponded to longer PFS and OS. A phase II study in 2002 by Kahn et al143 evaluating efficacy of extended low-dose temozolomide also used radiographic response via contrast enhancement to determine efficacy, in addition to PFS and OS. The choice to use these metrics was largely based on radiographic response rates initially observed in the phase I study.144
In 2003, Fine et al145 used objective radiographic response compared with historical controls as a measure of efficacy to evaluate thalidomide and carmustine in patients with recurrent high-grade gliomas as part of a phase II trial, noting that the favorable radiographic response was evidence of antitumor activity. A 2003 phase I study by Sampson et al146 evaluating intracerebral micro-infusion of a recombinant chimeric targeted toxin composed of an epidermal growth factor receptor binding ligand transforming growth factor alpha and pseudomonas exotoxin (PE-38) showed that patients with radiographic responses via contrast enhancing tumor had long-term survival advantages compared with patients exhibiting no signs of radiographic response. This was followed up by a study by Sampson et al147 showing that radiographic response as indicated by a shrinkage in contrast enhancing tumor corresponded directly with histologic evidence of response via biopsy as well as hypometabolism on positron emission tomography (PET).
In 2004, a series of both phase I and phase II trials examining irinotecan plus BCNU148,149 demonstrated “clear antitumor activity as measured by radiographic response,” again supporting the use of change in contrast enhancing tumor size as a measure of early drug efficacy in clinical trials. In 2005, Pipas et al150 reported low radiographic response rates, but high rates of radiographic stability in a phase II trial of paclitaxel and topotecan with filgrastim in patients with recurrent malignant gliomas, which the investigators used as evidence that this combination has only modest activity. In a 2005 phase II study of 131-I-labeled anti-tenascin 81C6 murine monoclonal antibody in patients with newly diagnosed malignant gliomas, regions showing an increase in contrast enhancement indicative of treatment failure were found to be hypermetabolic on PET, providing molecular imaging evidence that change in enhancing tumor often corresponds with nonresponsive tumor.151 Also in 2005, Galanis et al152 showed that median time to tumor progression was significantly longer in patients who demonstrated radiographic response via contrast enhancement compared with patients not showing a response (5.4 vs 2.3 mo) in a phase II trial of temsirolimus in recurrent GBM. Similarly, a phase I trial of irinotecan plus temozolomide in recurrent malignant gliomas by Reardon et al153 also showed that radiographic response via a decrease in contrast enhancing tumor size corresponded to a longer median time to tumor progression compared with patients who did not show a response. A phase II study of imatinib mesylate plus hydroxyurea in recurrent GBM also reported similar findings in terms of radiographic response rate and PFS.154
A 2007 study by Lustig et al155 explicitly examined whether imaging response via change in contrast enhancement on CT or MRI correlated with survival in 453 patients enrolled in RTOG 90-06, a phase I/II study to compare standard radiation with BCNU versus hyperfractionated radiation with BCNU in newly diagnosed malignant gliomas. Results of the study suggested that CT or MRI response was a significant predictor of median survival and 2-year OS, further establishing the relationship of response to treatment and survival in malignant gliomas. A 2010 phase II trial of intratumoral BCNU injection and radiotherapy on newly diagnosed malignant glioma156 used radiographic response via contrast enhancing tumor as the primary endpoint for the trial. The 2 of 12 patients who exhibited a partial response had PFS and OS of more than 5 years, which was substantially longer than the other patients (average, PFS = 9 mo, OS = 15 mo).
Bevacizumab and anti-angiogenic agents
As mentioned in single-center studies above, the use of bevacizumab and other anti-angiogenic agents often results in radiographic response as measured using contrast enhancement on postcontrast T1-weighted images. Generally speaking, most of the clinical trials involving bevacizumab or other anti-angiogenic agents have used radiographic response as either a primary or a secondary endpoint for the trial,157,158 but relatively few studies have explored whether radiographic response in bevacizumab-treated patients results in patient benefit or biological changes within the tumor associated with favorable drug activity. Of the studies that did examine these associations, the decrease in contrast enhancement can often be associated with increased drug activity in malignant gliomas. For example, a study in 2007 by Vredenburgh et al159 was one of the first phase II trials of bevacizumab in recurrent malignant gliomas, reporting only complete shrinkage of contrast enhancing tumor in 1 of 32 patients, but 19 of 32 patients exhibited a partial radiographic response via a decrease in contrast enhancement. Despite noting higher than expected radiographic response rates, the authors failed to mention whether an association was found between radiographic responders and prolonged survival. This was addressed in a 2009 phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan in recurrent tumors reported by Kreisl et al,160 which showed that early response on postcontrast MRI was predictive of long-term PFS (ie, longer local control of the disease).
A phase II trial of metronomic chemotherapy with daily oral etoposide plus bevacizumab for recurrent malignant gliomas demonstrated that patients with sustained radiographic response had a favorable prognosis, including no evidence of hypermetabolic tumor on PET scans. In a phase II trial of cetuximab, bevacizumab, and irinotecan for patients with primary and recurrent GBM,161 approximately 26% (11 of 32 patients) showed some favorable radiographic response, which translated to a significant difference in TTP. A 2010 phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma162 also showed a significant correlation between radiographic response and PFS in GBM along with a significant correlation between radiographic response and both PFS and OS in anaplastic astrocytomas. In 2011, Prados et al163 performed landmark analysis to evaluate the association between radiographic response as measured by a change in contrast enhancing tumor at 9, 18, or 26 weeks and OS for 167 patients with recurrent GBM who participated in the BRAIN trial, a phase II trial evaluating the efficacy of bevacizumab alone or in combination with irinotecan. After correcting for known prognostic factors, including age, neurological status, number of prior relapses, and treatment arms, investigators clearly showed that radiographic response measured at each time point evaluated (9, 18, and 26 wk) predicted OS.
A 2011 study by Wefel et al164 also examined the same patients in the BRAIN trial and found that patients who exhibited radiographic response as indicated by a decrease in contrast enhancing tumor size had stable or improved performance on a variety of neurocognitive tests at the time of the response as well as 24 weeks from the start of therapy. In 2013, Boxerman et al165 examined whether radiographic response as indicated by a change in contrast enhancing tumor size (volume and bidirectional) was predictive of OS in ACRIN 6677/RTOG 0625, a multicenter, randomized, phase II trial of bevacizumab with irinotecan or temozolomide in recurrent GBM. Results suggested that patients with stable or partial response at 8 or 16 weeks had significantly longer survival compared with those who showed growing contrast enhancing tumor. Lastly, a 2014 study by Ellingson et al98 reexamined the BRAIN trial data using contrast enhanced digital subtraction of precontrast T1-weighted images from postcontrast T1-weighted images to highlight areas of subtle enhancement. Results of this study clearly indicated that contrast enhancement was apparent in almost all patients when using T1 subtraction, suggesting that the high radiographic response rates previously reported in bevacizumab may have lacked sensitivity for detecting subtle enhancement in the underlying tissue. Further, results indicated that patients exhibiting >25% decrease in contrast enhancing tumor volume after the first dose of bevacizumab had significantly longer PFS and OS. A follow-up study166 subsequently showed that prediction of PFS and OS could be improved by including a second confirmatory scan, again using T1 subtraction to better delineate areas of true contrast enhancement from surrounding tissues.
The radiographic response as measured by a change in contrast enhancing tumor size has not been appreciable for anti-angiogenic agents other than bevacizumab. In a phase II trial of pazopanib,167 an oral multitargeted angiogenesis inhibitor in recurrent GBM, radiographic response was low (2 partial responses out of 35 patients), which translated to equally low PFS and OS. In 2010, Batchelor et al168 presented results from a phase II study of cediranib, an oral pan-VEGF receptor tyrosine kinase inhibitor, in recurrent GBM. Results showed that patients exhibiting a partial or minor radiographic response via contrast enhancement corresponded with longer PFS as well as improvement in Karnofsky performance status. In 2011, de Groot et al169 described results from a phase II trial of aflibercept, a VEGF trap, in recurrent malignant glioma, noting that patients with anaplastic gliomas or GBM exhibiting a partial radiographic response had longer median PFS compared with the entire study cohort stratified by grade (23 wk vs 12 wk for GBM; 45 wk vs 24 wk for anaplastic gliomas).
In summary, a very large number of phase I–III clinical trials, including additional noteworthy studies not described in detail,129,130,169–189 have used radiographic response via change in contrast enhancement as a measure of drug efficacy or as a primary or secondary endpoint and/or have shown a clear relationship between radiographic response and outcomes, including histologic response, metabolic changes, patient reported outcomes, quality of life measures, PFS, or OS.
Limitations to Interpretation
It is important to note that other clinical, genetic, neurological, and medical factors (isocitrate dehydrogenase status, O6-methylguanine-DNA methyltransferase methylation status, age, performance status, prior therapies, etc.) may have significantly impacted outcomes and may not have been considered at the time of the initial studies. Additionally, many early studies had differing eligibility requirements, which may have biased recurrent studies by allowing enrollment or identifying early progression in patients with potential pseudoprogression, as well as slightly different definitions for disease progression, which may slightly adjust measures of TTP or PFS. Also, many studies may not have used centralized, blinded review, which may account for some additional discrepancies. Despite these and other potential caveats, including radiation and treatment effects that can mimic disease (summarized by Ellingson et al190), there appears to be overwhelming evidence to suggest that contrast enhancement is a significant biomarker for baseline risk stratification and as a posttherapeutic measure of drug efficacy.
Conclusion
Altogether, there is an abundance of scientific evidence spanning decades supporting the use of contrast enhancement as a surrogate biomarker for disease burden and as a tool for measuring treatment response in malignant glioma. The current article explicitly describes multiple levels of verification, or evidentiary standards, to support the use of contrast enhancement in these specific contexts of use. This comprehensive review should be valuable for regulatory institutions involved in drug approval and for use in the biomarker qualification process.
Funding
None.
Conflict of interest statement. Consultant and/or Advisory Role: B. Ellingson: MedQIA, Roche/Genentech, Agios, Insys, OmniOx, Nativis, BMS, Siemens, Janssen, Medicenna, Imaging Endpoints, Novogen; P. Wen: Agios, Genentech/Roche, Cavion, Cortice Biosciences, Foundation Medicine, Insys, Monteris, Novartis, Vascular Biogenics, VBI Vaccines; T. Cloughesy: Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, MedQIA.
References
- 1. Kirkpatrick DB. The first primary brain-tumor operation. J Neurosurg. 1984;61(5):809–813. [DOI] [PubMed] [Google Scholar]
- 2. Pfahler G. Cerebral skiagraphy: transactions of the American Roentgen Ray Society—5th Annual Meeting. Am J Roentgenol Radium Ther Nucl Med. 1904;4:174–186. [Google Scholar]
- 3. Kerr PB, Caputy AJ, Horwitz NH. A history of cerebral localization. Neurosurg Focus. 2005;18(4):e1. [DOI] [PubMed] [Google Scholar]
- 4. Scott WG, Seaman WB. Developments in cerebral angiography with rapid serialized X-ray exposures on roll film 9½ inches wide. Radiology. 1951;56(1):15–30. [DOI] [PubMed] [Google Scholar]
- 5. Moore RC. Cerebral arteriography in general hospital practice. Radiology. 1951;57(4):487–499. [DOI] [PubMed] [Google Scholar]
- 6. NEW PF. Carotid angiography in the localisation of supratentorial neoplasms and hamartomata. Radiology. 1959;72(1):35–41. [DOI] [PubMed] [Google Scholar]
- 7. Baker HL Jr, Houser OW, Campbell JK. National Cancer Institute study: evaluation of computed tomography in the diagnosis of intracranial neoplasms. I. Overall results. Radiology. 1980;136(1):91–96. [DOI] [PubMed] [Google Scholar]
- 8. Brismar J, Strömblad LG, Salford LG. Impact of CT in the neurosurgical management of intracranial tumors. Neuroradiology. 1978;16:506–509. [DOI] [PubMed] [Google Scholar]
- 9. Salcman M. Glioblastoma multiforme. Am J Med Sci. 1980;279(2):84–94. [DOI] [PubMed] [Google Scholar]
- 10. Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171(3976):1151–1153. [DOI] [PubMed] [Google Scholar]
- 11. Lauterbur PC. Image formation by induced interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 12. Mansfield P. Multi-planar image formation using NMR spin echoes. J Phys C Solid State PHys. 1977;10(3):L55. [Google Scholar]
- 13. Carr DH, Brown J, Bydder GM et al. Intravenous chelated gadolinium as a contrast agent in NMR imaging of cerebral tumours. Lancet. 1984;1(8375):484–486. [DOI] [PubMed] [Google Scholar]
- 14. Felix R, Schörner W, Laniado M et al. Brain tumors: MR imaging with gadolinium-DTPA. Radiology. 1985;156(3):681–688. [DOI] [PubMed] [Google Scholar]
- 15. Graif M, Bydder GM, Steiner RE, Niendorf P, Thomas DG, Young IR. Contrast-enhanced MR imaging of malignant brain tumors. AJNR Am J Neuroradiol. 1985;6(6):855–862. [PMC free article] [PubMed] [Google Scholar]
- 16. Earnest F 4th, Kelly PJ, Scheithauer BW et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988;166(3):823–827. [DOI] [PubMed] [Google Scholar]
- 17. Haughton VM, Rimm AA, Czervionke LF et al. Sensitivity of Gd-DTPA-enhanced MR imaging of benign extraaxial tumors. Radiology. 1988;166(3):829–833. [DOI] [PubMed] [Google Scholar]
- 18. Dean BL, Drayer BP, Bird CR et al. Gliomas: classification with MR imaging. Radiology. 1990;174(2):411–415. [DOI] [PubMed] [Google Scholar]
- 19. Claussen C, Laniado M, Kazner E, Schörner W, Felix R. Application of contrast agents in CT and MRI (NMR): their potential in imaging of brain tumors. Neuroradiology. 1985;27(2):164–171. [DOI] [PubMed] [Google Scholar]
- 20. Butler AR, Horii SC, Kricheff II, Shannon MB, Budzilovich GN. Computed tomography in astrocytomas. A statistical analysis of the parameters of malignancy and the positive contrast-enhanced CT scan. Radiology. 1978;129(2):433–439. [DOI] [PubMed] [Google Scholar]
- 21. Lewander R, Bergström M, Boëthius J et al. Stereotactic computer tomography for biopsy of gliomas. Acta Radiol Diagn (Stockh). 1978;19(6):867–888. [DOI] [PubMed] [Google Scholar]
- 22. Amundsen P, Dugstad G, Syvertsen AH. The reliability of computer tomography for the diagnosis and differential diagnosis of meningiomas, gliomas, and brain metastases. Acta Neurochir (Wien). 1978;41(1-3):177–190. [DOI] [PubMed] [Google Scholar]
- 23. Burger PC. Pathologic anatomy and CT correlations in the glioblastoma multiforme. Appl Neurophysiol. 1983;46(1-4):180–187. [PubMed] [Google Scholar]
- 24. Burger PC, Dubois PJ, Schold SC Jr et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58(2):159–169. [DOI] [PubMed] [Google Scholar]
- 25. Lilja A, Bergström K, Spännare B, Olsson Y. Reliability of computed tomography in assessing histopathological features of malignant supratentorial gliomas. J Comput Assist Tomogr. 1981;5(5):625–636. [PubMed] [Google Scholar]
- 26. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 27. Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB. Stereotactic histologic correlations of computed tomography– and magnetic resonance imaging–defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62(6):450–459. [DOI] [PubMed] [Google Scholar]
- 28. Burger PC, Heinz ER, Shibata T, Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988;68(5):698–704. [DOI] [PubMed] [Google Scholar]
- 29. Barajas RF Jr, Hodgson JG, Chang JS et al. Glioblastoma multiforme regional genetic and cellular expression patterns: influence on anatomic and physiologic MR imaging. Radiology. 2010;254(2):564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barajas RF Jr, Phillips JJ, Parvataneni R et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro Oncol. 2012;14(7):942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kubben PL, Wesseling P, Lammens M et al. Correlation between contrast enhancement on intraoperative magnetic resonance imaging and histopathology in glioblastoma. Surg Neurol Int. 2012;3:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reeves GI, Marks JE. Prognostic significance of lesion size for glioblastoma multiforme. Radiology. 1979;132(2):469–471. [DOI] [PubMed] [Google Scholar]
- 33. Andreou J, George AE, Wise A et al. CT prognostic criteria of survival after malignant glioma surgery. AJNR Am J Neuroradiol. 1983;4(3):488–490. [PMC free article] [PubMed] [Google Scholar]
- 34. Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21(2):201–206. [DOI] [PubMed] [Google Scholar]
- 35. Wood JR, Green SB, Shapiro WR. The prognostic importance of tumor size in malignant gliomas: a computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6(2):338–343. [DOI] [PubMed] [Google Scholar]
- 36. Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ. The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53(6):466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Curran WJ Jr, Scott CB, Horton J et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. [DOI] [PubMed] [Google Scholar]
- 38. Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78(5):767–775. [DOI] [PubMed] [Google Scholar]
- 39. Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34(1):45–60; discussion 60. [DOI] [PubMed] [Google Scholar]
- 40. Laws ER, Parney IF, Huang W et al. ; Glioma Outcomes Investigators Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 41. McGirt MJ, Chaichana KL, Gathinji M et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110(1):156–162. [DOI] [PubMed] [Google Scholar]
- 42. Bauchet L, Mathieu-Daudé H, Fabbro-Peray P et al. ; Société Française de Neurochirurgie (SFNC); Club de Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC); Société Française de Neuropathologie (SFNP); Association des Neuro-Oncologues d’Expression Française (ANOCEF) Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J, Wang M, Won M et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 45. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oppenlander ME, Wolf AB, Snyder LA et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 47. Ho J, Ondos J, Ning H et al. Chemoirradiation for glioblastoma multiforme: the national cancer institute experience. PLoS One. 2013;8(8):e70745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gutman DA, Cooper LA, Hwang SN et al. MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology. 2013;267(2):560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zinn PO, Colen RR, Kasper EM, Burkhardt JK. Extent of resection and radiotherapy in GBM: a 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol. 2013;42(3):929–934. [DOI] [PubMed] [Google Scholar]
- 50. Pan IW, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: a USA population-based study from 2000–2010. J Clin Neurosci. 2015;22(10):1575–1581. [DOI] [PubMed] [Google Scholar]
- 51. Qin JJ, Liu ZX, Wang JM et al. Prognostic factors influencing clinical outcomes of malignant glioblastoma multiforme: clinical, immunophenotypic, and fluorescence in situ hybridization findings for 1p19q in 816 chinese cases. Asian Pac J Cancer Prev. 2015;16(3):971–977. [DOI] [PubMed] [Google Scholar]
- 52. Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996;27(1):65–73. [DOI] [PubMed] [Google Scholar]
- 53. Lacroix M, Abi-Said D, Fourney DR et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 54. Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D?AJNR Am J Neuroradiol. 2005;26(4):770–776. [PMC free article] [PubMed] [Google Scholar]
- 55. Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol. 2011;13(12):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carrillo JA, Lai A, Nghiemphu PL et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol. 2012;33(7):1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leu K, Enzmann DR, Woodworth DC et al. Hypervascular tumor volume estimated by comparison to a large-scale cerebral blood volume radiographic atlas predicts survival in recurrent glioblastoma treated with bevacizumab. Cancer Imaging. 2014;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Litofsky NS, Bauer AM, Kasper RS, Sullivan CM, Dabbous OH; Glioma Outcomes Project Investigators Image-guided resection of high-grade glioma: patient selection factors and outcome. Neurosurg Focus. 2006;20(4):E16. [DOI] [PubMed] [Google Scholar]
- 60. Vidiri A, Carapella CM, Pace A et al. Early post-operative MRI: correlation with progression-free survival and overall survival time in malignant gliomas. J Exp Clin Cancer Res. 2006;25(2):177–182. [PubMed] [Google Scholar]
- 61. Stummer W, Reulen HJ, Meinel T et al. ; ALA-Glioma Study Group Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576; discussion 564. [DOI] [PubMed] [Google Scholar]
- 62. Senft C, Franz K, Blasel S et al. Influence of iMRI-guidance on the extent of resection and survival of patients with glioblastoma multiforme. Technol Cancer Res Treat. 2010;9(4):339–346. [DOI] [PubMed] [Google Scholar]
- 63. Ramakrishna R, Barber J, Kennedy G et al. Imaging features of invasion and preoperative and postoperative tumor burden in previously untreated glioblastoma: correlation with survival. Surg Neurol Int. 2010;1: doi: 10.4103/2152-7806.68337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ewelt C, Goeppert M, Rapp M, Steiger HJ, Stummer W, Sabel M. Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neurooncol. 2011;103(3):611–618. [DOI] [PubMed] [Google Scholar]
- 65. Ngwenya LB, Chiocca EA. Extent of resection. J Neurosurg. 2011;115(1):1–2; discussion 2. [DOI] [PubMed] [Google Scholar]
- 66. Zachenhofer I, Maier R, Eiter H et al. Overall survival and extent of surgery in adult versus elderly glioblastoma patients: a population based retrospective study. Wien Klin Wochenschr. 2011;123(11-12):364–368. [DOI] [PubMed] [Google Scholar]
- 67. Oszvald A, Güresir E, Setzer M et al. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. J Neurosurg. 2012;116(2):357–364. [DOI] [PubMed] [Google Scholar]
- 68. Murakami R, Hirai T, Nakamura H et al. Recurrence patterns of glioblastoma treated with postoperative radiation therapy: relationship between extent of resection and progression-free interval. Jpn J Radiol. 2012;30(3):193–197. [DOI] [PubMed] [Google Scholar]
- 69. Salvati M, Pichierri A, Piccirilli M et al. Extent of tumor removal and molecular markers in cerebral glioblastoma: a combined prognostic factors study in a surgical series of 105 patients. J Neurosurg. 2012;117(2):204–211. [DOI] [PubMed] [Google Scholar]
- 70. Raysi Dehcordi S, De Paulis D, Marzi S et al. Survival prognostic factors in patients with glioblastoma: our experience. J Neurosurg Sci. 2012;56(3):239–245. [PubMed] [Google Scholar]
- 71. Langsenlehner T, Groll MJ, Quehenberger F, Payer F, Mokry M, Kapp KS. Interdisciplinary treatment of glioblastoma: analysis of prognostic factors and treatment results in unselected patients. Neoplasma. 2012;59(6):662–668. [DOI] [PubMed] [Google Scholar]
- 72. Patil CG, Yi A, Elramsisy A et al. Prognosis of patients with multifocal glioblastoma: a case-control study. J Neurosurg. 2012;117(4):705–711. [DOI] [PubMed] [Google Scholar]
- 73. Dea N, Fournier-Gosselin MP, Mathieu D, Goffaux P, Fortin D. Does extent of resection impact survival in patients bearing glioblastoma?Can J Neurol Sci. 2012;39(5):632–637. [DOI] [PubMed] [Google Scholar]
- 74. Orringer D, Lau D, Khatri S et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117(5):851–859. [DOI] [PubMed] [Google Scholar]
- 75. Bloch O, Han SJ, Cha S et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117(6):1032–1038. [DOI] [PubMed] [Google Scholar]
- 76. Smets T, Lawson TM, Grandin C, Jankovski A, Raftopoulos C. Immediate post-operative MRI suggestive of the site and timing of glioblastoma recurrence after gross total resection: a retrospective longitudinal preliminary study. Eur Radiol. 2013;23(6):1467–1477. [DOI] [PubMed] [Google Scholar]
- 77. Ahmadloo N, Kani AA, Mohammadianpanah M et al. Treatment outcome and prognostic factors of adult glioblastoma multiforme. J Egypt Natl Canc Inst. 2013;25(1):21–30. [DOI] [PubMed] [Google Scholar]
- 78. Field KM, Rosenthal MA, Yilmaz M, Tacey M, Drummond K. Comparison between poor and long-term survivors with glioblastoma: review of an Australian dataset. Asia Pac J Clin Oncol. 2014;10(2):153–161. [DOI] [PubMed] [Google Scholar]
- 79. Daigle K, Fortin D, Mathieu D et al. Effects of surgical resection on the evolution of quality of life in newly diagnosed patients with glioblastoma: a report on 19 patients surviving to follow-up. Curr Med Res Opin. 2013;29(10):1307–1313. [DOI] [PubMed] [Google Scholar]
- 80. Hrabalek L, Kalita O, Vaverka M et al. Resection versus biopsy of glioblastomas in eloquent brain areas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(1):150–155. [DOI] [PubMed] [Google Scholar]
- 81. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coburger J, Hagel V, Wirtz CR, König R. Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS One. 2015;10(6):e0131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Coburger J, Wirtz CR, König RW. Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field magnetic resonance imaging. J Neurosurg Sci. 2017;61(3):233–244. [DOI] [PubMed] [Google Scholar]
- 84. Majós C, Cos M, Castañer S et al. Early post-operative magnetic resonance imaging in glioblastoma: correlation among radiological findings and overall survival in 60 patients. Eur Radiol. 2016;26(4):1048–1055. [DOI] [PubMed] [Google Scholar]
- 85. Gehan EA, Walker MD. Prognostic factors for patients with brain tumors. Natl Cancer Inst Monogr. 1977;46:189–195. [PubMed] [Google Scholar]
- 86. Kiwit JC, Floeth FW, Bock WJ. Survival in malignant glioma: analysis of prognostic factors with special regard to cytoreductive surgery. Zentralbl Neurochir. 1996;57(2):76–88. [PubMed] [Google Scholar]
- 87. Sawaya R. Extent of resection in malignant gliomas: a critical summary. J Neurooncol. 1999;42(3):303–305. [DOI] [PubMed] [Google Scholar]
- 88. Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71(4):487–493. [DOI] [PubMed] [Google Scholar]
- 89. Jakacki RI, Siffert J, Jamison C, Velasquez L, Allen JC. Dose-intensive, time-compressed procarbazine, CCNU, vincristine (PCV) with peripheral blood stem cell support and concurrent radiation in patients with newly diagnosed high-grade gliomas. J Neurooncol. 1999;44(1):77–83. [DOI] [PubMed] [Google Scholar]
- 90. Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved?World Neurosurg. 2014;82(1-2):e257–e265. [DOI] [PubMed] [Google Scholar]
- 91. Grabowski MM, Recinos PF, Nowacki AS et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 92. Rostomily RC, Spence AM, Duong D, McCormick K, Bland M, Berger MS. Multimodality management of recurrent adult malignant gliomas: results of a phase II multiagent chemotherapy study and analysis of cytoreductive surgery. Neurosurgery. 1994;35(3):378–388; discussion 388. [DOI] [PubMed] [Google Scholar]
- 93. Jeremic B, Shibamoto Y, Grujicic D et al. Pre-irradiation carboplatin and etoposide and accelerated hyperfractionated radiation therapy in patients with high-grade astrocytomas: a phase II study. Radiother Oncol. 1999;51(1):27–33. [DOI] [PubMed] [Google Scholar]
- 94. Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien). 2003;145(1):5–10. [DOI] [PubMed] [Google Scholar]
- 95. Hauch H, Sajedi M, Wolff JE. Treatment arms summarizing analysis of 220 high-grade glioma studies. Anticancer Res. 2005;25(5):3585–3590. [PubMed] [Google Scholar]
- 96. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 97. Suchorska B, Weller M, Tabatabai G et al. 136 complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma results from the DIRECTOR trial. Neurosurgery. 2015;62(Suppl 1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ellingson BM, Kim HJ, Woodworth DC et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ellingson BM, Kim E, Woodworth DC et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int J Oncol. 2015;46(5):1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ellingson BM, Harris RJ, Woodworth DC et al. Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 102. Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47(3):329–335. [DOI] [PubMed] [Google Scholar]
- 103. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. [DOI] [PubMed] [Google Scholar]
- 104. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 105. Cairncross G, Wang M, Shaw E et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 107. Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008;29(3):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5(11):634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s Criteria. J Clin Oncol. 2009;27(18):2905–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mehta AI, Kanaly CW, Friedman AH, Bigner DD, Sampson JH. Monitoring radiographic brain tumor progression. Toxins (Basel). 2011;3(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13(5):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep. 2011;11(3):336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13(1):50–56. [DOI] [PubMed] [Google Scholar]
- 114. Pope WB, Hessel C. Response assessment in neuro-oncology criteria: implementation challenges in multicenter neuro-oncology trials. AJNR Am J Neuroradiol. 2011;32(5):794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Reardon DA, Galanis E, DeGroot JF et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Khan SN, Linetsky M, Ellingson BM, Pope WB. Magnetic resonance imaging of glioma in the era of antiangiogenic therapy. PET Clin. 2013;8(2):163–182. [DOI] [PubMed] [Google Scholar]
- 117. McCullough DC, Huang HK, DeMichelle D, Manz HJ, Sinks LF. Correlation between volumetric CT imaging and autopsy measurements of glioblastoma size. Comput Tomogr. 1979;3(2):133–141. [DOI] [PubMed] [Google Scholar]
- 118. Kumar PP, Good RR, Jones EO, Leibrock LG. Intraoperative cobalt-60 treatment of glioblastoma multiforme. Radiat Med. 1988;6(5):219–228. [PubMed] [Google Scholar]
- 119. Finlay JL, August C, Packer R et al. High-dose multi-agent chemotherapy followed by bone marrow ‘rescue’ for malignant astrocytomas of childhood and adolescence. J Neurooncol. 1990;9(3):239–248. [DOI] [PubMed] [Google Scholar]
- 120. Couldwell WT, Weiss MH, DeGiorgio CM et al. Clinical and radiographic response in a minority of patients with recurrent malignant gliomas treated with high-dose tamoxifen. Neurosurgery. 1993;32(3):485–489; discussion 489. [DOI] [PubMed] [Google Scholar]
- 121. See SJ, Levin VA, Yung WK, Hess KR, Groves MD. 13-cis-retinoic acid in the treatment of recurrent glioblastoma multiforme. Neuro Oncol. 2004;6(3):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Patel M, Siddiqui F, Jin JY et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009;92(2):185–191. [DOI] [PubMed] [Google Scholar]
- 123. Gladwish A, Koh ES, Hoisak J et al. Evaluation of early imaging response criteria in glioblastoma multiforme. Radiat Oncol. 2011;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. [DOI] [PubMed] [Google Scholar]
- 125. Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008;112(10):2267–2273. [DOI] [PubMed] [Google Scholar]
- 126. Norden AD, Young GS, Setayesh K et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 127. Zuniga RM, Torcuator R, Jain R et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91(3):329–336. [DOI] [PubMed] [Google Scholar]
- 128. Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96(2):259–269. [DOI] [PubMed] [Google Scholar]
- 129. Huang RY, Rahman R, Hamdan A et al. Recurrent glioblastoma: volumetric assessment and stratification of patient survival with early posttreatment magnetic resonance imaging in patients treated with bevacizumab. Cancer. 2013;119(19):3479–3488. [DOI] [PubMed] [Google Scholar]
- 130. Desjardins A, Quinn JA, Vredenburgh JJ et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83(1):53–60. [DOI] [PubMed] [Google Scholar]
- 131. Kreisl TN, Lassman AB, Mischel PS et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM). J Neurooncol. 2009;92(1):99–105. [DOI] [PubMed] [Google Scholar]
- 132. Kreisl TN, Smith P, Sul J et al. Continuous daily sunitinib for recurrent glioblastoma. J Neurooncol. 2013;111(1):41–48. [DOI] [PubMed] [Google Scholar]
- 133. Greenberg HS, Ensminger WD, Layton PB et al. Phase I-II evaluation of intra-arterial diaziquone for recurrent malignant astrocytomas. Cancer Treat Rep. 1986;70(3):353–357. [PubMed] [Google Scholar]
- 134. Macdonald D, Cairncross G, Stewart D et al. Phase II study of topotecan in patients with recurrent malignant glioma. National Clinical Institute of Canada Clinical Trials Group. Ann Oncol. 1996;7(2):205–207. [DOI] [PubMed] [Google Scholar]
- 135. Hochberg F, Prados M, Russell C et al. Treatment of recurrent malignant glioma with BCNU-fluosol and oxygen inhalation. A phase I-II study. J Neurooncol. 1997;32(1):45–55. [DOI] [PubMed] [Google Scholar]
- 136. Fine HA, Wen PY, Robertson M et al. A phase I trial of a new recombinant human beta-interferon (BG9015) for the treatment of patients with recurrent gliomas. Clin Cancer Res. 1997;3(3):381–387. [PubMed] [Google Scholar]
- 137. Fetell MR, Grossman SA, Fisher JD et al. Preirradiation paclitaxel in glioblastoma multiforme: efficacy, pharmacology, and drug interactions. New Approaches to Brain Tumor Therapy Central Nervous System Consortium. J Clin Oncol. 1997;15(9):3121–3128. [DOI] [PubMed] [Google Scholar]
- 138. Chang SM, Barker FG 2nd, Huhn SL et al. High dose oral tamoxifen and subcutaneous interferon alpha-2a for recurrent glioma. J Neurooncol. 1998;37(2):169–176. [DOI] [PubMed] [Google Scholar]
- 139. Yung WK, Prados MD, Yaya-Tur R et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17(9):2762–2771. [DOI] [PubMed] [Google Scholar]
- 140. Hess KR, Wong ET, Jaeckle KA et al. Response and progression in recurrent malignant glioma. Neuro Oncol. 1999;1(4):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fine HA, Figg WD, Jaeckle K et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18(4):708–715. [DOI] [PubMed] [Google Scholar]
- 142. Yung WK, Albright RE, Olson J et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4(1):39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Brock CS, Newlands ES, Wedge SR et al. Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res. 1998;58(19):4363–4367. [PubMed] [Google Scholar]
- 145. Fine HA, Wen PY, Maher EA et al. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J Clin Oncol. 2003;21(12):2299–2304. [DOI] [PubMed] [Google Scholar]
- 146. Sampson JH, Akabani G, Archer GE et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65(1):27–35. [DOI] [PubMed] [Google Scholar]
- 147. Sampson JH, Reardon DA, Friedman AH et al. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro Oncol. 2005;7(1):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Quinn JA, Reardon DA, Friedman AH et al. Phase 1 trial of irinotecan plus BCNU in patients with progressive or recurrent malignant glioma. Neuro Oncol. 2004;6(2):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Reardon DA, Quinn JA, Rich JN et al. Phase 2 trial of BCNU plus irinotecan in adults with malignant glioma. Neuro Oncol. 2004;6(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pipas JM, Meyer LP, Rhodes CH et al. A Phase II trial of paclitaxel and topotecan with filgrastim in patients with recurrent or refractory glioblastoma multiforme or anaplastic astrocytoma. J Neurooncol. 2005;71(3):301–305. [DOI] [PubMed] [Google Scholar]
- 151. Akabani G, Reardon DA, Coleman RE et al. Dosimetry and radiographic analysis of 131I-labeled anti-tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: a phase II study. J Nucl Med. 2005;46(6):1042–1051. [PubMed] [Google Scholar]
- 152. Galanis E, Buckner JC, Maurer MJ et al. ; North Central Cancer Treatment Group Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. [DOI] [PubMed] [Google Scholar]
- 153. Reardon DA, Quinn JA, Rich JN et al. Phase I trial of irinotecan plus temozolomide in adults with recurrent malignant glioma. Cancer. 2005;104(7):1478–1486. [DOI] [PubMed] [Google Scholar]
- 154. Reardon DA, Egorin MJ, Quinn JA et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23(36):9359–9368. [DOI] [PubMed] [Google Scholar]
- 155. Lustig RA, Seiferheld W, Berkey B et al. Imaging response in malignant glioma, RTOG 90-06. Am J Clin Oncol. 2007;30(1):32–37. [DOI] [PubMed] [Google Scholar]
- 156. Jenkinson MD, Smith TS, Haylock B et al. Phase II trial of intratumoral BCNU injection and radiotherapy on untreated adult malignant glioma. J Neurooncol. 2010;99(1):103–113. [DOI] [PubMed] [Google Scholar]
- 157. Raizer JJ, Grimm S, Chamberlain MC et al. A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer. 2010;116(22):5297–5305. [DOI] [PubMed] [Google Scholar]
- 158. Gerstner ER, Eichler AF, Plotkin SR et al. Phase I trial with biomarker studies of vatalanib (PTK787) in patients with newly diagnosed glioblastoma treated with enzyme inducing anti-epileptic drugs and standard radiation and temozolomide. J Neurooncol. 2011;103(2):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 160. Kreisl TN, Kim L, Moore K et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hasselbalch B, Lassen U, Hansen S et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12(5):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sathornsumetee S, Desjardins A, Vredenburgh JJ et al. Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol. 2010;12(12):1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Prados M, Cloughesy T, Samant M et al. Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wefel JS, Cloughesy T, Zazzali JL et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(6):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Boxerman JL, Zhang Z, Safriel Y et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013;15(7):945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ellingson BM, Kim HJ, Woodworth DC et al. Contrast-enhanced T1-weighted subtraction maps for response assessment on recurrent glioblastoma treated with bevacizumab. J Clin Oncol. 2013;31:abstr 2055. [Google Scholar]
- 167. Iwamoto FM, Lamborn KR, Robins HI et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol. 2010;12(8):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Batchelor TT, Duda DG, di Tomaso E et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. de Groot JF, Lamborn KR, Chang SM et al. Phase II study of aflibercept in recurrent malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2011;29(19):2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Reardon DA, Quinn JA, Vredenburgh J et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005;103(2):329–338. [DOI] [PubMed] [Google Scholar]
- 171. Reardon DA, Quinn JA, Vredenburgh JJ et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12(3 Pt 1):860–868. [DOI] [PubMed] [Google Scholar]
- 172. Mamelak AN, Rosenfeld S, Bucholz R et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol. 2006;24(22):3644–3650. [DOI] [PubMed] [Google Scholar]
- 173. Badruddoja MA, Penne K, Desjardins A et al. Phase II study of Cloretazine for the treatment of adults with recurrent glioblastoma multiforme. Neuro Oncol. 2007;9(1):70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Fine HA, Kim L, Albert PS et al. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13(23):7101–7106. [DOI] [PubMed] [Google Scholar]
- 175. Sampson JH, Akabani G, Archer GE et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10(3):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Reardon DA, Fink KL, Mikkelsen T et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34): 5610–5617. [DOI] [PubMed] [Google Scholar]
- 177. Reardon DA, Desjardins A, Vredenburgh JJ et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol. 2010;96(2):219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Reardon DA, Dresemann G, Taillibert S et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101(12):1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kreisl TN, Kotliarova S, Butman JA et al. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Reardon DA, Vredenburgh JJ, Desjardins A et al. Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol. 2011;101(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Iwamoto FM, Lamborn KR, Kuhn JG et al. A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro Oncol. 2011;13(5):509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Torok JA, Wegner RE, Mintz AH, Heron DE, Burton SA. Re-irradiation with radiosurgery for recurrent glioblastoma multiforme. Technol Cancer Res Treat. 2011;10(3):253–258. [DOI] [PubMed] [Google Scholar]
- 183. Reardon DA, Vredenburgh JJ, Coan A et al. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105(3):621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kreisl TN, McNeill KA, Sul J, Iwamoto FM, Shih J, Fine HA. A phase I/II trial of vandetanib for patients with recurrent malignant glioma. Neuro Oncol. 2012;14(12):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hu J, Wen PY, Abrey LE et al. A phase II trial of oral gimatecan for recurrent glioblastoma. J Neurooncol. 2013;111(3):347–353. [DOI] [PubMed] [Google Scholar]
- 186. Lou E, Peters KB, Sumrall AL et al. Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med. 2013;2(2):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gerstner ER, Ye X, Duda DG et al. A phase I study of cediranib in combination with cilengitide in patients with recurrent glioblastoma. Neuro Oncol. 2015;17(10):1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Wen PY, Schiff D, Cloughesy TF et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13(4):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33(4):1657–1660. [PubMed] [Google Scholar]
- 190. Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;doi: 10.1007/s11060-017-2375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bauman G, Lote K, Larson D et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45(4):923–929. [DOI] [PubMed] [Google Scholar]
- 192. Jeremic B, Grujicic D, Antunovic V, Djuric L, Stojanovic M, Shibamoto Y. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21(2):177–185. [DOI] [PubMed] [Google Scholar]
- 193. Nitta T, Sato K. Prognostic implications of the extent of surgical resection in patients with intracranial malignant gliomas. Cancer. 1995;75(11):2727–2731. [DOI] [PubMed] [Google Scholar]
- 194. Iliadis G, Kotoula V, Chatzisotiriou A et al. Volumetric and MGMT parameters in glioblastoma patients: survival analysis. BMC Cancer. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Li WB, Tang K, Chen Q et al. MRI manifestions correlate with survival of glioblastoma multiforme patients. Cancer Biol Med. 2012;9(2):120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Friedman HS, Prados MD, Wen PY et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 197. Lamborn KR, Yung WK, Chang SM et al. ; North American Brain Tumor Consortium Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ballman KV, Buckner JC, Brown PD et al. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Wen PY, Prados MD, Schiff D et al. Phase II study of XL184 (BMS 907351), an inhibitor of MET, VEGFR2, and RET, in patients (pts) with progressive glioblastoma (GB). J Clin Oncol. 2010;28(15s); abstr 2006. [Google Scholar]
- 200. Han K, Ren M, Wick W et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro Oncol. 2014;16(5):696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Wick W, Puduvalli VK, Chamberlain MC et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Batchelor TT, Mulholland P, Neyns B et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Chinot OL, Wick W, Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2049. [DOI] [PubMed] [Google Scholar]
- 204. Chinot OL, Wick W, Mason W et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]