Abstract
Phospholipase D is an enzyme that catalyzes the hydrolysis of phosphatidylcholine, the major phospholipid in the plasma membrane, to generate an important signaling lipid, phosphatidic acid (PA). PA is a second messenger that regulates vesicular trafficking, cytoskeletal reorganization, cell signaling in immune cells and other cell types. Published studies by using pharmacological inhibitors or protein overexpression indicate that PLD plays a positive role in T cell receptor (TCR)-mediated signaling and cell activation. Here we used mice deficient in PLD1, PLD2, or both to study the function of these enzymes in T cells. Our data showed that PLD1 deficiency impaired TCR-mediated signaling, T cell expansion, and effector function during immune responses against Listeria monocytogene; however, PLD2 deficiency had a minimal impact on T cells it. Biochemical analysis indicated that PLD1 deficiency affected Akt and PKCθ activation. In addition, PLD1 deficiency also impaired TCR downregulation and secondary T cell response. Together, our results suggested that PLD1 plays an important role in T cell activation.
Keywords: T cells, cytotoxicity, PI3K pathway, and signal transduction
Introduction
Phospholipase D (PLD) is a widely expressed enzyme that catalyzes the hydrolysis of phosphatidylcholine, the major phospholipid in the membrane, to produce the water-soluble choline and phosphatidic acid (PA). PA is a second messenger that is thought to function in vesicular trafficking and cytoskeletal reorganization (1-3). PA can also bind important signaling proteins, such as Raf and mTOR, to regulate their activation and function in different signaling pathways (4, 5). PLD is not the only enzyme that can generate PA. PA is mainly produced by two ways: the hydrolysis of phosphocholine by PLD and the phosphorylation of diacylglycerol (DAG) by diacylglyercol kinase (DGK). The PLD family consists of two closely related members, PLD1 and PLD2. Interestingly, although these two proteins share similar sequences and structural domains, they have different subcellular localizations. PLD1 is primarily localized to the secretory granules; however, it redistributes to the plasma membrane after stimulation. In contrast, PLD2 is present at the plasma membrane (6).
The function of PLD proteins in the immune system has been extensively studied in the past decade. Published data have clearly indicated that PLD proteins are important in immunoreceptor signaling and immune cell function. It has been shown that PLDs can function in Fcγ-mediated phagocytosis by macrophages and are involved in the activation of NADPH oxidase in neutrophils (7). The PLD function in mast cells has also been studied previously. Those studies, which were done mostly by using pharmacological inhibitors, such as 1-butanol, or by overexpression of the WT or DN (dominant negative) forms of PLD proteins, indicate that PLD1 and PLD2 play positive roles in FcεRI-mediated signaling and mast cell function (6, 8, 9). However, we recently generated PLD1 and PLD2-deficienct mice to study their function in mast cells. Our data demonstrate that PLD1 deficiency impairs FcεRI-mediated F-actin disassembly and mast cell degranulation; however, PLD2 deficiency enhances microtubule formation and degranulation. In addition, PLD deficiency affects FcεRI-mediated activation of the PI3K pathway and RhoA (10). Our results suggest that PLD1 and PLD2, two proteins that catalyze the same enzymatic reaction, can regulate different steps in mast cell degranulation.
In addition to studies on PLD in macrophages, neutrophils, and mast cells, it has been demonstrated that PLD proteins play an important role in T cells. Early studies using the Jurkat T cell line show that crosslinking of the TCR or PMA treatment increases PLD activity (11, 12). 1-butanol can reduce TCR-mediated calcium flux and overall tyrosine phosphorylation of proteins, including the TCRζ chain and block AP-1 activation. Overexpression of either PLD1 or PLD2 in Jurkat cells enhances TCR-mediated NFAT activation. In contrast, overexpression of the DN form of PLD1 or PLD2 inhibits it, indicating that PLD proteins play a positive role in TCR-mediated signaling(13, 14). Interestingly, overexpression of DN PLD2, but not PLD1, impairs TCRζ phosphorylation, suggesting that PLD1 and PLD2 function differently in this signaling pathway. Another study using the Jurkat T cell line shows that PLD2 can function upstream of Ras during T cell activation (12). The engagement of the TCR and LFA-1 leads to the activation of PLD2 and production of PA, which might be converted to DAG by phosphatidic acid phosphatase (PAP). DAG then recruits and activates RasGRP1. It is also possible that PA may be involved in Ras activation through binding to Sos directly. Recent data showed that PLD1-specific inhibitor, VU0359595, could inhibit TCR-mediated Ras-MAPK activation and block HIV infection(15), suggesting that PLD1 is required for activation of this pathway. In addition, PLD may have different roles in T regulatory cells (Treg) and conventional T cells. PLD1 expression is significantly lower in Treg cells than in conventional T cells. Consequently, anti-CD3ε stimulation only increases PLD activity in conventional T cells, but not in Treg cells (13). However, it has also been shown that exocytosis of CTLA-4 in Treg cells is dependent on PLD activity as 1-butanol inhibits the surface expression of CTLA-4 (16). While these data strongly suggest that PLD1 and PLD2 may play an important role in TCR-mediated signaling and T cell activation, most of these studies were done using pharmacological inhibitors or by overexpression of PLD; therefore, the function of PLDs in TCR signaling and T cell activation has yet to be fully completely delineated.
In this study, we used PLD-deficient mice to study the function of PLD proteins in TCR-mediated signaling and T cell function in vitro and during immune responses against Listeria monocytogenes infection. Our data showed that among the two members of PLD family, PLD1 plays a more dominant role in T cells than PLD2. PLD1 deficiency impairs TCR-mediated signaling and T cell expansion during primary and memory response while PLD2 deficiency had a minimal impact on T cell function.
Materials and Methods
Mice
PLD1-/- (PLD1KO), PLD2-/- (PLD2KO), and PLD1-/-PLD2-/- (PLDdKO) mice were generated as described previously (10). They were crossed with the OT-I transgenic mice (Jackson laboratory, Bar Harbor, ME) to generate PLD1KO/OT-1, PLD2KO/OT-1, and PLDdKO/OT-1 mice. All mice were used in accordance with the National Institutes of Health guidelines. The procedures in this study were approved by the Duke University Institutional Animal Care and Use Committee. Mice were housed in a specific pathogen-free facility.
Listeria Infection and adoptive transfer
For adoptive transfer of naïve OT-I cells, 1 × 105 CD45.1+ WT and CD45.2+ PLD1KO, PLD2KO, and PLDdKO OT-I CD8+ T cells were adoptively transferred into non-irradiated naïve recipient mice (CD45.1+CD45.2+) at day 0. One day after OT-I cell transfer, recipient mice were injected i.v. with 1.5 × 104 CFU Listeria monocytogenes expressing ovalbumin (Lm-OVA). To induce a secondary response, mice were injected with 1.5 × 105 CFU Lm-OVA.
FACS analysis
Most of the antibodies used for flow cytometry were purchased from Biolegend unless indicated. Single-cell suspensions were prepared from the spleens, lymph nodes, and blood from different mice after lysis of RBCs with ammonium-chloride-potassium lysis buffer. To detect OVA-specific CD8 T cells, cells were stained with DimerX (H-2Kb-Ig recombinant fusion protein, BD Biosciences) loaded with the OVA257–264 peptide (SIINFEKL). To determine the activation status of T cells, cells were stained with PE- or APC-conjugated antibodies, such as PE-anti-CD62L and CD11a, APC–anti-CD44 and TCR-β. To detect the percentage of OT-I T cells among total CD8+ T cells after adoptive transfer, cells were stained with PE-anti-Vα2, APC-Cy7–anti-CD8, PE-Cy7–anti-CD45.1, FITC–anti-CD45.2, and Pacific Blue-live/dead (Invitrogen). To detect granzyme and Ki-67 expression, cells were first stained with surface markers, fixed, and permeabilized using the Cytofix/Cytoperm kit (eBiosciences), and then stained with APC–anti-granzyme A and PE–anti-granzyme B or anti–Ki-67. For intracellular staining of cytokines, splenocytes were restimulated with 1 μM OVA257–264 peptide for 5 hours in the presence of monensin (Biolegend), stained with anti-CD8, fixed, permeabilized, and then stained again with APC-anti–IFN-γ. Samples were analyzed on FACS-Canto II (BD Biosciences). Flow plots shown were analyzed with FlowJo (Ashland, OR).
TCR down-regulation
Down-regulation of the TCR on CD4+ and CD8+ T cells was performed as described previously (17). Briefly, splenocytes were treated with biotin-anti-CD3ε (2C11, 10μg/ml) and anti-CD28 (2μg/ml) on ice for 30min followed by washing with cold RPMI. Cells were then moved to 37°C at the indicated time points. The level of the surface TCR remaining was quantitated by FACS analysis after staining with streptavidin-conjugated APC. The TCR down-regulation% was calculated based on the mean fluorescence intensity (MFI) of CD3 surface expression. The TCR down-regulation%= 100×|MFI(0)-MFI(t)}/MFI0.
In vitro cytotoxicity assays
CD8+ T cells were purified from PLDKO OT-1+ and WT OT-1+ splenocytes using MojoSort streptavidin nanobeads (Biolegend). These cells were then primed with 1μM OVA257–264 peptide for 2 days. EL4 cells loaded with 10μM OVA peptide for 1 hour were labeled with 10μM cell tracker orange (Invitrogen) as target cells (Cell tracker orangeHigh). EL4 cells without the OVA peptide loading were labeled with 0.5μM cell tracker orange as controls (Cell tracker orangelow). These two populations of EL4 cells were mixed in a ratio of 1:1. CTLs were mixed with 1×105 EL4 cell mixture at effector/target ratios of 1:1, 2:1, 4:1 in a 96-well round bottom plate at 37°C for 3 hours. Specific killing was determined as 100-(100× (% of peptide-loaded targets/% of control targets in the presence of CTLs)/(% of peptide-loaded targets/% of control target in the absence of CTLs).
Western Blotting
For detection of phosphorylated proteins, CD4+ and CD8+ T cells were purified by negative selection by using MojoSort streptavidin nanobeads (Biolegend). These T cells were stimulated with biotin-anti- CD3ε and anti-CD28, followed by streptavidin for the indicated time points. Cell lysates were analyzed by Western blotting with the following antibodies: pTyr(4G10), pAKT(Ser473), pAKT(Thr308), pERK1/2 (Thr202/Tyr204), pPKCθ(Thr538), pPKCδ(Thr505 and Y311), AKT, and PKCθ from Cell Signaling. For Western blotting, samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with primary antibodies, nitrocellulose membranes were washed three times and probed with either anti–mouse or rabbit Ig conjugated to AlexaFluor 680 (Molecular Probes) or IRDye800 (Rockland). Membranes were then visualized with the LI-COR Bioscience Odyssey system (LI-COR).
Results
PLD1 is required for optimal expansion of CD8+ T cell
We recently generated PLD1-/- (PLD1KO), PLD2-/- (PLD2KO), and PLD1-/-PLD2-/- (PLDdKO) mice, and used them to study PLD function in FcεRI-mediated signaling and mast cell function(10). In this study, we used these mice to study PLD function in T cells during immune responses. To this end, we infected WT, PLD1KO, PLD2KO, and PLDdKO mice with a low dose (1.5 × 104 CFU /mouse) of Listeria monocytogene that express ovalbumin (LmOVA) by intravenous injection. Seven days after infection, T cells from these mice were analyzed by flow cytometry for their activation and expansion.
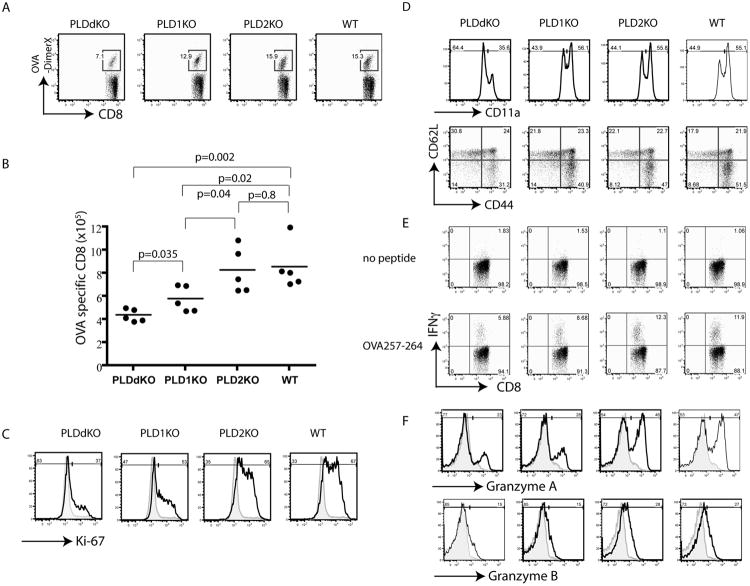
To identify antigen-specific T cells, we loaded H-2Kb DimerX with the OVA 257-264 peptide and used it in combination with other cell surface and activation markers in FACS analysis. As shown in Fig.1A, there was a clear difference in the percentages of OVA-specific T cells (CD8+DimerX+) in these mice. Close to 15% of CD8+ T cells were present in WT and PLD2kO mice; however, the percentages of OVA-specific T cells in PLD1KO and PLDdKO mice were reduced to ∼12.9% and 7.1%, respectively. We also enumerated total numbers of OVA-specific T cells in the spleens of these mice. Corresponding to the reduced percentages of these T cells, both PLD1KO and PLDdKO mice had an apparent reduction in the number of OVA-specific T cells (Fig.1B). In addition, there was a significant difference in the numbers of antigen-specific T cells between PLD1KO and PLDdKO mice (p=0.035, Fig.1B). These data suggested that PLD1 may play a more dominant role in T cells than PLD2; however, in the absence of PLD1, PLD2 may also contribute to antigen-driven T cell expansion.
Figure 1.
CD8+ T cell response to Lm-OVA infection in PLD-deficient mice. PLD1KO, PLD2KO, PLDdKO, and WT mice were infected with Lm-OVA. At day 7 after infection, splenocytes from these mice were analyzed by flow cytometry. (A) OVA-specific CD8+ T cells in the spleen of infected mice. (B) The number of OVA-specific CD8+ T cells in the spleen. Data are shown as mean ± SEM. (C) Ki-67 expression in CD8+ T cells. (D) Representative plots of CD11a and CD62L/CD44 on CD8+T cells. (E) IFNγ production. Splenocytes were restimulated with 1μM OVA peptide for 5 h in the presence of monensin before intracellular staining. (F) Intracellular staining of Granzymes A and B. Data are representative of three independent experiments.
To further determine whether PLD deficiency affected T cell proliferation, we performed intracellular staining of these cells with antibodies against the Ki-67 protein, which marks proliferating cells. At day 7 after listeria infection, WT and PLD2KO CD8+ T cells had similar percentages of Ki-67+ cells, at 67% and 65%, respectively. PLD1KO T cells had slightly reduced percentage (53%). In contrast, PLDdKO T cells had the lowest percentage of Ki-67+ cells, at only ∼37% (Fig.1C). These data suggested that PLD deficiency impaired T cell proliferation.
Upon T cell activation, CD8+T cells upregulate CD11a, which is involved in CD8+ T cell expansion and differentiation (18). As shown in Fig.1D, CD11a upregulation on CD8+ T cells was impaired by deficiency in both PLD1 and PLD2; however not by PLD1 deficiency alone. T cell activation also leads to downregulation of CD62L and upregulation of CD44. At day 7 after infection, the percentage of activated CD8+T cells (CD44+CD62L-) cells in WT and PLD2KO mice was ∼ 51.5% and 47%, respectively; however, it was reduced to 40.9% in PLD1KO mice and reduced further to 31.2% in PLDdKO mice.
Upon activation and differentiation into effector cells, CD8+ T cells produce IFN-γ and granzymes, which are important effector molecules in T cell function. We next examined the ability of these CD8+ T cells to produce IFN-γ. Splenocytes from these mice were stimulated in vitro in the presence of the OVA 257-264 peptide. The percentage of IFN-γ producing cells was analyzed by intracellular staining followed by flow cytometry. As shown in Fig.1E, IFN-γ production by CD8+ T cells was impaired by PLD1 or PLD1 and PLD2 double deficiency. We further analyzed expression of granzyme A and B by intracellular staining. As seen in Fig.1F, the percentages of WT and PLD2KO CD8+ T cells expressing granzyme A were highest, at 47% and 46%, respectively. In contrast, only 28% of PLD1KO and 23% of PLDdKO T cells expressed granzyme A. Similar reduction in the expression of granzyme B was also seen in PLD1KO and PLDdKO T cells (Fig.1F), indicating that PLD deficiency affected the expression of these effector molecules in CD8+ effector T cells.
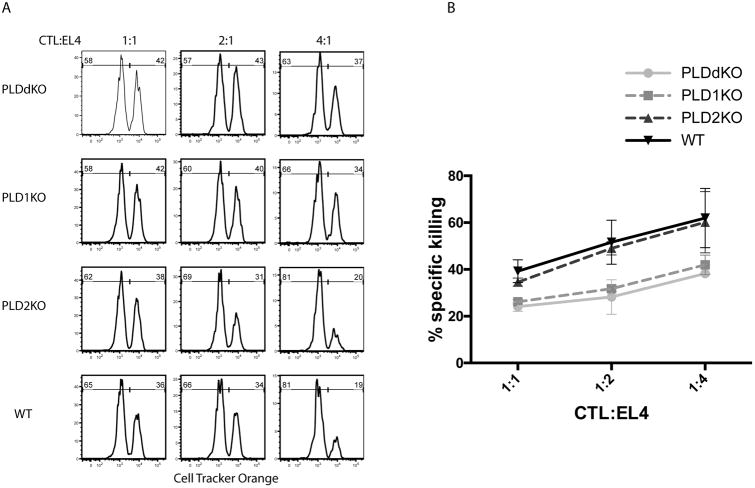
We further tested whether CD8 T cell function was affected by PLD deficiency in vitro. WT, PLD1KO, PLD2KO, and PLDdKO OT-1 T cells were activated by antigen presenting cells in the presence of the OVA peptide and differentiated into CTLs. To test whether their cytotoxicity was affected, we loaded EL-4 cells with the OVA peptide and 10 μM Cell Tracker Orange as target cells (Cell Tracker Orangehigh). EL-4 cells without the OVA peptide were loaded with a lower concentration (0.5μM) of Cell Tracker Orange as control cells (Cell Tracker OrangeLow). CTLs were incubated in ratios of 1:1, 2:1, and 4:1 with these EL-4 cells that were pre-mixed in a ratio of 1:1. After incubation for 3 hours, these cell mixtures were analyzed by flow cytometry. As shown in Fig.2A, in the presence of WT CTLs, the percentage of Cell tracker orangehigh cells was gradually reduced with the increased ration of CTL:EL4. At the ratio of 4:1, the percentage of EL-4 cells loaded with the OVA peptide was reduced from 50% to 19%. PLD2KO cells killed similarly as WT CTLs; however, PLD1KO and PLDdKO CTLs killed less efficiently compared with WT and PLD2KO cells. At the ratio of 4:1, there was still 34% and 37% of Cell tracker Orangehigh cells. Together, these data indicated that PLD1 deficiency affected T cell activation, expansion, cytokine production, granzyme expression, and cytotoxicity. In the absence of PLD1, PLD2 deficiency may cause additional impairment on T cell function.
Figure 2. Effect of PLD-deficiency on CTL cytotoxicity.
(An) In vitro cytotoxicity assay. EL-4 cells loaded with the OVA257–264 (SIINFEKL) peptide were labeled with 10 μM cell tracker orange (cell tracker orangehigh). EL-4 cells without the peptide were labeled with 0.5 μM cell tracker orange(cell tracker orangelow). These EL-4 cells were pre-mixed in a ratio of 1:1. CTLs were incubated with the EL-4 mixture at effector/target ratios of 1:1, 2:1, and 4:1 for 3 hours before FACS analysis. (B) Quantification of in vitro cytotoxicity. Data are representative of three independent experiments.
The impairment caused by PLD deficiency is T cell intrinsic
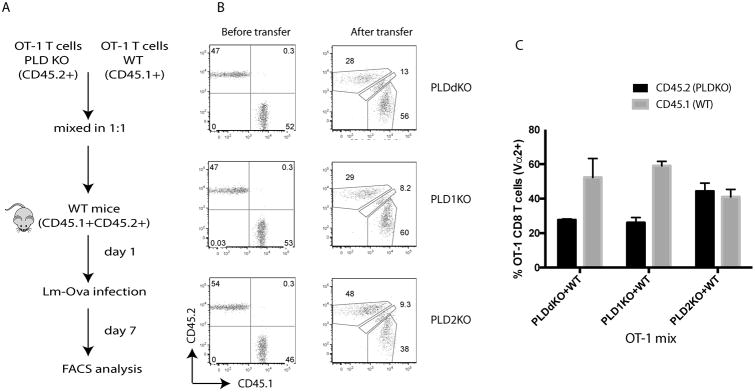
PLD proteins are ubiquitously expressed in different cell types including various immune cells. To exclude the possibility that impaired T cell expansion and function in PLD-deficient mice were due to defects in other immune cells, such as antigen-presenting cells, we performed an adoptive transfer experiments as described in Fig.3A. OT-1 T cells from CD45.1+ WT were mixed with the ones from CD45.2+ PLDKO in a ratio of 1:1 and injected intravenously into CD45.1+CD45.2+ WT mice. One day after transfer, these mice were infected intravenously with Listeria-OVA. Seven days later, splenocytes and lymph node cells were analyzed by flow cytometry. As seen in Fig.3B, similar percentages of WT and PLD KO T cells were mixed before the adoptive transfer. Seven days after infection, a similar ratio of CD45.2+/CD45.1+ cells was seen in mice received WT and PLD2KO OT-1 T cells before and after the transfer (Fig.2B and 2C); however, the ratios of CD45.2+/CD45.1+ cells were decreased in mice with PLD1KO/WT and PLDdKO/WT OT-1 T cells after listeria infection. These data indicated that the defect in antigen-induced T cell expansion was T cell-intrinsic, although we could not exclude the possibility that deficiency of PLD proteins in other cell types may also contribute to T cell defect.
Figure 3. T cell-intrinsic requirement of PLDs in T cell expansion.
(A) A schematic diagram of the adoptive transfer procedure. A mixture of 1×105 WT OT-I cells (CD45.1+) and 1×105 PLDKO OT-I cells (CD45.2+) were mixed and transferred intravenously into WT (CD45.1+CD45.2+) recipient mice. On the following day, these recipients were infected i.v. with Lm-OVA. (B) Left panel, FACS analysis of donor naïve OT-1 T cell mixture before adoptive transfer. Right panel, FACS analysis of OT-1 T cells at day 7 after adoptive transfer. Cells were gated on live CD8+Vα2+ T cells from the spleen. (C) The percentages of WT and PLD-deficient OT-I cells among total CD8+Vα2+ T cells in the spleen after Lm-OVA infection. Data are shown as mean ± SEM. Data are representative of two independent experiments with five mice in each experiment.
PLD deficiency on TCR signaling
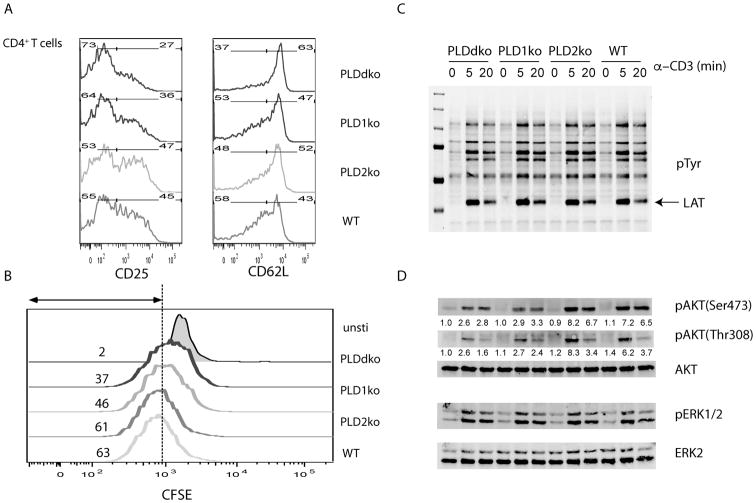
The data above suggested that PLD proteins, especially PLD1, play an important role in T cell activation, expansion, and function. Next, we determined the impact of PLD deficiency on TCR-mediated signaling and T cell activation in vitro. First, we examined whether PLD deficiency affected T cell activation in vitro. Splenocytes were isolated from WT, PLD1KO, PLD2KO and PLDdKO mice and activated by anti- CD3ε antibodies. Twenty-four hours after activation, expression of surface markers, such as CD25 and CD62L, was analyzed on CD4+ T cells. As seen in Fig.4A, ∼45% WT cells and ∼47% of PLD2KO CD4+ T cell upregulated CD25; however, only 36% PLD1KO and 27% PLDdKO T cells were CD25+. Similar results were also seen for CD62L expression. TCR-mediated downregulation of CD62L in PLD1KO and PLDdKO T cells was impaired. We also assayed T cell proliferation by CFSE labeling. T cell proliferation leads to dilution of CFSE. As seen in Fig.4B, PLDdKO and PLD1KO T cells had slower dilution of CFSE than WT and PLD2KO T cells. Similar results were also seen for CD8+ T cells (data not shown).
Figure 4. PLD-deficiency on T cell activation and TCR signaling.
(A) Representative flow analysis of CD25 and CD62L. Splenocytes were activated with anti-CD3ε for 20 hours before analysis. (B) CFSE dilution in CD4+ T cells after anti- CD3ε stimulation for 2 days. (C) Biochemical analysis of TCR-mediated signaling pathway. Purified CD4+ T cells were stimulated with biotin-anti-CD3ε and anti-CD28, followed by streptavidin crosslinking for different time points. Cell lysates were analyzed by Western blotting with antibodies against pTyr, pAKT(Ser473), pAKT(Thr308), and pERK1/2 (Thr202/Tyr204). AKT and ERK proteins were normalized by reblotting with anti-AKT and ERK2 antibodies respectively. The numbers shown are relative intensities for the phosphorylated form of proteins normalized to total proteins. Data are representative of three independent experiments.
We next performed biochemical analysis of TCR signaling in these T cells. T cells were purified from the spleens and lymph nodes of WT, PLD1KO, PLD2KO and PLDdKO mice by negative selection. These cells were then activated by cross-linking with anti-CD3ε and anti-CD28 antibodies for 0, 5, and 20 mins before lysis. Crosslinking of the TCR leads to activation of protein tyrosine kinases, such as Lck and ZAP-70, and subsequent phosphorylation of proteins, including LAT, SLP-76, and PLC-γ1(19). As shown in Fig.4C, overall tyrosine phosphorylation of proteins was similar in T cells from these mice. LAT is an adaptor protein that mediates TCR-calcium flux and Ras-Erk activation. LAT phosphorylation was not affected by PLD deficiency. We further assayed whether PLD deficiency affected TCR-mediated calcium flux by loading T cells with calcium indicator, indo-1, followed by analysis by flow. No significant differences were seen in calcium mobilization induced by anti- CD3ε crosslinking (data not shown). We further assayed activation of the Ras-Erk and PI3K pathways by Western blotting with anti- pERK1/2 (Thr202/Tyr204), pAKT(Ser473), and pAKT(Thr308) antibodies. As shown in Fig.4D, TCR-mediated Erk activation was similar in these T cells; however, PLD1-deficiency or PLD1 and PLD2 double deficiency significantly impaired phosphorylation of Akt at both Ser473 and Thr308, suggesting that PLD1 is important for TCR-mediated Akt activation.
PLD deficiency on TCR downregulation
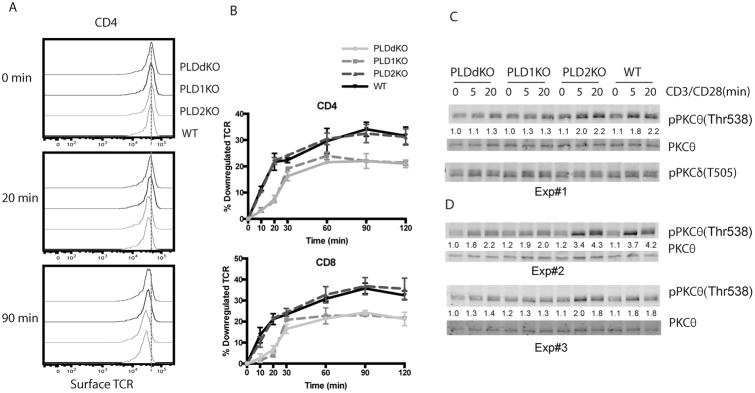
It has been shown that PLD proteins play an important role in receptor-mediated endocytosis. Epidermal growth factor (EGF) endocytosis can be accelerated by overexpression of WT PLD and retarded by overexpression of catalytically inactive forms (20). We examined whether deficiency in either PLD1 or PLD2 protein also affected downregulation of the TCR from the cell surface following T cell activation. To this end, we first coated splenocytes from WT, PLD1KO, PLD2KO and PLDdKO mice with biotinylated anti- CD3ε antibodies on ice, then activated them at 37°C for 0, 10, 20, 30, 60, 90, and 120 mins in the presence of anti-CD28 antibodies. The expression level of the surface TCR was then determined by staining these cells with streptavidin-APC and analyzed by flow. As shown in Fig.5A and B, TCR was gradually downregulated after crosslinking with anti- CD3ε and anti-CD28. At 60 mins after activation, TCR surface expression was reduced by ∼30% for WT and PLD2KO T cells, however, for PLD1KO and PLDdKO T cells, maximal downregulation only reached to 20%, indicating that PLD1 deficiency impairs downregulation of the TCR during T cell activation.
Figure 5. PLD-deficiency on TCR downregulation.
(A) Representative plots of surface TCR expression on splenic CD4+ T cells from PLDKO and WT mice before and after anti-CD3 and anti-CD28 stimulation. The dash line shows the mean MFI of CD3 before stimulation. (B) Quantitated TCR downregulation in CD4+ and CD8+ T cells. (C) PKCθ activation by Western blotting. Purified CD4+ T cells were stimulated with biotin-anti-CD3ε and biotin-anti-CD28, followed by streptavidin crosslinking for different time points. Cell lysates were analyzed by Western blotting with pPKCθ(Thr538), PKCθ, and anti-pPKCδ(Thr505). The relative intensities of pPKCθ was normalized by the intensity of pan-PKCθ. (D) PKCθ activation. Additional experiments showing that PLD1-deficiency impaired PKCθ activation.
PKCs play important roles in the TCR downregulation. While PKCα is involved in the downregulation of the unengaged TCR, PKCθ is important for the downregulation of engaged TCRs by phosphorylating the Ser126 residue in CD3γ. Phosphorylation of Ser126 makes the di-leucine motif accessible to binding by AP2, which leads to TCR endocytosis (21). It is possible that PLD deficiency affects PKCθ activation and subsequent CD3γ phosphorylation. To determine PKCθ activation, T cells were activated with anti-CD3ε and anti-CD28 antibodies for 0, 5, and 20 minutes. Protein lysates were prepared and analyzed by Western blotting with anti- pPKCθ(Thr538) and pan-PKCθ antibodies. As seen in Fig.5C, in WT and PLD2KO T cells, phosphorylation of PKCθ was increased at 5 and 20 minutes after TCR cosslinking; however, PKCθ phosphorylation was reduced in PLDdKO and PLD1KO T cells. As a control, phosphorylation of PKCδ (Thr505) was not affected. Similar reduction in PKCθ phosphorylation was consistently seen in other experiments we performed (Fig.5D). These data indicated that in addition to Akt activation, PLD1 deficiency also affected TCR-mediated activation of PKCθ. Impaired PKCθ activation likely led to reduced CD3γ phosphorylation and slower downregulation of the TCR from the cell surface.
PLD deficiency affects secondary T cell response
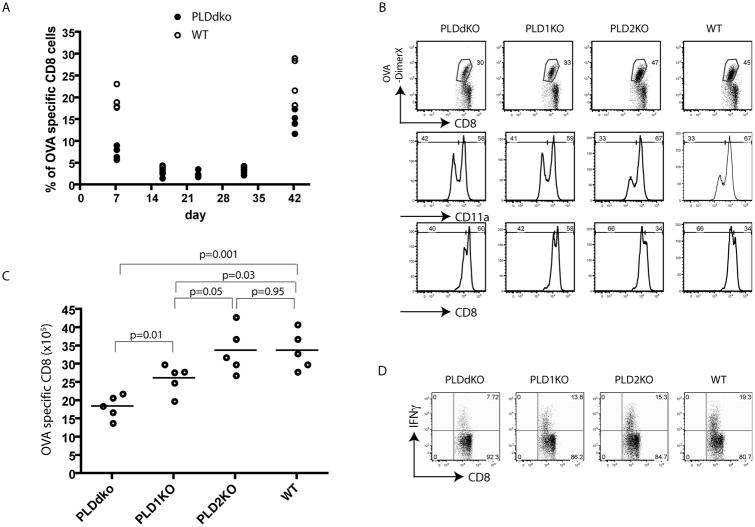
Our data clearly indicated that TCR signaling and T cell expansion in PLD1KO and PLDdKO mice were impaired during primary T cell response against Listeria infection. Next, we examined whether PLD proteins also had a similar role in secondary T cell response during infection. We infected WT and PLDdKO mice with 1.5 × 104 CFU Lm-OVA and bled them at day 7, 16, 23, 32 and 42. The percentage and number of OVA-specific T cells in the blood were analyzed by FACS after staining with H-2Kb DimerX with the OVA peptide. At day 37, mice were challenged again with 1.5 × 105 CFU Lm-OVA to induce memory responses. As shown in Fig.6A, the percentage of OVA-specific CD8+ T cells in PLDdKO mice was approximately 3 times less than in WT mice at day 7 after initial infection. After initial T cell expansion, T cells undergo contraction and differentiate into memory T cells. There were no significant differences in the percentages of OVA-specific WT and PLDdKO T cells at day 16, 23, and 32. However, upon secondary challenge with listeria, WT T cells expand more quickly than PLDdKO T cells.
Figure 6. Secondary CD8 T cell response in PLD-deficient mice.
Mice were infected i.v. with 1.5 × 104 Lm-OVA at day 0, and challenged with 1.5 × 105 Lm-OVA at day 37. (A) Kinetics of OVA-specific CD8+ T cell response in PLDdKO and WT mice. Representative plots of OVA-specific CD8+ T cells in the blood from infected mice. (B) OVA-specific T cells after challenge. At day 5 after reinfection, splenocytes were stained with OVA-DimerX, CD11a, and CD8 and analyzed by flow cytometry. (C) The number of OVA-specific CD8+ T cells in the spleen at day 5 after Lm-OVA reinfection. Data are shown as mean ± SEM. (D) IFNγ production by CD8+ T cells. Splenocytes were restimulated with 1μM OVA peptide for 5 hours in the presence of Monensin before intracellular staining. Data are representative of three independent infections.
We also determined the memory responses of PLD1KO and PLD2KO T cells following the same procedure. Five days after secondary challenge (Day 42), splenocytes were analyzed by flow cytometry after staining with H-2Kb DimerX and antibodies against CD11a, CD4, CD8, and other surface markers. As shown in Fig.6B, tetramer staining showed that similar to the data in Fig.6A, PLDdKO T cells had reduced OVA-specific CD8+ T cell expansion upon challenge. ∼30% CD8+ T cells were OVA specific (CD8+DimerX+), while ∼45% of WT CD8+ T cells and 47% of PLD2KO T cells were OVA-specific. PLD1 deficiency alone also affected memory T cell expansion as only 33% of PLD1KO T cells were OVA-specific. We quantitated the numbers of OVA-specific T cells in the spleens of these mice after secondary challenge. As shown in Fig.6C, the number of OVA-specific T cells in PLD2KO and WT mice was similar; however, it was reduced in PLD1KO mice and further reduced in PLDdKO mice. The difference in the numbers of T cells in in PLD1KO and PLDdKO mice was statistically significant (p=0.01). In addition, PLD deficiency also affected the number of IFN-γ producing of CD8 T cells in PLD1KO and PLDdKO mice (Fig.6D). Together, these data indicated that in addition to their roles on initial T cell activation, PLD deficiency, especially PLD1 deficiency, impaired memory T cell expansion and interferon production.
Discussion
In this study, we used mice deficient in PLD1, PLD2, or both to study the function of PLD in T cells in vivo and in vitro. Although PLD1 and PLD2 catalyze the same enzymatic reaction, their deficiencies had differential effects on T cell function. Our data clearly showed that PLD1 had a more dominant role in T cells than PLD2. While PLD2 deficiency alone had a minimal impact on T cells, PLD1 deficiency affected TCR-mediated signaling, T cell activation, and effector function. Our adoptive transfer experiment showed that the defects in T cell activation in PLD1KO and PLDdKO mice were T cell intrinsic as these T cells had reduced expansion when transferred into WT mice. We further investigated how PLD deficiency affected T cell activation by analyzing TCR proximal signaling events. Our data showed that PLD1 deficiency did not affected overall tyrosine phosphorylation of proteins and main proximal signaling events, such as Ca2+ flux and Ras-MAPK activation; however, it impaired Akt and PKCθ activation. How does PLD1 deficiency affects activation of these proteins is not clear. It is known that PA can bind and activate mTORC1 and mTORC2 complexes, which regulate cell growth and proliferation (22). While mTOR is a target protein of the PI3K/AKT signaling pathway, interestingly, mTORC2 can also phosphorylate Akt on Serine 473. Thus, it is possible that in the absence of PLD1, activation of mTORC complexes was impaired, further leading to defective Akt activation. It is known that PKC can interact with both PLD1 and PLD2 and enhance their lipase activity (23, 24). On the other hand, PA can also be converted to DAG by phosphatidic acid phosphatase (PAP). It is possible that PLD deficiency impairs DAG production and subsequent PKCθ activation in T cells.
PLD1 deficiency affects TCR-mediated signaling and T cell activation. In contrast, PLD2 deficiency had no apparent effect. Deficiency in both proteins had more severe effects on T cells than PLD1 deficiency alone, suggesting that in the absence of PLD1, PLD2 could compensate for the loss of PLD1 to some extent. Why deficiency in PLD1 or PLD2, two proteins with similar functions, affected T cells differently is not clear. T cells express both PLD1 and PLD2; but their relative abundance in T cells is not known. In addition, their intrinsic enzymatic activity or activation could be different. Moreover, PLD1 and PLD2 are known to localize to different subcellular compartments. PLD1 or PLD2 deficiency could lead to changes of the local concentrations of PA. Thus, the more severe impact of PLD1 deficiency on T cells could be because there is more PLD1 protein in T cells or PLD1 has higher enzymatic activity than PLD2. When T cells were only deficient in PLD1, TCR-mediated signaling and T cell activation were significantly impaired. When they were only deficient in PLD2, T cells were largely unaffected due to the presence of PLD1. Deficiency in both PLD proteins led to the total absence of PLD activity and more severe impairment on T cells.
Previous study using Jurkat cells indicated that PLD2 is required in LFA-1-mediated Ras activation (12). Interestingly, in Jurkat cells, PLD1 was mostly found in intracellular membranes while PLD2 was exclusively present in the plasma membrane. Silencing PLD2 expression by siRNA could block Ras activation stimulated through the TCR and LFA-1. It was speculated that the engagement of TCR and LFA-1 leads to PLD2 activation and production of PA, which may activate Ras through direct binding to Sos. PA can also be converted to DAG, which activates RasGRP1 and PKCθ. However, in this published study, activation of Ras was determined by imaging the localization of Raf RBD, which might not accurately reflect the activation status of Ras (12). This result needs to be confirmed by biochemical analysis of Ras activation in T cells. Our biochemical data indicated that in PLD1KO and PLDdKO T cells, TCR-mediated Erk was normal; however, the study by Taylor et al. showed that PLD1-specific small molecule inhibitor, VU0359595, could inhibit Erk1/2 phosphorylation similarly to a Mek/Erik inhibitor (15), suggesting that PLD1 is required for TCR-mediated Ras-MAPK activation. Why this result differs from ours is not clear. One possibility is that this PLD1 inhibitor, VU0359595, might not only inhibit PLD1. It might act nonspecifically to impair the activity of other proteins that function in the Ras-MAPK pathway. Previous studies also showed that 1-butanol could inhibit TCR-mediated overall tyrosine phosphorylation of proteins and calcium flux (11, 12); however, we did not observe any deficiencies in these proximal signaling events in PLD-deficient T cells. While it is possible that primary alcohols have non-specific effects, it is equally possible that PLD deficiency might be compensated for by other enzymes in lipid metabolism. Considering the reported importance of PLD enzymes in many cellular functions, it is puzzling that deficiency in PLD1 and PLD2 did not cause lethality or more severe phenotypes. The main production of PLD enzymatic reaction is PA, which is a secondary message that acts on other signaling proteins. PA can also be produced by hydrolysis of DAG by DGK or synthesized from lysophosphatidic acid by LPAAT (lysophosphatidic acid acyltransferase). FcεRI-mediated PA production is affected by DGKζ deficiency, suggesting that DGKζ also contributes to FcεRI-mediated PA production (25). Thus, it is possible that the activity of LPAAT or DGK is enhanced in PLD-deficient T cells to compensate for the loss of PLD proteins.
Previous studies using 1-butanol as a PLD inhibitor showed that PLD may function differently in T regulatory cells (Treg) and conventional T cells because of its lower expression in Treg cells (13). Interestingly, it was found that 1-butanol inhibits the surface expression of CTLA-4 (16), which suggested that PLD might be involved in Treg cell function. Our flow analysis of PLD-deficient mice showed that Treg cell development and CTLA-4 expression were not affected (data not shown). Thus, it is possible that reduced CTLA-4 expression by 1-butanol is a consequence of non-specific effect of this inhibitor.
It has been reported that PLD proteins are involved in receptor-mediate endocytosis (20). In this study, we showed that TCR downregulation from the cell surface was also affected by PLD1 or PLD double deficiency likely due to reduced PKCθ activation. PKCθ phosphorylates Ser126 in CD3γ, leading to AP-2 binding and TCR endocytosis (21). It is also possible that PLD1 deficiency affects actin reorganization, which is required the endocytosis of the TCR. Our published results indicate that PLD1 deficiency in mast cells impairs FcεRI-mediated F-actin disassembly. On the other hand, PLD2 deficiency enhances microtubule formation (10). It is possible that PLD proteins function differently in T and mast cells as we did not observe any significant effect of PLD2 deficiency on T cells. Still, the role of PLD proteins in cytoskeletal rearrangement in T cells needs to further investigated in order to fully understand how they function in T cells.
Although many previous studies suggested that PLD proteins play important roles in TCR signaling, T cell activation, and immune responses, many of these experiments were done using inhibitors that are not highly specific or by overexpression of the WT and DN forms of PLD1 and PLD2 proteins. Our results using T cells deficient in PLD1, PLD2, or both, suggest that out of the two members of the PLD family, PLD1 is required for optimal TCR-mediated signaling, T cell expansion, and T cell function during primary and secondary immune response. While currently PLD1 and PLD2 specific inhibitors are being tested against HIV, influenza, and other infections, or used to treat cancers, our data suggest that we must carefully consider the utility of these inhibitors, which will likely also inhibit immune responses.
Acknowledgments
The authors thank the Duke University Cancer Center Flow Cytometry and Transgenic Mouse facilities for their excellent service.
This work was supported by National Institutes of Health Grant AI093717
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Cockcroft S. Signalling roles of mammalian phospholipase D1 and D2. Cell Mol Life Sci. 2001;58:1674–1687. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehman N, Di Fulvio M, McCray N, Campos I, Tabatabaian F, Gomez-Cambronero J. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood. 2006;108:3564–3572. doi: 10.1182/blood-2006-02-005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Cambronero J, Di Fulvio M, Knapek K. Understanding phospholipase D (PLD) using leukocytes: PLD involvement in cell adhesion and chemotaxis. J Leukoc Biol. 2007;82:272–281. doi: 10.1189/jlb.0107033. [DOI] [PubMed] [Google Scholar]
- 4.Andresen BT, Rizzo MA, Shome K, Romero G. The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade. FEBS Lett. 2002;531:65–68. doi: 10.1016/s0014-5793(02)03483-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9:706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 6.Choi WS, Kim YM, Combs C, Frohman MA, Beaven MA. Phospholipases D1 and D2 regulate different phases of exocytosis in mast cells. J Immunol. 2002;168:5682–5689. doi: 10.4049/jimmunol.168.11.5682. [DOI] [PubMed] [Google Scholar]
- 7.Melendez AJ, Bruetschy L, Floto RA, Harnett MM, Allen JM. Functional coupling of FcgammaRI to nicotinamide adenine dinucleotide phosphate (reduced form) oxidative burst and immune complex trafficking requires the activation of phospholipase D1. Blood. 2001;98:3421–3428. doi: 10.1182/blood.v98.12.3421. [DOI] [PubMed] [Google Scholar]
- 8.Chahdi A, Choi WS, Kim YM, Fraundorfer PF, Beaven MA. Serine/threonine protein kinases synergistically regulate phospholipase D1 and 2 and secretion in RBL-2H3 mast cells. Mol Immunol. 2002;38:1269–1276. doi: 10.1016/s0161-5890(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown FD, Thompson N, Saqib KM, Clark JM, Powner D, Thompson NT, Solari R, Wakelam MJ. Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr Biol. 1998;8:835–838. doi: 10.1016/s0960-9822(98)70326-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhu M, Zou J, Li T, O'Brien SA, Zhang Y, Ogden S, Zhang W. Differential Roles of Phospholipase D Proteins in FcepsilonRI-Mediated Signaling and Mast Cell Function. J Immunol. 2015;195:4492–4502. doi: 10.4049/jimmunol.1500665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid PA, Gardner SD, Williams DM, Harnett MM. The antigen receptors on mature and immature T lymphocytes are coupled to phosphatidylcholine-specific phospholipase D activation. Immunology. 1997;90:250–256. doi: 10.1046/j.1365-2567.1997.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR. The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol. 2007;9:713–719. doi: 10.1038/ncb1592. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Seki Y, Takami M, Baban B, Chandler PR, Khosravi D, Zheng X, Takezaki M, Lee JR, Mellor AL, Bollag WB, Iwashima M. Enrichment of regulatory CD4(+)CD25(+) T cells by inhibition of phospholipase D signaling. Nat Methods. 2006;3:629–636. doi: 10.1038/nmeth903. [DOI] [PubMed] [Google Scholar]
- 14.Mollinedo F, Gajate C, Flores I. Involvement of phospholipase D in the activation of transcription factor AP-1 in human T lymphoid Jurkat cells. J Immunol. 1994;153:2457–2469. [PubMed] [Google Scholar]
- 15.Taylor HE, Simmons GE, Jr, Mathews TP, Khatua AK, Popik W, Lindsley CW, D'Aquila RT, Brown HA. Phospholipase D1 Couples CD4+ T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication. PLoS Pathog. 2015;11:e1004864. doi: 10.1371/journal.ppat.1004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mead KI, Zheng Y, Manzotti CN, Perry LC, Liu MK, Burke F, Powner DJ, Wakelam MJ, Sansom DM. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J Immunol. 2005;174:4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- 17.Yang CW, Hojer CD, Zhou M, Wu X, Wuster A, Lee WP, Yaspan BL, Chan AC. Regulation of T Cell Receptor Signaling by DENND1B in TH2 Cells and Allergic Disease. Cell. 2016;164:141–155. doi: 10.1016/j.cell.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Bose TO, Pham QM, Jellison ER, Mouries J, Ballantyne CM, Lefrancois L. CD11a regulates effector CD8 T cell differentiation and central memory development in response to infection with Listeria monocytogenes. Infect Immun. 2013;81:1140–1151. doi: 10.1128/IAI.00749-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Xu L, Foster DA. Role for phospholipase D in receptor-mediated endocytosis. Mol Cell Biol. 2001;21:595–602. doi: 10.1128/MCB.21.2.595-602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Essen M, Nielsen MW, Bonefeld CM, Boding L, Larsen JM, Leitges M, Baier G, Odum N, Geisler C. Protein kinase C (PKC) alpha and PKC theta are the major PKC isotypes involved in TCR down-regulation. J Immunol. 2006;176:7502–7510. doi: 10.4049/jimmunol.176.12.7502. [DOI] [PubMed] [Google Scholar]
- 22.Jaafar R, Zeiller C, Pirola L, Di Grazia A, Naro F, Vidal H, Lefai E, Nemoz G. Phospholipase D regulates myogenic differentiation through the activation of both mTORC1 and mTORC2 complexes. J Biol Chem. 2011;286:22609–22621. doi: 10.1074/jbc.M110.203885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JS, Exton JH. Regulation of phospholipase D2 activity by protein kinase C alpha. J Biol Chem. 2004;279:22076–22083. doi: 10.1074/jbc.M311033200. [DOI] [PubMed] [Google Scholar]
- 24.Oishi K, Takahashi M, Mukai H, Banno Y, Nakashima S, Kanaho Y, Nozawa Y, Ono Y. PKN regulates phospholipase D1 through direct interaction. J Biol Chem. 2001;276:18096–18101. doi: 10.1074/jbc.M010646200. [DOI] [PubMed] [Google Scholar]
- 25.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]