Introduction
Patients with mycosis fungoides (MF) are at a greater risk (relative risk, 1.73; range, 1.32-2.4) to have secondary malignancies than healthy subjects, and co-occurrence of cutaneous T-cell lymphoma with other hematoproliferative diseases have been reported.1, 2 Chronic myelomonocytic leukemia (CMML) is a rare myeloproliferative disease with features of myelodysplastic syndrome and myeloproliferative disorders leading to monocytosis in the peripheral blood.3 Cutaneous manifestations of CMML have been reported and seem to be associated with a poor prognosis and more likely progression to acute myeloid leukemia.4, 5, 6 Specific infiltrates in myeloproliferative disorders may clinically and histologically be difficult to distinguish from neutrophilic dermatoses, and neoplastic cells might accompany inflammatory or infectious dermatoses, as reported for B-cell chronic lymphocytic leukemia.7, 8, 9, 10, 11 We describe a case of CMML coinciding with MF presenting with Sweet-like infiltrates and neoplastic cells in MF plaques.
Case report
A 65-year old, otherwise healthy patient was first seen in our department in 2005 with suberythroderma and histologically confirmed diagnosis of MF. Treatment with systemic psoralen ultraviolet A (PUVA) initially led to a complete remission of disease symptoms over several years.
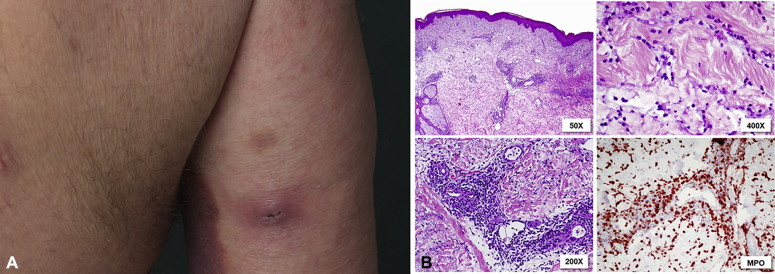
Initial staging did not show any signs of visceral or blood involvement. In 2014, the patient experienced a relapse with erythematous plaques and patches predominantly on the trunk. Systemic PUVA was reintroduced, however, the skin condition worsened with erythematous patches affecting 80% body surface area and severe pruritus. In addition, the patient subsequently had multiple abscess-like lesions positive for methicillin-resistant staphylococcus aureus (Fig 1, A).
Fig 1.
A, example of an abcess-like lesion on the patient's arm. B, Histologic picture of an abcess-like lesion with abundant superficial and deep interstitial and perivascular infiltrate of neutrophilic granulocytes with signs of leucocytoclasia. Additionally, larger MPO-positive myelomonocytic cells can be found.
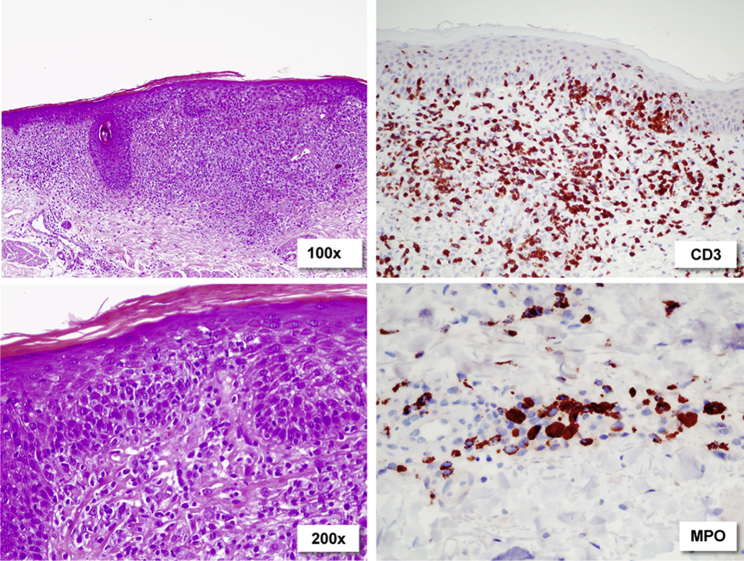
A first biopsy taken from an abscess found a dense superficial and deep perivascular and interstitial infiltrate of neutrophils and poorly differentiated myelomonocytic cells (myeloperoxidase [MPO]+, elastase+, CD3−, C4−) raising the suspicion of a secondary hematologic malignancy (Fig 1, B). Biopsies from erythematous skin lesions showed features typical of MF with a band-like and partly epidermotropic infiltrate of atypical T lymphocytes (CD4+, CD3+, CD8–) next to a deep perivascular infiltrate of poorly differentiated myelomonocytic cells (MPO+, CD68+, CD5+; Fig 2). T-cell receptor rearrangement was negative in skin specimens, peripheral blood, and bone marrow.
Fig 2.
Subepidermal infiltrate of CD3+ cells with hyperchromatic, atypical nuclei, and abundant epidermotropism. Single myelomonocytic cells around the deep dermal vessels.
Differential blood count showed a leukocytosis (36,000/μL) with monocytosis (11.000/μL), a microcytic and hypochromic anemia, thrombopenia (88.000/μL), elevated lactate dehydrogenase level (793 U/L) and C-reactive protein (8.8 mg/dL). Repeated bone marrow biopsies and smears found a myelomonocytic infiltrate expressing CD33, lysozyme, and myeloperoxidase compatible with CMML.
Further workup, including abdominal and lymph node ultrasound scan, showed splenomegaly of 13.8 cm and palpable axillary and inguinal lymphadenopathies. We initiated an antibiotic treatment and high-dose oral corticosteroids (1 mg/kg). This finding led to a transient regression of erythematous lesions, relapsing on corticosteroid dose tapering to less than 0.5 mg/kg. Treatment with low-dose methotrexate (15 mg weekly) had to be stopped after 1 injection because of dyspnea, vertigo, and radiologic signs of pneumonitis.
Monocytosis persisted over more than 6 months, and repeat bone marrow biopsies found blast infiltrates of up to 20%. The diagnosis of CMML was made, and an association to the exacerbation of MF had to be assumed. Consequently, a treatment with hydroxicarbamide, 500 mg/d, was started. Owing to lack of response, the treatment was changed to azacitidine, 75 mg/m2. The patient responded well to the treatment with improvement of skin manifestations and pruritus (Fig 3). However, pruriginous erythematous patches and plaques remained, and a parallel treatment with bexarotene, 300 mg/m2, was initiated.
Fig 3.
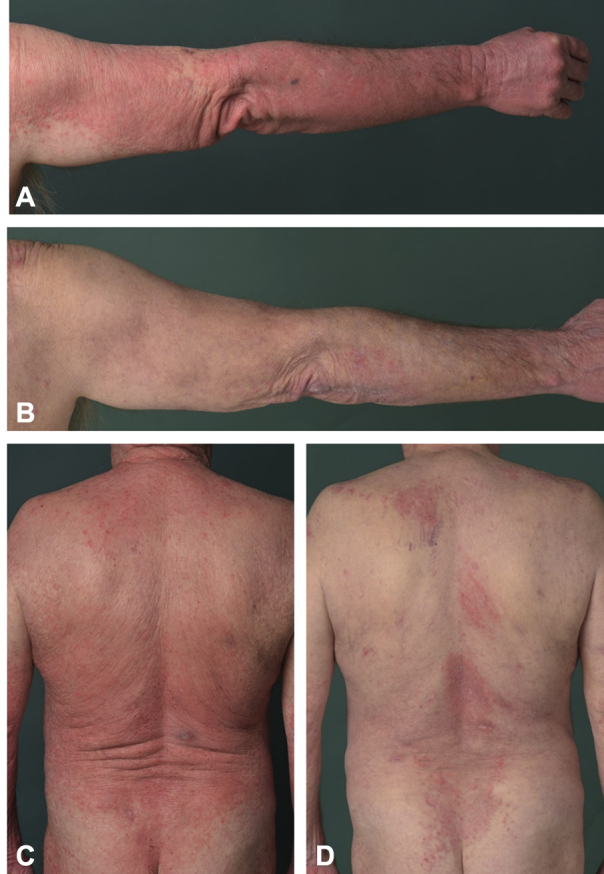
A and C, Clinical picture shortly after reintroduction of PUVA treatment. B and D, Clinical picture 4 months after introduction of azacitidine and bexarotene combination therapy.
The administration of azacitidine was reduced to 5 instead of 7 days in a 28-day cycle after the first 6 treatment cycles because of thrombocytopenia (33.000/μL). Under this combined treatment, the skin condition improved dramatically, lymphadenopathy was regressing, and blood count improved with near normalization of thrombocyte count and differential blood count. Neither CMML nor MF worsened. Bexarotene was continued at a dose of 300 mg/m2. One year after initiation of the combination therapy, the patient had an increasing leukocytosis with up to 80% of blasts and progression to acute myeloid leukemia (AML). Treatment was changed from azacitidine to cytarabine, 20 mg, days 1 to 10 every 4 weeks. Treatment with bexarotene was continued. The patient died of leukemic disease 4 months after diagnosis of AML.
Discussion
Various hypotheses on the pathogenesis of secondary hemoproliferative diseases exist; identical stem cell clones, immunosuppression, mutagenic therapies, and specific cytokine milieus may be possible causes.1 With regard to ultraviolet light treatment, such as PUVA irradiation, for cutaneous T-cell lymphoma, no correlation with hemoproliferative diseases has been shown.1 Changes in microenvironment and increased immunosuppression may also be explanations for the worsening of MF in our case. Interestingly, we could observe neoplastic myeloid cell infiltrates in MF plaques. One may speculate on a direct interaction between CMML and MF neoplastic cells or a locally altered immune response as potential triggers of MF exacerbation. Also, a “pseudo” MF could be discussed as reported in B-cell chronic lymphocytic leukemia.12, 13 It has been described as lesions clinically and histologically indistinguishable from MF, but without evidence of a positive T-cell receptor rearrangement. Yet, the long duration from diagnosis of MF to diagnosis of CMML and the typical histologic picture are in favor of a true cutaneous lymphoma in our case.
In general, differentiation between specific infiltrates and reactive infiltrates containing neoplastic cells may be impossible.10 We observed Sweet-like infiltrates and abscess formation probably caused by skin infection triggered by immunosuppression in the context of CMML and MF. Here too, a nonnegligible part of the infiltrate may have consisted of neoplastic CMML cells.
Treating 2 different neoplasms at a time may be challenging; azacitidine is a hypomethylating agent approved for the treatment of CMML with a white blood count of less than 13 G/L. In highly replicative CMML it seems to be less efficient, which is why our patient first received a cytoreductive agent (hydroxycarbamide) and azacitidine as second-line treatment.14, 15, 16
Adding bexarotene meant combining 2 drugs with each specific and dose-dependent side effects and potential unknown interactions. Fortunately, except for thrombocytopenia, no other side effects led to interruption or tapering of medication dose. The clinical picture could be stabilized under combination therapy over 12 months before progression to AML.
This case shows how 2 hemoproliferative diseases may influence one another and lead to therapeutic challenges that call for individual solutions. It also shows that neoplastic cells in CMML may be found not only in inflammatory or infectious dermatoses but also in cutaneous lymphoma plaques.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Hodak E., Lessin S., Friedland R. New insights into associated co-morbidities in patients with cutaneous T-cell lymphoma (mycosis fungoides) Acta Derm Venereol. 2013;93:451–455. doi: 10.2340/00015555-1496. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl L.M., Fenger-Grøn M., Iversen L. Subsequent cancers, mortality, and causes of death in patients with mycosis fungoides and parapsoriasis: a Danish nationwide, population-based cohort study. J Am Acad Dermatol. 2014;71:529–535. doi: 10.1016/j.jaad.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Vardiman J., Hyjek E. World Health Organization classification, evaluation, and genetics of the myeloproliferative neoplasm variants. Hematology Am Soc Hematol Educ Program. 2011;2011:250–256. doi: 10.1182/asheducation-2011.1.250. [DOI] [PubMed] [Google Scholar]
- 4.Mathew R.A., Bennett J.M., Liu J.J. Cutaneous manifestations in CMML: indication of disease acceleration or transformation to AML and review of the literature. Leuk Res. 2012;36:72–80. doi: 10.1016/j.leukres.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Duguid J.K., Mackie M.J., McVerry B.A. Skin infiltration associated with chronic myelomonocytic leukaemia. Br J Haematol. 1983;53:257–264. doi: 10.1111/j.1365-2141.1983.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 6.Vitte F., Fabiani B., Bénet C. Specific skin lesions in chronic myelomonocytic leukemia: a spectrum of myelomonocytic and dendritic cell proliferations: a study of 42 cases. Am J Surg Pathol. 2012;36:1302–1316. doi: 10.1097/PAS.0b013e31825dd4de. [DOI] [PubMed] [Google Scholar]
- 7.Cerroni L., Zenahlik P., Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26–31. doi: 10.1002/1097-0142(19950701)76:1<26::aid-cncr2820760105>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Sokumbi O., Gibson L.E., Comfere N.I., Peters M.S. Granuloma annulare-like eruption associated with B-cell chronic lymphocytic leukemia. J Cutan Pathol. 2012;39:996–1003. doi: 10.1111/j.1600-0560.2012.01967.x. [DOI] [PubMed] [Google Scholar]
- 9.Ziemer M., Bornkessel A., Hahnfeld S., Weyers W. “Specific” cutaneous infiltrate of B-cell chronic lymphocytic leukemia at the site of a florid herpes simplex infection. J Cutan Pathol. 2005;32:581–584. doi: 10.1111/j.0303-6987.2005.00377.x. [DOI] [PubMed] [Google Scholar]
- 10.Magro C.M., De Moraes E., Burns F. Sweet's syndrome in the setting of CD34-positive acute myelogenous leukemia treated with granulocyte colony stimulating factor: evidence for a clonal neutrophilic dermatosis. J Cutan Pathol. 2001;28:90–96. doi: 10.1034/j.1600-0560.2001.280205.x. [DOI] [PubMed] [Google Scholar]
- 11.Hensley C.D., Caughman S.W. Neutrophilic dermatoses associated with hematologic disorders. Clin Dermatol. 2000;18:355–367. doi: 10.1016/s0738-081x(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 12.Ingen-Housz-Oro S., Franck N., Beneton N. Folliculotropic T-cell infiltrates associated with B-cell chronic lymphocytic leukaemia or MALT lymphoma may reveal either true mycosis fungoides or pseudolymphomatous reaction: seven cases and review of the literature. J Eur Acad Dermatol Venereol. 2015;29:77–85. doi: 10.1111/jdv.12454. [DOI] [PubMed] [Google Scholar]
- 13.Metzman M.S., Stevens S.R., Griffiths C.E., Ross C.W., Barnett J.M., Cooper K.D. A clinical and histologic mycosis fungoides simulant occurring as a T-cell infiltrate coexisting with B-cell leukemia cutis. J Am Acad Dermatol. 1995;33:341–345. doi: 10.1016/0190-9622(95)91430-7. [DOI] [PubMed] [Google Scholar]
- 14.Fenaux P., Mufti G.J., Hellstrom-Lindberg E. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adès L., Itzykson R., Fenaux P. Myelodysplastic syndromes. Lancet. 2014;383:2239–2252. doi: 10.1016/S0140-6736(13)61901-7. [DOI] [PubMed] [Google Scholar]
- 16.Silverman L.R., Demakos E.P., Peterson B.L. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]