Abstract
Withaferin-A (WA) has anti-oxidant activities however, its therapeutic potential in acetaminophen (APAP) hepatotoxicity is unknown. We performed a proof-of-concept study to assess the therapeutic potential of WA in a mouse model that mimics APAP-induced liver injury (AILI) in humans. Overnight fasted C57BL/6NTac (5–6 wk. old) male mice received 200 mg/kg APAP intraperitoneally (i.p.). After 1 h mice were treated with 40 mg/kg WA or vehicle i.p., and euthanized 4 and 16 h later; their livers were harvested and serum collected for analysis. At 4 h, compared to vehicle-treated mice, WA-treated mice had reduced serum ALT levels, hepatocyte necrosis and intrahepatic hemorrhage. All APAP-treated mice had reduced hepatic glutathione (GSH) levels however, reduction in GSH was lower in WA-treated when compared to vehicle-treated mice. Compared to vehicle-treated mice, livers from WA-treated mice had reduced APAP-induced JNK activation, mitochondrial Bax translocation, and nitrotyrosine generation. Compared to vehicle-treated mice, WA-treated mice had increased hepatic up-regulation of Nrf2, Gclc and Nqo1, and down-regulation of Il-6 and Il-1β. The hepatoprotective effect of WA persisted at 16 h. Compared to vehicle-treated mice, WA-treated mice had reduced hepatocyte necrosis and hepatic expression of Il-6, Tnf-α and Il-1β, increased hepatic Gclc and Nqo1 expression and GSH levels, and reduced lipid peroxidation. Finally, in AML12 hepatocytes, WA reduced H2O2-induced oxidative stress and necrosis by preventing GSH depletion. Collectively, these data show mechanisms whereby WA reduces necrotic hepatocyte injury, and demonstrate that WA has therapeutic potential in AILI.
Keywords: Acetaminophen, Withaferin-A, Necrosis, Gclc, Oxidative stress
Graphical Abstract

1. Introduction
In the United States, drug-induced acute liver injury is a major cause for liver transplantation [1, 2]. Acetaminophen (APAP), a commonly used over-the-counter anti-pyretic and analgesic, is safe in therapeutic doses, however after an overdose, causes acute liver injury. In US, annually, APAP overdose accounts for majority of acute liver failure cases [3], leading to thousands of emergency department visits and hospitalizations [4]. Medical therapies for AILI are limited. N-acetyl cysteine (NAC), the only FDA-approved antidote, has partial efficacy and is most effective within 8–10 h after APAP overdose [5].
APAP ingested in therapeutic doses is metabolized by sulfation and glucuronidation; only a small fraction is metabolized via cytochrome P450 enzymes, mainly CYP2E1, to generate N-acetyl-p-benzoquinone imine (NAPQI), the toxic metabolite. NAPQI is detoxified by conjugation to glutathione (GSH) and excreted in the urine. However, APAP overdose saturates glucuronidation and sulfation pathways leading to increased generation and accumulation of NAPQI which depletes cellular GSH, binds to cellular proteins, and promotes JNK activation and peroxynitrite production, thus generating overwhelming oxidative stress that leads to hepatocyte necrosis [6, 7].
In response to oxidative stress, the cellular machinery in the liver mounts anti-oxidant response by inducing cytoprotective enzymes. One major example of cellular defense is activation of Nrf2 (Nuclear factor (erythroid-derived 2)-like 2), a key sensor of oxidative stress [8]. Activation of Nrf2 leads to its translocation into the nuclei where it upregulates anti-oxidant response element (ARE) genes such as glutamate-cysteine ligase, catalytic subunit (Gclc) and NAD (P) H dehydrogenase, quinone 1 (Nqo1) [8]. We hypothesized that therapeutic modulation of ARE pathway by Withaferin-A (WA; 4β, 27-dihydroxy-1-oxo-5β, 6β-epoxy witha-2, 24-dienolide), a plant-derived biomolecule, is a potential strategy to treat APAP-induced acute liver injury.
WA, a steroidal lactone derived from a plant Withania somnifera, interacts with Nrf2 [9]. In experimental models of cancer, WA induces apoptosis, promotes radio-sensitization, and has anti-angiogenic effects [10–12]. A few reports indicate that WA has anti-oxidant activities [13–15]. However, the role of WA in APAP hepatotoxicity has not been assessed. We performed a proof-of-concept study to assess the effects of WA in a mouse model that mimics AILI in humans. To mimic “real-world scenario”, we employed an experimental strategy of clinical importance by investigating the effect of WA after induction of AILI. In addition, we confirmed our in vivo findings by assessing the role of WA in modulating oxidative stress in hepatocytes in vitro. We found that treatment with WA reduces AILI in mice, which is associated with inhibition of necrosis signaling and up-regulation of anti-oxidant response. Further, WA reduced oxidative stress-induced hepatocyte necrosis in vitro. These data indicate that WA-like compounds have therapeutic potential in alleviating AILI via modulating APAP-induced oxidative stress to reduce hepatocyte necrosis.
2. Materials and Methods
2.1. Experimental design and animal procedures
All studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals prepared by the United States National Academy of Sciences (National Institutes of Health) and approved by the Institutional Animal Care and Use Committee (#0411005). All surgeries were performed under ketamine and xylazine anesthesia, and all efforts were made to minimize suffering. Male C57BL/6NTac mice (Taconic, Petersburg, NY) were housed under identical conditions in a pathogen-free environment with a 12:12 h light/dark cycle and free access to laboratory chow and water. Mice were acclimatized for 1 week before the study. After overnight fasting, mice (5–6 weeks old, n= 20) received 200 mg/kg APAP (Sigma-Aldrich, St. Louis, MO) i.p. and randomly separated into two groups. An hour later one group (n=10) received 40 mg/kg WA (ChromaDex Inc., Irvine, CA) (PubChem CID: 265237) and the other (n=10), vehicle (ethanol 2 μl/g) i.p. The WA dose and route of administration were selected based on previous studies [12, 16–18]. In mice, serum levels after administration of 4 mg/kg WA i.p. indicate that WA has short half-life (1.3 h) and clears rapidly (AUCo-t : 541 ng-h/ml [1.09 μM.h]) [16]. Therefore, to achieve maximal and prolonged effect on necrosis signaling—which follows GSH depletion within 2 h after APAP injection and leads to peak necrosis by 16 h in mice [19, 20]—we administered one log higher dose (40 mg/kg WA i.p.) than used previously [16].
Mice were euthanized 4 h (n=5/group) and 16 h (n=5/group) after APAP injection; their serum was collected and livers harvested. Five additional mice, who neither received APAP nor WA or vehicle, were euthanized as no treatment controls.
2.2. Blood and tissue collection
Portions of mouse livers were stored in formalin, RNAlater (Sigma-Aldrich, St. Louis, MO), and snap frozen in liquid nitrogen for further analysis. The blood collected by cardiac puncture was centrifuged to obtain serum using microcontainer tubes (Becton Dickinson, Franklin Lakes, NJ).
2.3. Histology
Formalin-fixed paraffin-embedded liver sections were stained using Hematoxylin and Eosin (H&E). Semi-quantitative scoring system was used to assess hepatocyte necrosis and intrahepatic hemorrhage (0, none; 1, <10% of the total area; 2, <30% of the total area; 3, <50% of the total area; 4, >50% of the total area) [20] by two investigators blinded to the study.
2.4. Serum ALT
The serum ALT was determined by measuring the rate of NADH oxidation at 340 nm using ALT (SGPT) Kinetic Kit as per manufacturer’s instructions (TECO Diagnostics Anaheim, CA).
2.5. Immunohistochemistry
Peroxynitrite generation was assessed by immunolocalization of nitrotyrosine adducts. Paraffin-embedded liver sections were deparaffinized with xylene and rehydrated using series of graded ethanol and water. Heat-induced antigen retrieval was carried out using citric acid buffer, and non-specific peroxidase activity was blocked by incubating sections with 2% H2O2. After washing with PBS, sections were treated with 1% bovine serum albumin (60 min) and goat serum (30 min) to block non-specific protein binding, and incubated with polyclonal rabbit anti-nitrotyrosine antibody (Millipore, Billerica, MA; dilution 1:200) overnight at 4°C in a humidified chamber. Next day, sections were washed with PBS and incubated with secondary antibody (biotinylated goat anti-rabbit IgG (H+L) antibody) for 60 min at room temperature. After carrying out avidin-biotin reaction using VECTASTAIN Elite ABC kit (Vector Laboratories, Burlingame, CA), sections were treated with diaminobenzidine (Vector Laboratories, Burlingame, CA) to visualize nitrotyrosine. Subsequently, sections were counterstained with hematoxylin (Sigma-Aldrich, St. Louis, MO), mounted using DPX (Sigma-Aldrich, St. Louis, MO) and examined under a microscope. Two investigators independently evaluated the blinded stained-sections.
2.6. Measurement of reduced glutathione and lipid peroxidation
A 10% (w/v) liver homogenate was prepared using mitochondria isolation buffer (250 mM sucrose containing 5 mM MOPS and 1 mM EDTA with 0.25 mg BSA/ml at pH 7.4) using a Polytron homogenizer (PT2100, Kinematica, Switzerland). Cellular debris were removed by centrifugation of homogenate at 650 g for 10 min (4°C). The resultant supernatant was further centrifuged at 10000 g for 10 min (4°C) to sediment mitochondrial pellet, which was washed twice with and suspended in isolation buffer, and protein content was quantified using Bradford assay (Sigma-Aldrich, St. Louis, MO). Reduced glutathione (GSH) was measured spectrophotometrically by assessing the reduction of DTNB (dithiobis-2-nitrobenzoic acid) to a yellow anion at 412 nm as described previously [20]. The GSH content was calculated as μg/mg protein using a standard GSH curve. Mitochondrial lipid peroxidation was assessed by measuring malondialdehyde (MDA) formation [20, 21]. Commercially available 1, 1, 3, 3-tetraethoxypropane (Sigma-Aldrich, St. Louis, MO) was used as a standard for calculating MDA content and expressed as MDA formed (nmoles/mg protein).
2.7. Quantitative real-time polymerase chain reaction (qPCR)
Mouse liver (100 mg) was homogenized in Trizol (Life Technologies, Grand Island, NY) using a Polytron PT2100 homogenizer. RNA was extracted, and its quantity and quality were assessed using Thermo Scientific 2000 Nanodrop Spectrophotometer (Thermo Scientific, Wilmington, DE). cDNA was prepared using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and subjected to real-time PCR. Assays were performed in 96-well PCR plates using Quantifast SYBR green PCR kit (Qiagen, Valencia, CA). The reaction volume of 25 μl contained 12.5 μl SYBR green master mix (2X), 1 μl cDNA (25 ng), 1 μl of each primer (10 pmol/μl) and 9.5 μl nuclease-free water. Primer sequences are listed in Table 1. The following two-step thermal cycling profile was used (StepOnePlus Real-Time PCR, Life Technologies, Grand Island, NY): Step I (cycling): 95 °C for 5 min, 95 °C for 10 s and 60 °C for 30 s for 40 cycles. Step II (melting curve): 60 °C for 15 s, 60 °C 1 min and 95 °C for 30 s. The template amplification was confirmed by melting curve analysis. Changes in mRNA expression were assessed by ΔΔCt method.
Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| IL-1β | TCTATACCTGTCCTGTGTAATG | GCTTGTGCTCTGCTTGTG |
| Tnf-α | GTGGAACTGGCAGAAGAG | AATGAGAAGAGGCTGAGAC |
| IL-6 | CTTCTGGAGTACCATAGC | TCTGTTAGGAGAGCATTG |
| Gclc | AACACAGACCCAACCCAGAG | CCGCATCTTCTGGAAATGTT |
| Nrf-2 | CGAGATATACGCAGGAGAGGTAAGA | GCTCGACAATGTTCTCCAGCTT |
| Nqo1 | CAGATCCTGGAAGGATGGAA | TCTGGTTGTCAGCTGGAATG |
| Gapdh | ACAACTTTGGCATTGTGGAA | GATGCAGGGATGATGTTCTG |
2.8. Immunoblotting
To extract protein, liver samples were homogenized using a Polytron PT2100 homogenizer in cell lysis buffer (Cell Signaling, Danvers, MA), containing phosphatase and protease inhibitors, and quantified using Bradford reagent (Sigma-Aldrich, St. Louis, MO). Mitochondrial fractions were prepared as described previously [20]. Equal amount of protein (40 μg) from each sample was resolved on SDS-PAGE, trans-blotted onto a PVDF membrane (Bio-Rad, USA) and subjected to immunoblot assay by using primary antibodies (1:1000) for JNK, Bax and p-JNK (Cell Signaling, Danvers, MA) followed by secondary anti-rabbit horseradish peroxidase antibody (1:5000; Cell Signaling, Danvers, MA). Blots were developed with chemiluminescence reagent (Bio-Rad, Hercules, CA) using autoradiography films (Genesee Scientific, San Diego, CA). Blots were stripped using stripping buffer (Thermo Scientific, Wilmington, DE) and re-probed with goat anti-rabbit β-actin antibody (1:5000; Cell Signaling, USA) to determine equivalent loading. Scanned images of blots were used to quantify protein expression using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
2.9. Cell culture
AML12 hepatocytes (ATCC, Manassas, VA) were cultured using 1:1 mixture of Dulbecco’s modified Eagle’s and Ham’s F12 medium (Genesee Scientific, San Diego, CA) with 0.005 mg/ml insulin, 0.005 mg/ml transferrin, 5 ng/ml selenium, 10% fetal bovine serum at 37°C with 5% CO2. Cells were sub-cultured (1:4–1:6) using a 0.25% (w/v) trypsin-0.53 mM EDTA solution (Genesee Scientific, San Diego, CA).
2.10. Cell viability assay
AML12 cells (6.0 × 103 cells/well) were treated with 1 mM H2O2 and WA (0.01–1.0 μM) or vehicle (0.02% methanol) (n=4–8 for all treatment groups). After 6 h, culture media was replaced with 100 μl MTT (Sigma-Aldrich, St. Louis, MO) solution (0.5 mg/ml in culture media) and incubated at 37°C. After 2.5 h, MTT solution was discarded, all wells was washed with PBS and 150 μl DMSO was added to each well and absorbance was read at 540 nm (VersaMax Microplate Reader, Molecular Device, Sunnyvale, CA).
2.11. LDH release assay
LDH released in the cell culture media was assessed using Pierce LDH Cytotoxicity Assay Kit as per manufacturer’s instruction (Thermo Scientific, Wilmington, DE). In 96-well plates, AML12 cells (6.0 × 103 cells/well) were maintained for 2 or 6 h in the presence of vehicle (0.02% methanol), H2O2 (1 mM) plus vehicle, H2O2 (1 mM) plus WA (0.01–1.0 μM), and WA alone (n=3–6 for all treatment groups). At the end of incubation, 50 μl media was mixed with 50 μl reaction mixture and incubated in dark at room temperature. After 30 min, 50 μl stop solution was added and absorbance was read at 490 and 680 nm on a VersaMax Microplate Reader (Molecular Device, Sunnyvale, CA). LDH release is presented as % cytotoxicity, which was calculated per manufacture’s instruction as follows:
2.12. Measurement of intracellular ROS
H2O2-induced oxidative stress was assessed using CellROX green reagent (Life Technologies, Grand Island, NY) as per manufacturer’s instructions. In 6-well plates, AML12 cells (1.0 × 106 cells/well) were maintained for 2 h in the presence of vehicle (methanol 0.02%), H2O2 (1 mM) plus vehicle, H2O2 (1 mM) plus WA (0.01–0.05 μM), and WA alone (n=2 for all treatment groups). At the end of incubation, CellROX green reagent (5 μM) was added to each well and incubated at 37°C in dark. After 30 min, cells were washed with PBS three times and images were observed and captured using Zeiss LSM 780 Inverted Confocal microscope (Zeiss, Thornwood, NY) using green filter and bright field modes (at 10X). Additionally, in 96-well plates, AML12 cells (6.0 × 103 cells/well) were incubated with vehicle, H2O2 (1 mM) plus vehicle, H2O2 plus WA (0.01 and 0.05 μM), and WA alone (n=3/treatment group). After 2 h, 5 μM CellROX green reagent was added to each well and incubated at 37°C. After 30 min, cells were washed with PBS three times and fluorescence intensity was recorded using excitation and emission wavelengths of 485/520 nm in a Synergy 2 Multi-Mode Microplate Reader (Bio-Tek, USA).
2.13. Statistical analysis
All data are expressed as mean ± S.E.M. Student’s t-test (normally distributed data) or the Mann-Whitney U test (nonparametric data) were used to determine significance. Analysis was performed using SigmaPlot (version 12.0; Systat Software, Inc. San Jose, CA). Significance was defined as P < 0.05.
3. Results
3.1. WA attenuates early APAP-induced hepatocyte injury
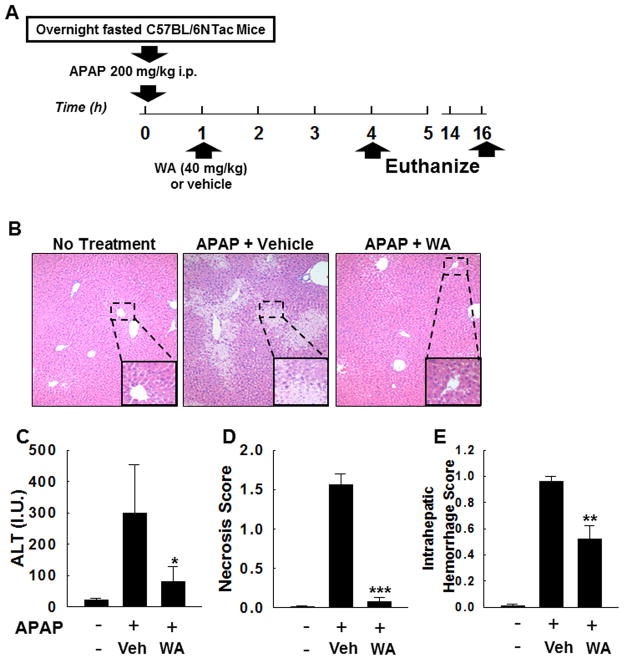
Figure 1A illustrates our study design—see Methods section for details. Four hours after APAP overdose, compared to vehicle-treated mice, WA-treated mice had significant reduction in serum ALT levels, hepatocyte necrosis and intrahepatic hemorrhage (Fig. 1B–E). These data indicated that after induction of APAP hepatotoxicity, treatment with WA significantly reduces hepatocyte injury.
Figure 1.
Effect of WA on early APAP-induced hepatocyte injury. (A) Study design: After overnight fasting, age-matched male mice (n=20) received an overdose of APAP intraperitoneally. An hour later, mice received WA (n=10) or vehicle (n=10), and euthanized at 4 h and 16 h (n=5 in WA-treated and vehicle-treated group); their livers were harvested and serum collected. Five mice that received neither APAP nor WA or vehicle were also euthanized (no treatment controls). (B) Representative photomicrographs (100x) of H&E-stained liver sections from mice euthanized at 4 h are shown. Hepatocytes undergoing necrosis were identified using the following criteria: increased eosinophilia, cell swelling and lysis, loss of architecture, karyolysis and karyorrhexis [43]. Summary data show that after APAP overdose, compared to vehicle-treated mice WA-treated mice had reduced serum ALT (C), hepatocyte necrosis (D), and intrahepatic hemorrhage (E) (n = 5 mice/group). Results are shown as mean ± S.E.M. * P < 0.05, ** P < 0.01, *** P<0.001 when compared among APAP-treated groups. Vehicle (Veh).
3.2. WA reduces APAP-induced GSH depletion
During the course of APAP hepatotoxicity, GSH depletion peaks by 4 h [22], which contributes to oxidative stress-mediated hepatocyte necrosis. To determine the effect of WA in modulating APAP-induced oxidative stress, we assessed for changes in mitochondrial GSH content and lipid peroxidation. Four h after APAP-overdose, hepatic mitochondrial GSH levels decreased markedly. However, compared to vehicle-treated mice, livers from WA-treated mice had significantly higher GSH levels (Fig. 2A). APAP-treated mice had a marginal increase in hepatic lipid peroxidation, which was significantly reduced in mice that received WA (Fig. 2B) [23]. These data indicated that treatment with WA significantly prevented APAP-induced GSH depletion in mouse livers.
Figure 2.
Effect of WA treatment on GSH depletion and necrosis signaling. (A) Four h after APAP overdose, GSH content declined in hepatic mitochondrial fractions, which was blunted in WA-treated mice. (B) Concomitantly, lipid peroxidation was reduced in WA-treated mice when compared to vehicle-treated mice (n=5 in WA-treated, vehicle-treated and no treatment groups). (C) Representative immunoblots for phospho-JNK, total JNK and β-actin (loading control) in mouse livers assessed 4 h after APAP injection. (D) Densitometric assessment of p-JNK indicates that APAP-induced hepatic JNK activation is reduced in mice that received WA (n = 3 mice/group). (E) Immunoblots for hepatic mitochondrial Bax, and their respective loading controls assessed 4 h after APAP injection followed by WA or vehicle treatment. (F) Densitometry indicates that APAP-induced hepatic mitochondrial Bax translocation is reduced in WA-treated mice. Results are shown as mean ± S.E.M. * P < 0.05, ** P < 0.01, *** P<0.001 when compared among APAP-treated groups. Vehicle (Veh).
3.3. WA reduces APAP-induced JNK activation
In AILI, JNK activation is a major event that follows GSH depletion [22]. Activated JNK translocates to mitochondria where it promotes peroxynitrite and H2O2 generation. We observed that 4 h after APAP injection, hepatic JNK phosphorylation increased in vehicle-treated mice, which was reduced in the livers of WA-treated mice (Fig. 2C–D).
3.4. WA reduces APAP-induced mitochondrial Bax translocation
APAP overdose-mediated mitochondrial Bax translocation induces mitochondrial release of proteins that cause nuclear DNA fragmentation. Therefore, we assessed the effect of WA treatment on mitochondrial Bax translocation 4 h after APAP overdose. Western blot analysis showed that WA treatment reduced mitochondrial Bax 4 h after APAP administration (Fig. 2E–F).
3.5. WA reduces APAP-induced peroxynitrite formation
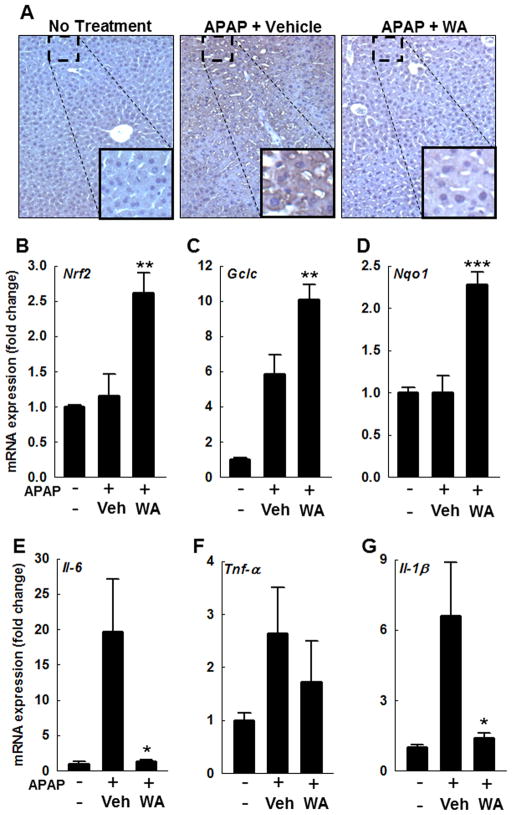
To determine the impact of WA treatment on peroxynitrite generation, liver sections were stained for nitrotyrosine adducts using anti-3-nitrotyrosine (3-NT) antibody. Compared to vehicle-treated mice, liver sections from WA-treated mice had reduced 3-NT staining (Fig. 3A), indicating that treatment with WA attenuates necrosis signaling by reducing JNK activation, which attenuates peroxynitrite generation in mouse livers.
Figure 3.
Effect of WA treatment on hepatic peroxynitrite generation, transcription of cytoprotective genes, and inflammatory cytokines in mice 4 h after APAP injection. (A) Representative photomicrographs of 3-NT-stained (brown color) liver sections are shown. In mouse livers, Nrf2, Gclc and Nqo1 expression was determined by real-time PCR. Compared to vehicle, treatment with WA increased the transcription of Nrf2 (B), Gclc (C) and Nqo1 (D) (n = 4/group). Similarly, hepatic expression of Il-6 (E), Tnf-α (F), and Il-1β (G) was assessed by real-time PCR. WA treatment significantly reduced expression of inflammatory cytokines; Il-6 (E) and Il-1β (G) (n = 4 mice/group). Results are shown as mean ± S.E.M. * P < 0.05, ** P < 0.01, *** P<0.001 when compared among APAP-treated groups. Vehicle (Veh).
3.6. WA augments induction of cytoprotective genes
Nrf2, a key oxidative stress-sensitive transcription factor, regulates expression of cytoprotective genes. To determine if Nrf2 plays a role in WA-mediated reduction of AILI, we assessed in mouse livers harvested at 4 h, expression of Nrf2 and its downstream target genes Gclc and Nqo1; Gclc is the key enzyme involved in GSH synthesis. While the vehicle-treated mice only had increased Gclc expression, WA-treated mice had a further upregulation of Gclc, as well as Nrf2 and Nqo1 (Fig. 3B–D). These data indicate that in AILI, treatment with WA upregulates ARE genes to modulate anti-oxidant response.
3.7. WA reduces APAP-induced inflammatory response cytokines
AILI is associated with sterile inflammation, which is thought be secondary to recruitment of inflammatory cells by damage-associated molecular pattern molecules. To assess the effect of WA on generation of inflammatory cytokines, we measured hepatic expression of Il-1β, Il-6 and Tnf-α in mouse livers harvested at 4 h. Treatment with WA significantly reduced APAP-induced upregulated levels of Il-1β and Il-6 (Figure 3E–G). Tnf-α expression was similar in both WA- and vehicle-treated groups. These findings demonstrate that WA treatment attenuates APAP-induced inflammation in mouse livers.
3.8. WA attenuates late APAP-induced liver injury
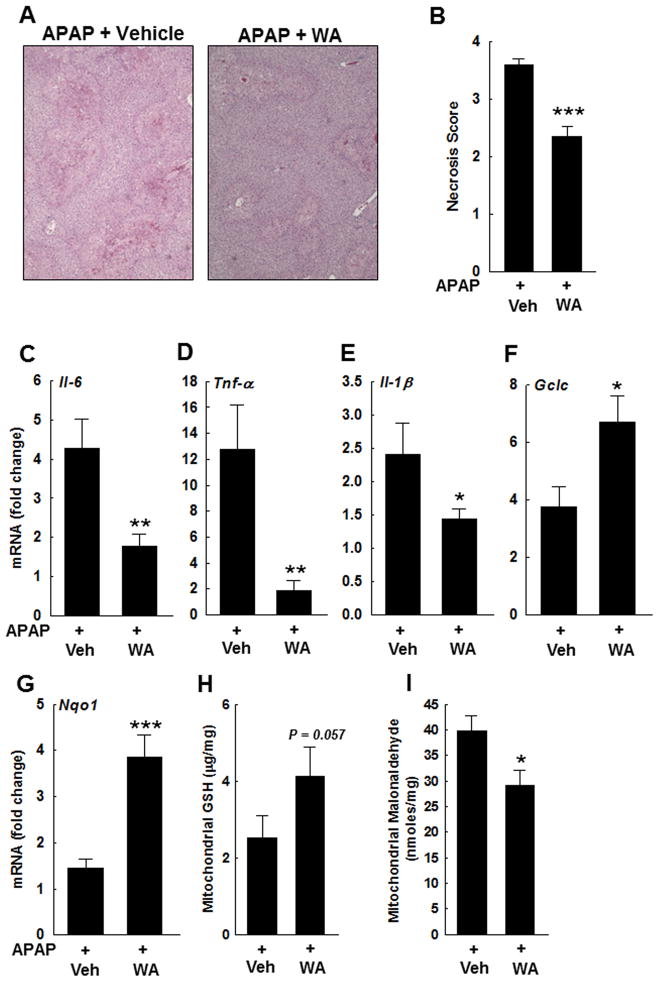
By 16 h, WA- and vehicle-treated mice had similar serum ALT levels 1317.16 ± 453 vs. 1050.2 ± 136.8 (mean ± S.E.M.), respectively. However, the liver sections from WA-treated compared to vehicle-treated mice, demonstrated reduced necrosis (Fig. 4A–B). APAP-induced up-regulation of hepatic Il-1β, Il-6 and Tnf-α was also reduced in WA-treated mice (Fig. 4C–E). Moreover, hepatic Gclc and Nqo1 transcription remained upregulated in WA-treated mice when compared to vehicle-treated mice (Fig. 4F–G), which were associated with increased hepatic GSH levels and reduced lipid peroxidation (Fig. 4H–I). These data suggest that during the course of AILI, early treatment with WA renders lasting hepatoprotective effects.
Figure 4.
Effect of WA on late APAP-induced hepatocyte injury. (A) Representative photomicrographs (100x) of H&E-stained sections of livers harvested 16 h after APAP injection. Hepatocytes undergoing necrosis were identified as described previously [43]. (B) Summary data. APAP-treated mice that received WA compared to vehicle, had reduced hepatocyte necrosis (n = 5 mice/group). Effect of WA treatment on expression of inflammatory cytokines and cytoprotective genes was assessed by real-time PCR. APAP-mediated upregulation of hepatic (C) Il-6, (D) Tnf-α, and (E) Il-1β was significantly reduced in WA-treated mice compared to those treated with vehicle (n = 4 mice/group). Compared to vehicle, treatment with WA increased the expression of (F) Gclc and (G) Nqo1 (n = 4 mice/group), which was associated with (H) elevated GSH levels and (I) reduced lipid peroxidation. Results are shown as mean ± S.E.M. * P < 0.05, ** P < 0.01, *** P<0.001 when compared among APAP-treated groups. Vehicle (Veh).
3.9. WA attenuates oxidative stress-induced hepatocyte injury in vitro
To confirm the role of WA in modulating the response to oxidative stress in hepatocytes, we assessed WA’s effects on oxidative injury in AML12 hepatocytes. H2O2 is a major mediator of APAP-induced oxidative stress [22], and previous studies showed that 1 mM H2O2 induces hepatocyte necrosis in vitro [24, 25]. To determine the effect of WA on H2O2-mediated cell death, we performed MTT assay in hepatocytes incubated for 6 h with 1 mM H2O2, and assessed LDH release in their media, in the presence and absence of WA (0.01–1.0 μM). Methanol, the solvent for WA, was added to all samples. As shown in Figure 5A–B, treatment with WA enhanced cell survival and significantly reduced H2O2–induced LDH release; incubation with WA alone had no effect (not shown). Since lower doses of WA appeared to render most hepatocyte protection, subsequent experiments were performed using 0.01 and 0.05 μM WA.
Figure 5.
Effect of WA treatment on H2O2-mediated oxidative stress and injury in AML-12 hepatocytes. (A) MTT assay indicates that incubation with 1 mM H2O2 plus vehicle for 6 h markedly increased AML12 cell death. Treatment with WA (0.01–0.75 μM) significantly enhanced cell viability; WA alone had no effect (not shown here) (n=4–8 for all treatment groups). (B) LDH release in the cell culture media was assessed 6 h after incubation with 1 mM H2O2. Compared to vehicle, treatment with WA (0.01–1.0 μM) attenuated H2O2-induced LDH release; treatment with WA alone had no effect (not shown here). Results are expressed as percentage of a cell lysis control provided by the manufacturer (n=3–6 for all treatment groups). (C) Effect of WA on H2O2-mediated oxidative stress in AML12 hepatocytes was determined using CellRox green reagent. Cells were incubated for 2 h with vehicle, 1 mM H2O2 plus vehicle, H2O2 plus WA (0.01–0.05 μM) and WA alone (n=2 for all treatment groups). Cells were analyzed for oxidative stress by assessing fluorescence. Cells incubated with H2O2 that were treated with WA had reduced fluorescence. Fluorescence-intensity, as described in Results section, declined significant in H2O2-treated cells that were co-treated with WA (n=3/group). Results are shown as mean ± S.E.M. * P<0.05, ** P < 0.01, *** P<0.001 when compared to H2O2 plus vehicle-treated group.
To determine the effect of WA on oxidative stress in H2O2-treated AML12 hepatocytes, we used confocal microscopy to assess the oxidation of CellROX green reagent by ROS to generate fluorescence. Treatment with 1 mM H2O2 for 2 h increased green fluorescence in AML12 hepatocytes, which was reduced in cells co-incubated with 0.01 and 0.05 μM WA (Fig. 5C). Change in fluorescence was quantified as described previously [20]. Incubation with vehicle + H2O2 for 2 h increased fluorescence 2.33-fold when compared to vehicle alone (P < 0.01). Co-treatment with 0.01 and 0.05 μM WA reduced fluorescence to 1.16- and 1.37-fold respectively (P <0.05 for both when compared to H2O2 + vehicle–treated cells). Treatment with 0.01 and 0.05 μM WA alone had no effect (1.09- and 1.08-fold change when compared to vehicle).
3.10. WA prevents H2O2-mediated GSH depletion in vitro
We assessed the effect of WA on oxidative stress-mediated GSH depletion in AML12 hepatocytes. Cells incubated with 1 mM H2O2 were treated with vehicle (methanol) or WA (all samples contained methanol, the vehicle for WA). GSH content was measured in cytosolic and mitochondrial fractions at 30, 60 and 120 min (Fig. 6A–B). At 30 min, GSH was significantly reduced in cells incubated with H2O2 but co-treatment with WA had no effect on cytoplasmic or mitochondrial GSH content. By 60 min, WA (0.05 μM) prevented decline of GSH levels only in cytoplasm (P = 0.054). However, by 120 min, WA (0.01–0.05 μM) prevented GSH decline in both cytoplasmic and mitochondrial fractions.
Figure 6.
Effect of WA on H2O2-mediated GSH depletion in AML-12 hepatocytes. GSH was measured in (A) cytoplasmic and (B) mitochondrial fractions, 30-, 60- and 120-min after incubation with 1 mM H2O2. Compared to cells treated with vehicle alone, GSH levels were significantly reduced in H2O2-treated cells. Treatment with 0.01 and 0.05 μM WA prevented H2O2-mediated GSH depletion. In the absence of H2O2, WA had no effect (n=3 for all treatment groups). (C) LDH release was assessed in the media 2 h after treatment with vehicle, H2O2 plus vehicle, and H2O2 plus WA, with or without pre-incubation with BSO (100 μM, a GCLC inhibitor, for 18 h). Pre-incubating cells with BSO abolished the effects of WA on LDH release (n=3 for all treatment groups). Results are shown as mean ± S.E.M. * P<0.05 and ** P<0.01 when compared to H2O2 plus vehicle-treated group.
Further, we assessed the effect of GCLC inhibition on WA-mediated hepatocyte protection. We assessed the effect of WA on 1 mM H2O2-mediated LDH release from AML12 hepatocytes pre-treated with or without 100 μM BSO (a GCLC inhibitor). WA-mediated cytoprotection was lost in hepatocytes pre-incubated for 18 h with BSO; LDH release from these cells was similar to that from cells treated with 1 mM H2O2 alone (Fig. 6C). These findings indicate a key role for GSH in WA-mediated hepatocyte protection. Collectively, these in vitro data are consistent with our in vivo findings, and support therapeutic potential for WA in modulating anti-oxidant responses.
4. Discussion
In US, AILI is the major cause of drug-induced liver injury (DILI) that requires liver transplantation. APAP overdose leads to oxidative stress that overwhelms hepatocyte defenses and activates necrosis signaling cascade [26]. Currently, NAC is the only FDA-approved antidote, which is most effective when administered within 8–10 h after APAP overdose. Since AILI is associated with depletion of hepatocyte GSH, NAC provides the necessary cysteine to promote GSH generation and replete GSH stores [27]. The goal of this study was to determine if therapies targeted at augmenting ARE signaling pathways and deactivating necrosis signaling have a role in treatment of AILI. WA induces apoptosis in cancer cells and potentiates the effects of other chemotherapeutic agents by activating tumor suppressor proteins and inducing oxidative stress [28–33]. On the other hand, WA also has anti-oxidant activity [10, 13–15, 34–36]. In this study, we tested the hypothesis that anti-oxidant effects of WA will reduce oxidative stress-induced liver injury. Our results indicate that WA reduces AILI by augmenting ARE target genes induction and reducing necrosis signaling.
To mimic a scenario of patients seeking care after APAP overdose, we employed an experimental strategy where mice were administered WA 1 h after APAP overdose. This strategy tested the therapeutic potential of WA after the initiation of APAP-induced oxidative stress and necrosis signaling.
Previous studies showed that in osteosarcoma cells, WA interacts with Nrf2 [9]. Nrf2 knockout mice are highly susceptible to APAP hepatotoxicity [37]. We showed previously that in response to APAP-induced oxidative stress, Nrf2 transcription is upregulated in hepatocytes, and augmented anti-oxidant response correlates with Nrf2 up-regulation [20]. In this study we observed that treatment with WA enhanced hepatic transcription of Nrf2-responsive genes Gclc and Nqo1, indicating that after APAP overdose, WA potentiates anti-oxidant response.
In APAP-induced necrosis signaling, JNK activation follows GSH depletion and JNK inhibition can reduce AILI despite GSH depletion [22]. Our findings indicate that WA-treated mice had reduced JNK activation and peroxynitrite generation. These events were associated with reduced GSH depletion and increased Gclc upregulation. In liver, GSH is synthesized predominantly in hepatocytes, utilizing amino acids glutamate, glycine and cysteine. NAC that replenishes GSH stores by providing the necessary cysteine to increase GSH synthesis is most effective when used early after APAP overdose. GSH not only quenches NAPQI but other reactive species such as peroxynitrite. Compared to NAC, WA neither has cysteine nor glycine and glutamate or sulfhydryl groups amenable to oxidation. Since WA is unlikely to be GSH precursor, we speculate that WA-mediated reduction of peroxynitrite generation is likely due to reduced JNK activation [6], which in turn is due to reduced GSH depletion secondary to Gclc upregulation.
In addition to GSH depletion, another early event that enhances APAP-induced hepatocyte necrosis is mitochondrial Bax translocation, which contributes to release of endonuclease G and AIF that induce hepatocyte DNA fragmentation and necrosis [38]. Our data show that WA-treatment reduces APAP-induced mitochondrial Bax translocation. JNK inhibition has been shown to reduce APAP-mediated mitochondrial translocation of Bax [39]. However, Bax deficiency in mice had no effect on APAP-induced peroxynitrite generation, an event downstream of JNK activation. Therefore, we hypothesize that APAP-induced JNK activation and mitochondrial Bax translocation are independent of each other, and WA treatment reduces both these events, thereby attenuating peroxynitrite generation and mitochondrial toxicity.
Our previous studies showed that after APAP (200 mg/kg) overdose in mice, liver injury peaks by 16 h and resolves by 24 h [20]. Therefore, in this study we assessed the effect of WA on APAP-induced hepatotoxicity at 4 and 16 h. At 16 h, compared to vehicle-treated mice, WA-treated mice had reduced hepatocyte necrosis and expression of injury cytokines however, the serum ALT levels were similar. We currently do not understand the reasons underlying this inconsistency, though other yet unidentified factors may affect the timing of APAP-induced ALT release [40]. Since, we employed only single dose of WA to determine the effect on AILI, we speculate that by 16 h, WA effects were waning. However, increased hepatic expression of Gclc and Nqo1, elevated GSH levels and reduced expression of cytokines indicate that treatment with WA exerted hepatoprotective even in later phases of AILI. Future studies to determine dose- and frequency-dependent effects of WA on APAP hepatotoxicity will enhance our understanding of mechanisms underlying hepatoprotective effects of WA.
AILI is associated with increased expression of inflammatory cytokines; however, the role of specific cytokines and the immune response in modulating hepatocyte injury is not well understood. We observed that livers from WA-treated mice had reduced APAP-induced up-regulation of injury cytokines. WA has been shown to reduce LPS-mediated activation of RAW macrophages [35]. Macrophages appear to have a protective role in APAP hepatotoxicity. Based on changes in GSH and Gclc, we believe that reduced expression of inflammatory cytokines in livers of WA-treated mice is a consequence and not the cause of reduced injury.
Our in vivo findings were confirmed using in vitro model of H2O2-induced necrosis in AML12 hepatocytes, the non-transformed hepatocytes derived from a mouse transgenic for human TGF-α [24, 25]. APAP hepatotoxicity is dependent on Cyp2e1 activity, the key enzyme required to metabolize APAP into toxic metabolites. Cyp2e1 is expressed at markedly low levels in AML12 cells [20]. Therefore, we used H2O2, a major mediator of APAP-induced liver injury instead of APAP, to assess the effects of WA [22]. Treatment of AML12 hepatocytes with WA reduced H2O2-induced oxidative stress and necrosis, which were associated with reduced GSH depletion (Fig. 5–6). The effect of WA on H2O2-induced LDH release was lost in hepatocytes pre-treated with BSO, a GCLC inhibitor (Fig. 6C). Based on these findings, we propose that WA reduces oxidative stress by regulating Gclc function.
Metabolism of APAP to its reactive metabolite by Cyp2e1, subsequent APAP-protein binding, and initiation of necrosis signaling are critical to induction of AILI [41]. Interference with these early events affect the severity of hepatotoxicity, especially when a compound or its vehicle is administered simultaneously with, or before APAP [41]. For example, resveratrol, α dietary polyphenol, when administered prior to induction of APAP-hepatotoxicity, reduces injury by suppressing Cyp2e1, and when administered after induction of APAP-hepatotoxicity, reduces injury by preventing protein nitration and release of endonucleases [41, 42]. In mice, we administered WA 1 h after APAP to avoid interfering with APAP’s metabolism to its reactive metabolite and the resulting protein binding [19]. Further, assessment of WA in AML12 cells in vitro confirmed that its anti-oxidant effects are exclusive of interference with early events, and due to enhanced GSH recovery.
In conclusion, we demonstrated that a therapeutic strategy aimed at enhancing anti-oxidant response which is inherent to cellular defense machinery, is able to reduce AILI in vivo. In future, combination therapies employing GSH substrates such as NAC plus activator of ARE pathway may be used to reduce the burden of APAP hepatotoxicity, non-APAP DILI, and the need for liver transplantation. Hence, further investigation is warranted into the role of WA-like agents in modulating AILI.
Acknowledgments
This work was supported by the National Institutes of Health (Grant T32-DK067872, K08-DK081479) and Medical College of Georgia (to SK) and University of Maryland Baltimore, School of Medicine (to NKS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95–106. doi: 10.1016/j.mayocp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36–45. doi: 10.1055/s-0032-1301733. [DOI] [PubMed] [Google Scholar]
- 4.Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- 5.Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856–64. 64 e1. doi: 10.1053/j.gastro.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicology and applied pharmacology. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. The Journal of pharmacology and experimental therapeutics. 2002;303:468–75. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. The Journal of biological chemistry. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaishnavi K, Saxena N, Shah N, Singh R, Manjunath K, Uthayakumar M, et al. Differential activities of the two closely related withanolides, Withaferin A and Withanone: bioinformatics and experimental evidences. PloS one. 2012;7:e44419. doi: 10.1371/journal.pone.0044419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochemical pharmacology. 2012;84:1282–91. doi: 10.1016/j.bcp.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Sabina EP, Chandal S, Rasool MK. Inhibition of monosodium urate crystal-induced inflammation by withaferin A. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2008;11:46–55. doi: 10.18433/j35k58. [DOI] [PubMed] [Google Scholar]
- 12.Nagalingam A, Kuppusamy P, Singh SV, Sharma D, Saxena NK. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer research. 2014;74:2617–29. doi: 10.1158/0008-5472.CAN-13-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian journal of experimental biology. 1997;35:236–9. [PubMed] [Google Scholar]
- 14.Bhondave PD, Devarshi PP, Mahadik KR, Harsulkar AM. ‘Ashvagandharishta’ prepared using yeast consortium from Woodfordia fruticosa flowers exhibit hepatoprotective effect on CCl4 induced liver damage in Wistar rats. Journal of ethnopharmacology. 2014;151:183–90. doi: 10.1016/j.jep.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya A, Ramanathan M, Ghosal S, Bhattacharya SK. Effect of Withania somnifera glycowithanolides on iron-induced hepatotoxicity in rats. Phytotherapy research : PTR. 2000;14:568–70. doi: 10.1002/1099-1573(200011)14:7<568::aid-ptr663>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. International journal of cancer Journal international du cancer. 2011;129:2744–55. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- 17.Kalthur G, Pathirissery UD. Enhancement of the response of B16F1 melanoma to fractionated radiotherapy and prolongation of survival by withaferin A and/or hyperthermia. Integrative cancer therapies. 2010;9:370–7. doi: 10.1177/1534735410378664. [DOI] [PubMed] [Google Scholar]
- 18.Uma Devi P, Kamath R. Radiosensitizing effect of withaferin A combined with hyperthermia on mouse fibrosarcoma and melanoma. Journal of radiation research. 2003;44:1–6. doi: 10.1269/jrr.44.1. [DOI] [PubMed] [Google Scholar]
- 19.McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, et al. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicology and applied pharmacology. 2013;269:240–9. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urrunaga NH, Jadeja RN, Rachakonda V, Ahmad D, McLean LP, Cheng K, et al. M1 muscarinic receptors modify oxidative stress response to acetaminophen-induced acute liver injury. Free radical biology & medicine. 2015;78:66–81. doi: 10.1016/j.freeradbiomed.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in enzymology. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 22.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. The Journal of biological chemistry. 2008;283:13565–77. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicological sciences : an official journal of the Society of Toxicology. 2003;76:229–36. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- 24.Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, et al. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. Journal of hepatology. 2006;44:918–29. doi: 10.1016/j.jhep.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Saberi B, Shinohara M, Ybanez MD, Hanawa N, Gaarde WA, Kaplowitz N, et al. Regulation of H(2)O(2)-induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. American journal of physiology Cell physiology. 2008;295:C50–63. doi: 10.1152/ajpcell.90654.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicological sciences : an official journal of the Society of Toxicology. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. The Journal of nutrition. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 28.Henrich CJ, Brooks AD, Erickson KL, Thomas CL, Bokesch HR, Tewary P, et al. Withanolide E sensitizes renal carcinoma cells to TRAIL-induced apoptosis by increasing cFLIP degradation. Cell death & disease. 2015;6:e1666. doi: 10.1038/cddis.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grogan PT, Sleder KD, Samadi AK, Zhang H, Timmermann BN, Cohen MS. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Investigational new drugs. 2013;31:545–57. doi: 10.1007/s10637-012-9888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Um HJ, Min KJ, Kim DE, Kwon TK. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochemical and biophysical research communications. 2012;427:24–9. doi: 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- 31.Fong MY, Jin S, Rane M, Singh RK, Gupta R, Kakar SS. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PloS one. 2012;7:e42265. doi: 10.1371/journal.pone.0042265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee TJ, Um HJ, Min do S, Park JW, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free radical biology & medicine. 2009;46:1639–49. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Mandal C, Dutta A, Mallick A, Chandra S, Misra L, Sangwan RS, et al. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis : an international journal on programmed cell death. 2008;13:1450–64. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Seal CJ, Howes MJ, Kite GC, Okello EJ. In vitro protective effects of Withania somnifera (L.) dunal root extract against hydrogen peroxide and beta-amyloid(1–42)-induced cytotoxicity in differentiated PC12 cells. Phytotherapy research : PTR. 2010;24:1567–74. doi: 10.1002/ptr.3261. [DOI] [PubMed] [Google Scholar]
- 35.Oh JH, Lee TJ, Park JW, Kwon TK. Withaferin A inhibits iNOS expression and nitric oxide production by Akt inactivation and down-regulating LPS-induced activity of NF-kappaB in RAW 264.7 cells. European journal of pharmacology. 2008;599:11–7. doi: 10.1016/j.ejphar.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Singh D, Aggarwal A, Maurya R, Naik S. Withania somnifera inhibits NF-kappaB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytotherapy research : PTR. 2007;21:905–13. doi: 10.1002/ptr.2180. [DOI] [PubMed] [Google Scholar]
- 37.Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O’Connor T, et al. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicological sciences : an official journal of the Society of Toxicology. 2001;59:169–77. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 38.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. The Journal of pharmacology and experimental therapeutics. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 39.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 40.Harrill AH, Watkins PB, Su S, Ross PK, Harbourt DE, Stylianou IM, et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome research. 2009;19:1507–15. doi: 10.1101/gr.090241.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du K, McGill MR, Xie Y, Bajt ML, Jaeschke H. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2015;81:62–70. doi: 10.1016/j.fct.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Jiang Y, Fan X, Tan H, Zeng H, Wang Y, et al. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicology letters. 2015;236:82–9. doi: 10.1016/j.toxlet.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicological sciences : an official journal of the Society of Toxicology. 2002;67:322–8. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]