Abstract
Introduction
National and professional organizations recommend oral health promotion in prenatal care to improve women’s oral health. However, few prenatal programs include education about oral health promotion. The objective of this study was to determine if women receiving a brief, low cost, and sustainable educational intervention entitled CenteringPregnancy Oral Health Promotion, had clinically improved oral health compared to women receiving standard CenteringPregnancy care.
Methods
Women (n=101) attending CenteringPregnancy, a group prenatal care model, at four health centers in the San Francisco Bay Area, participated in this non-randomized controlled pilot study in 2010–2011. The intervention arm (n=49) received the CenteringPregnancy Oral Health Promotion intervention consisting of two 15-minute skills-based educational modules addressing maternal and infant oral health, each module presented in a separate CenteringPregnancy prenatal care session. The present analysis focused on the maternal module that included facilitated discussions and skills-building activities including proper tooth brushing. The control arm (n=52) received standard CenteringPregnancy prenatal care. Dental examinations and questionnaires were administered prior to and approximately 9 weeks post intervention. Primary outcomes included the Plaque Index, percent bleeding on probing, and percent of gingival pocket depths 4mm or greater. Secondary outcomes were self-reported oral health knowledge, attitudes (importance and self-efficacy), and behaviors (tooth brushing and flossing). Regression models tested whether pre- to post-changes in outcomes differed between the intervention-versus the control-arms.
Results
The control and intervention arms did not vary significantly at baseline. Significant pre- to post-differences were noted between the arms with significant improvements in the intervention arm for the Plaque Index, bleeding on probing, and pocket depths 4mm or greater.
Discussion
Providing brief oral health education and skills-building activities within prenatal care may be effective in improving women’s oral health during pregnancy. These findings provide support for developing a full-scale randomized clinical trial of the CenteringPregnancy Oral Health Promotion intervention.
MESH Key words: prenatal care, pregnancy, oral health, health promotion
INTRODUCTION
The physiologic changes that accompany pregnancy may result in oral health problems.1 Shifts in hormonal, immunologic, and vascular function can exacerbate gingival inflammation and increase susceptibility to periodontal disease.2 While gingival inflammation usually subsides after childbirth, if left untreated during pregnancy, gingivitis will likely persist after pregnancy.3 Although the literature is mixed and recent research has failed to find an association between periodontal disease and adverse birth outcomes,4–6 oral health is associated with general health7 and maternal oral health can impact infant oral health through postpartum maternal-to-infant transmission of caries-causing bacteria, mutans streptococci and lactobacilli.8,9 Infants mainly acquire cariogenic bacteria from mothers through saliva,10 and mothers with high levels are more likely to transfer bacteria to infants.9 Thus, reducing vertical transmission of cariogenic bacteria may reduce infant risk for early childhood caries,8 an important goal because children with caries are likely to have caries as adolescents and adults.11,12
Despite the benefits of good maternal oral health in pregnancy, relatively few pregnant women utilize dental care, with lowest utilization among women from underserved communities.13 However, most women in the United States receive prenatal care.14 In 2012, the Maternal and Child Health Bureau released a landmark consensus statement to promote oral health care provision to pregnant women, emphasizing guidance for prenatal care providers stating: “Prenatal care health professionals may be the ‘first line’ in assessing pregnant women’s oral health and can provide referrals to oral health professionals and reinforce preventive messages.15” In 2013, the American College of Obstetricians and Gynecologists (ACOG) issued a Committee Opinion that prenatal care providers should assess oral health and emphasize the importance of receiving dental care during pregnancy and routinely, thereafter.16
Despite guidelines, a gap persists in provider practice to include oral health in prenatal care. A recent small study of obstetric providers found that although most providers had knowledge about the mother-to-infant vertical transmission of caries-causing bacteria, fewer than 50% referred their patients for dental care or conducted oral examinations.17 A study of pregnant or postpartum women found that only about 20% of women reported that their obstetric provider advised them to visit a dentist while pregnant and fewer than 50% were told the importance of good oral health.18 Those who were encouraged to see a dentist were more likely to pay attention to their oral health during pregnancy, supporting the importance of the provider’s advice.
A review of prenatal oral health interventions found that very few focused on the mother, compared to the infant.19 A few educational interventions resulted in higher rates of prenatal dental care utilization,20 tooth brushing and flossing improvements,21 and reduced sugar intake.22 One study showed decreased mutans streptococci through a nutritional education intervention.22 Only studies including a professionally applied dental treatment component assessed clinical oral health status and showed improvements.23,24
The present study, a non-randomized controlled pilot trial, was conducted to test a skills-building oral health educational intervention, “CenteringPregnancy Oral Health Promotion,” delivered by trained CenteringPregnancy facilitators (certified nurse-midwives) and integrated into routine CenteringPregnancy prenatal sessions. The intervention has two modules; one maternal and one infant. The current analysis evaluated the maternal module with primary clinical oral health outcomes: changes in oral hygiene and gingival health; and secondary self-reported outcomes: oral health knowledge, attitudes (importance of oral health and self-efficacy), and behaviors.
MATERIALS AND METHODS
Setting and Sample
The study took place at four San Francisco CenteringPregnancy prenatal care sites during 2010–11. Sites were assigned non-randomly to intervention or control arms: one English- and one Spanish-language site per arm. Two CenteringPregnancy sites with experienced facilitators willing to assist in the development and delivery of the interventions were selected as the intervention sites (n=6 facilitators). Facilitators in the two control sites (n=6) were not recruited because they had no study role. Patients at least 18 years were eligible to participate. All sites were CenteringPregnancy Site Approved, indicating fidelity to the CenteringPregnancy model. The study was approved by the University of California, San Francisco and California Pacific Medical Center Institutional Review Boards.
CenteringPregnancy, a group care model, often found in clinics serving low-income populations, emphasizes healthcare, interactive learning, and community building.25 Approximately ten women of similar gestational age comprise each group, attending 10 two-hour sessions following the ACOG schedule, led by a credentialed facilitator and a co-facilitator. Women begin each session by recording their blood pressure, weight, and gestational age and the facilitator conducts the standard prenatal assessment. Then, the women and facilitators gather in a circle for a general check-in, facilitated discussion, and interactive activities addressing health topics relevant to the group’s gestational age. While the standard CenteringPregnancy curriculum includes some mention of oral health, it is not addressed consistently.
A research associate visited the second CenteringPregnancy group session to describe study activities and recruit participants. Interested women remained after group or supplied contact information for follow-up. All women who participated in CenteringPregnancy groups in the intervention arm received the intervention as part of their CenteringPregnancy sessions; however only recruited participants completed the study assessments.
Curriculum Development and Training
The study team including public health dentists, a periodontist, a behavioral scientist, and CenteringPregnancy leadership and facilitators, developed the skills-based educational intervention. The intervention aligns with CenteringPregnancy principles, and the key oral health messages reflect evidence-based professional guidelines.15 The content was evaluated as informative and acceptable by CenteringPregnancy participant focus groups. An earlier oral health curriculum, CenteringSmiles, developed by different researchers for CenteringPregnancy sites in Kentucky included dental care, oral health topics in each session, and a dental hygienist instructor.23 CenteringSmiles was too comprehensive for broad implementation but was a helpful resource for this curriculum.
Intervention facilitators underwent a three-hour training on intervention content and delivery including didactic information and demonstration. Tool boxes were provided that included the learning objectives, prompts, and reminder instructions for the skills-based activities. Post-training, facilitators conducted a practice session in a non-study CenteringPregnancy group and received feedback on fidelity.
Implementation
Facilitators presented the maternal module during the 3rd or 4th CenteringPregnancy session. This module has three discussion topics: 1) importance of maternal oral health, 2) common oral health problems, 3) the safety and importance of dental care during pregnancy; and two hands-on activities: 1) proper tooth brushing and the 2) Eastman toothpick test, a self-test of gum health.26 For the toothpick test, a toothpick is inserted horizontally between teeth near the gums and pressed lightly onto the gums. Resultant bleeding indicates inflammation, which should decrease with proper brushing and flossing. Participants received: tool kits including tooth brushes, fluoride toothpaste, dental floss, a 2-minute timer for brushing; and illustrated instructions on flossing and oral health-promoting practices. At subsequent CenteringPregnancy sessions, participants performed the toothpick test at the time of completing their weight and other self-assessments in order to reinforce the importance of oral health. Each facilitator’s intervention was audiotaped and evaluated for fidelity. Control participants received standard CenteringPregnancy curricula.
Maternal Assessments
Study participants completed a questionnaire and dental examination at baseline and approximately 9 weeks post-intervention, outside of CenteringPregnancy sessions. The women received $20 for each questionnaire completed. More detail regarding the assessment methods is described in an earlier publication that describes the baseline oral health status for this sample.27
The questionnaire assessed demographic information and oral health status, knowledge, attitudes (importance of oral health and self-efficacy), health literacy, and oral hygiene behaviors (tooth brushing and flossing).
Oral health status questions included: “How would you describe the condition of your 1) “teeth” and 2) “gums?,” using responses ranging from “poor”=1 to “excellent”=5.
The 12-item knowledge Likert scale responses ranged from “strongly disagree”=1 to “strongly agree”=5. The questions assessed knowledge in the following areas: 1) the importance of receiving dental care during pregnancy; 2) the normalcy of increased bleeding of gums; 3) the effects of tobacco use on gum health; 4) prevention of gum bleeding with proper brushing and flossing; 5) the safety of getting X-rays during pregnancy; 6) the recommendation to use soft bristle toothbrushes; 7) not sharing tooth brushes with family members; 8) the role of sugary foods and drinks in forming dental decay; 9) the presence of bacteria in the mouth; 10) the role of bacteria in causing cavities; 11) the recommendation to use fluoridated tooth paste; and 12) the benefits to teeth of drinking fluoridated tap water.
The 7-item importance of oral health Likert scale responses ranged from “not at all important”=1 to “extremely important”=5 and had Cronbach’s alpha reliability of .71. The questions assessed the importance of: 1) brushing teeth for a healthy mouth; 2) flossing for a healthy mouth; 3) routine preventive dental care; 4) learning about proper oral health care; 5) routine professional teeth cleaning; 6) limiting sugary foods and drinks; and 7) drinking fluoridated water.
The 6-item self-efficacy Likert scale responses ranged from “not at all sure”=1 to “extremely sure”=4 and had Cronbach’s alpha reliability of .73. The questions assessed women’s self-efficacy: 1) to brush teeth twice daily; 2) floss teeth daily; 3) avoid frequent sweets; 4) attend dental visits twice annually; 5) see a dentist during pregnancy; 6) avoid getting cavities.
Health literacy, assessed with a single item, asked how often help is needed to read written materials from a healthcare provider and responses ranged from “always”=1 to “never”=5.28
Oral hygiene behavior items included: “How often do you” 1) “brush your teeth?” (recoded to optimal brushing of at least 2 times daily vs not brushed at least 2 times daily) and 2) “floss your teeth?” (recoded to optimal flossing of daily vs not flossed daily), as recommended by the American Dental Association. 29 The questionnaire took approximately 20 minutes to complete.
Dental examinations were conducted at CenteringPregnancy sites by an experienced, trained, and licensed dentist examiner. Exams lasted approximately 15 minutes. The measure of oral hygiene chosen was plaque level and measures of gingival health included indices of periodontal probing resulting in gingival bleeding and gingival pocket depths probed at 4 millimeters or greater. These outcomes were chosen because they are responsive to changes in personal hygiene over a short time period. The Plaque Index,30 was assessed at 4 areas per tooth for 6 teeth (maximum=24), using 0–3 coding of none, small, moderate, or large amount. Plaque Index scores were calculated as the mean plaque index value of observed sites.
The examination to assess gingival health included full-mouth periodontal probing of six gingival sites/tooth. Two outcomes were assessed at each probed site: gingival bleeding on probing and gingival pocket depth ≥4mm. Examiner training on the pocket depth assessment included calibration to a ‘gold standard’ examiner (periodontist co-investigator). The intraclass correlation coefficient for pocket depth=0.86.27
Analysis Plan
To determine the comparability of the intervention and control arms on demographic and baseline oral health factors, we conducted chi-square tests of race/ethnicity, income, education, insurance status, and past-visit dental visit; and t-tests for age, health literacy, and self-reported condition of teeth and gums. Difference in differences tests, comparing the degree of change in the intervention arm to the corresponding change in the control arm, were conducted for all outcomes. For primary outcomes, linear regression models tested the difference in changes between the arms for Plaque Index and logistic regression models tested the differences in the percentages of sites that bled on probing and sites probed at 4mm or greater.
For secondary outcomes, linear regression models tested the difference in the changes between groups in oral health knowledge, importance of oral health, and self-efficacy. Logistic regression models tested these changes between groups in rates of optimal tooth brushing and flossing. All regression models controlled for the nested structure of the data: CP sites, facilitators within sites, groups within facilitators, study participants within groups, and pre- and post-outcome measures for study participants. Cross-sectional comparisons of the intervention and control arms on outcomes at baseline and at post-intervention were also performed. Analyses were conducted using SAS 9.4 software.
RESULTS
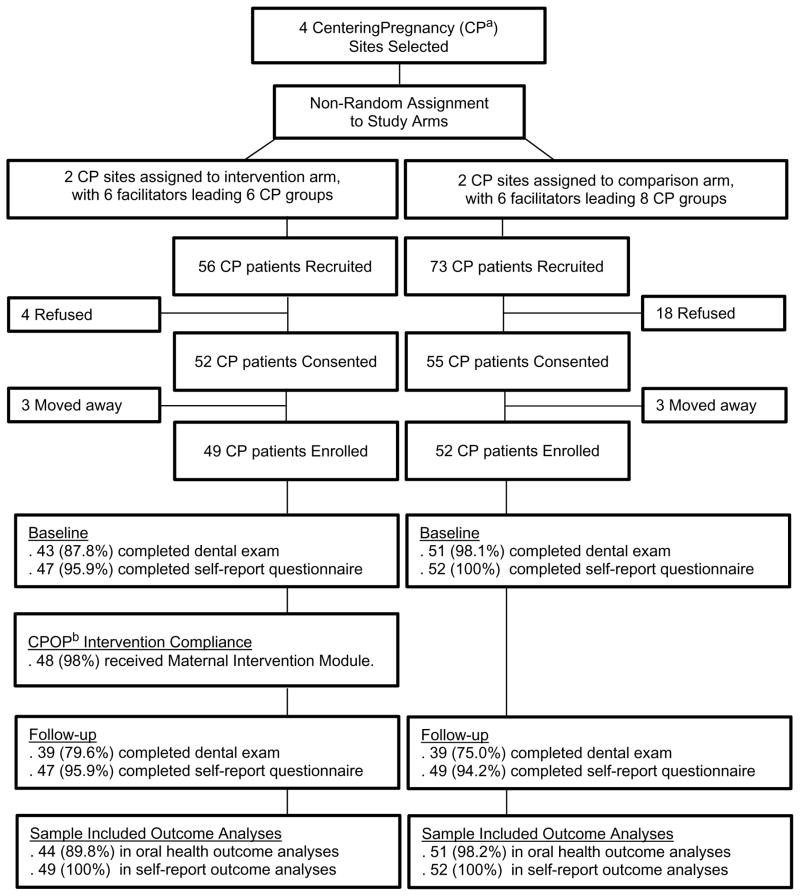
Eighty-three percent of the women recruited agreed to participate in the study (n=101; Figure 1). Mean age was 28.7 (SD=5.3) years and participants were primarily Hispanic (65%). Fifty percent had an annual income below $20,000; 52% had more than a high school education; 60% had a past-year dental visit; and 72% had either public or no dental insurance. Mean baseline values of self-reported variables included: condition of teeth= 2.6 (SD=1.0); condition of gums (gingival health)= 2.4 (SD=1.1); and health literacy= 4.1 (SD=1.1). There were no significant differences between the women in the intervention and control arms on baseline measures (Table 1).
Figure 1. CONSORT Diagram CenteringPregnancy Oral Health Promotion: Maternal Module.
Figure 1 describes the sample recruited for the CPOP study intervention and control arms.
Figure 1. CONSORT Diagram of CenteringPregnancy Oral Health Promotion Participants
a CenteringPregnancy
b CenteringPregnancy Oral Health Promotion
Table 1.
Demographic and Descriptive Factors of Women in the CenteringPregnancy Oral Health Promotion Intervention and Differences by Intervention versus Women in the Control Arm
| Characteristic | Intervention, n (%) (n =49) | Control, n (%) (n=52) | P value |
|---|---|---|---|
| Age, in years mean/SD | 28.5/5.8 | 28.9/4.8 | .70a |
| Race/ethnicity | .49b | ||
| White | 10 (20.4) | 10 (19.2) | |
| Hispanic | 34 (69.4) | 32 (61.5) | |
| Other | 5 (10.2) | 10 (19.2) | |
| Income | .47b | ||
| ≥ $20,000 | 25 (54.4) | 24 (47.1) | |
| < $20,000 | 21 (45.7) | 27 (52.9) | |
| Education level attained | .57b | ||
| < high school | 11 (23.4) | 12 (23.1) | |
| = high school | 14 (29.8) | 11 (21.2) | |
| > high school | 22 (46.8) | 29 (55.8) | |
| Health insurance | .86b | ||
| Private | 13 (28.9) | 14 (28.0) | |
| Public | 24 (53.3) | 29 (58.0) | |
| None | 8 (17.8) | 7 (14.0) | |
| Dental visit in past year | .68b | ||
| Yes | 27 (57.5) | 32 (61.5) | |
| No | 20 (42.6) | 20 (38.5) | |
| Health literacyc mean (SD) | 4.3 (1.1) | 3.9 (1.2) | .10a |
| Condition of teethc mean (SD) | 2.7 (1.1) | 2.4 (1.0) | .18a |
| Condition of gumsc mean (SD) | 2.4 (1.2) | 2.4 (1.0) | .85a |
Abbreviation: SD, standard deviation
P value of t-test
P value of Chi square
Measured on a scale of 1–5, higher number represents higher level of literacy and of oral health
Within the intervention arm, 98% of the women received the maternal module. On average, the module lasted 18 minutes (range 15–22 minutes). Overall, 97% of the learning objectives were delivered (range 91–100%). There were no significant differences between the women in the intervention and control arms in any of the outcomes at baseline.
Significant differences observed between women in the intervention arm pre- to post-intervention, based on the dental examination; plaque levels (F (1,75) = 27.95; P<.0001), bleeding on probing (F (1,75) = 8.88 (P<.01), and pocket depths 4mm or greater (F (1,75) = 23.95; P<.0001), indicated improvements on all three primary outcomes (Table 2). The women in the intervention arm showed a slight improvement in the self-reported importance of oral health compared to the control arm (F (1,92)=4.92; P<.05), but there were no other differences in assessments of knowledge, self-efficacy, optimal brushing or flossing (Table 2).
Table 2.
Effects of the Oral Health Intervention on Primary and Secondary Outcomes in the Intervention versus the Control Arm
| Outcome | Baseline | Follow-up | Fa/tb Value | P value |
|---|---|---|---|---|
| Primary outcomes: | ||||
| Plaque Index meanc, | 27.95a | <.0001 | ||
| Intervention group | 0.93 | 0.77 | ||
| Control group | 0.93 | 0.96 | ||
| Sites bleeding on probing, %d | 8.88a | .01 | ||
| Intervention group | 20.5 | 14.5 | ||
| Control group | 16.7 | 17.0 | ||
| Sites pocket depths ≥4mm, %d | 23.95a | <.0001 | ||
| Intervention group | 23.2 | 20.4 | ||
| Control group | 20.6 | 21.1 | ||
| Secondary outcomes: | ||||
| Mean oral health knowledgee | 0.09a | .77 | ||
| Intervention group | 4.0 | 4.0 | ||
| Control group | 3.9 | 3.9 | ||
| Mean oral health importancee | 4.92a | .05 | ||
| Intervention group | 4.0 | 4.2 | ||
| Control group | 4.1 | 4.1 | ||
| Mean oral health self-efficacyf | 1.08a | .30 | ||
| Intervention group | 2.8 | 2.9 | ||
| Control group | 2.8 | 2.8 | ||
| OptimalTooth brushing n, %g | 0.50b | .62 | ||
| Intervention group | 42 (89.4) | 44 (93.6) | ||
| Control group | 45 (86.5) | 43 (87.8) | ||
| Optimal Flossing n %h | 0.68b | .50 | ||
| Intervention group | 19 (40.4) | 22 (47.8) | ||
| Control group | 23 (46.0) | 21 (43.8) |
Lower number = better outcome; range 0–3, analyses controlled for CP site, CP facilitator, and CP group
Lower number = better outcome; range 0–100%, analyses controlled for CP site, CP facilitator, and CP group
Higher number represents better outcome; range 1–5:
Higher number represents better outcomes; range 1–4:
% Brushed at least 2 times/day; range 0–100%
% Flossed at least once daily; range 0–100%
DISCUSSION
This study found that facilitator-led, brief, skills-based oral health promotion within prenatal care improved maternal oral health: reduced plaque, gingival bleeding, and pocket depths 4mm or greater. These preliminary pilot results are the first, to our knowledge, to show clinically determined oral health improvements during pregnancy following an educational intervention. Given the evidence that oral health problems increase during pregnancy,1 these significant oral health improvements, found across a relatively short time, suggest that some problems can be mitigated through promoting oral health in prenatal care.
The modest but significant improvements in Plaque Index and gingival measures indicate that women in the intervention arm improved their oral hygiene. Baseline measures of bleeding on probing and pocket depths 4mm or greater were somewhat, but not significantly, higher in the intervention than the control arm; thus it is possible that there was more room for improvement in the intervention arm. While some studies that included dental treatment have shown improved clinical oral health during pregnancy,23,24 to our knowledge, there are no published educational interventions without dental treatment that have assessed clinical oral health with which to compare these findings. Other prenatal educational oral health interventions have resulted in improved knowledge, care utilization, or oral hygiene,20–22 and decreased mutans streptococci following a nutrition education intervention.22
In light of the significant improvements in oral hygiene and gingival measures, our finding that self-reported tooth brushing and flossing did not increase significantly in the intervention arm was unexpected. However, at baseline, about 90% of the sample reported brushing their teeth at least two times daily, leaving little room for improvement at follow-up. Further study is needed to develop self-report measures of the thoroughness of brushing and flossing that may affect outcomes. The finding that oral health knowledge did not increase in the intervention arm may be explained by high baseline scores on knowledge, which would make post-intervention increases less likely.
Despite the intervention’s positive impact on women’s oral health, the findings must be viewed as preliminary because they are derived from a small pilot study using a non-randomized design. A larger randomized controlled trial that addresses these limitations is needed to confirm the intervention’s efficacy. This intervention could possibly be adapted and tested for other prenatal care groups or traditional prenatal care. If prenatal care programs could not provide the oral health supplies utilized in this study, the women could bring their own tooth brushes for the tooth brushing activity and toothpicks could be provided at a minimum. Future studies could assess the effects of the educational component alone.
CONCLUSION
While it is ideal for pregnant women to receive dental care, these results suggest that prenatal care clinicians can provide integrated oral hygiene education and practice into their care that can enhance important skills for all women, particularly for those in low-income communities who lack access to dental care. As a facilitator-led, brief, low-cost, effective, and sustainable prenatal oral health intervention, the CenteringPregnancy Oral Health Promotion intervention has the potential to achieve this. Integrated into a group prenatal care setting and delivered by prenatal care facilitators, the intervention is consistent with current perinatal oral health guidelines15 and the Institute of Medicine’s recommendation to train and deploy non-dental healthcare clinicians to provide oral health services to vulnerable populations.31 These findings provide support for developing a full-scale randomized clinical trial of this intervention. If it were to show efficacy in a clinical trial, it could be implemented within CenteringPregnancy groups nationwide, reaching large populations at an important time in their family’s life course.
QUICK POINTS.
Providing an oral health education and oral hygiene skills-building curriculum within prenatal care can help improve pregnant women’s oral health.
Prenatal clinicians should be trained to provide oral health education and skills-building within their prenatal care.
If the CenteringPregnancy Oral Health Promotion intervention shows efficacy in a larger clinical trial, it could be implemented widely within group prenatal care and could positively affect the oral health of many women and their families.
Acknowledgments
Funding support for this research was provided by National Institute of Dental and Craniofacial Research awards: R21DE019221; U54 DE019285; R34 DE024574; and the Dental Trade Alliance Foundation
We acknowledge the valuable contributions of Dorian Hollis and Judy Gonzalez-Vargas.
Footnotes
“The authors report no conflicts of interest.”
A previous version of this research was presented in a poster at the National Oral Health Conference in 2014 at Fort Worth, Texas.
Contributor Information
Sally H. Adams, Specialist, Division of Adolescent and Young Adult Medicine, Dept. of Pediatrics, School of Medicine University of California, San Francisco (UCSF).
Steven E. Gregorich, Professor, Department of Medicine in the School of Medicine, and Department of Preventive and Restorative Dental Sciences in the School of Dentistry, UCSF.
Sharon Schindler Rising, Founder and President Emeritus of Centering Healthcare Institute, Silver Spring, MD.
Margaret Hutchison, Professor, Department of Obstetrics, Gynecology, and Reproductive Sciences, School of Medicine, UCSF.
Lisa H. Chung, Associate Clinical Professor, Div. of Oral Epidemiology & Dental Public Health. Co-Associate Director, Center to Address Disparities in Children’s Oral Health (known as CAN DO). School of Dentistry, UCSF.
References
- 1.Silk H, Douglass AB, Douglass JM, Silk L. Oral health during pregnancy. Am Fam Physician. 2008;77(8):1139–1144. [PubMed] [Google Scholar]
- 2.Laine MA. Effect of pregnancy on periodontal and dental health. Acta odontologica Scandinavica. 2002;60(5):257–264. doi: 10.1080/00016350260248210. [DOI] [PubMed] [Google Scholar]
- 3.Zeeman GG, Veth EO, Dennison DK. Focus on primary care: periodontal disease: implications for women’s health. Obstet Gynecol Surv. 2001;56(1):43–49. doi: 10.1097/00006254-200101000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107(1):29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 5.Michalowicz BS, Hodges JS, DiAngelis AJ, et al. Treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355(18):1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 6.Macones GA, Parry S, Nelson DB, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS) Am J Obstet Gynecol. 2010;202(2):147e141–148. doi: 10.1016/j.ajog.2009.10.892. [DOI] [PubMed] [Google Scholar]
- 7.Hancocks S. Reconnecting the mouth to the body. Br Dent J. 2016;221(3):122. doi: 10.1038/sj.bdj.2016.556. [DOI] [PubMed] [Google Scholar]
- 8.Berkowitz RJ. Causes, treatment and prevention of early childhood caries: a microbiologic perspective. J Can Dent Assoc. 2003;69(5):304–307. [PubMed] [Google Scholar]
- 9.Thorild I, Lindau-Jonson B, Twetman S. Prevalence of salivary Streptococcus mutans in mothers and in their preschool children. Int J Paediatr Dent. 2002;12(1):2–7. [PubMed] [Google Scholar]
- 10.Tanzer JM, Livingston J, Thompson AM. The microbiology of primary dental caries in humans. J Dent Educ. 2001;65(10):1028–1037. [PubMed] [Google Scholar]
- 11.Kohler B, Andreen I, Jonsson B. The earlier the colonization by mutans streptococci, the higher the caries prevalence at 4 years of age. Oral Microbiol Immunol. 1988;3(1):14–17. doi: 10.1111/j.1399-302x.1988.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 12.Thomson WM, Poulton R, Milne BJ, Caspi A, Broughton JR, Ayers KM. Socioeconomic inequalities in oral health in childhood and adulthood in a birth cohort. Community Dent Oral Epidemiol. 2004;32(5):345–353. doi: 10.1111/j.1600-0528.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 13.Boggess KA, Urlaub DM, Massey KE, Moos MK, Matheson MB, Lorenz C. Oral hygiene practices and dental service utilization among pregnant women. J Am Dent Assoc. 2010;141(5):553–561. doi: 10.14219/jada.archive.2010.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services HRaSA, Maternal and Child Health Bureau. Child Health USA. Rockville, Maryland: U.S. Department of Health and Human Services; 2013. [Google Scholar]
- 15.Maternal and Child Health Bureau. Oral Health Care During Pregnancy: A National Consensus Statement. Washington DC: National Maternal and Child Oral Health Resource Center; 2012. [Google Scholar]
- 16.American College of O, Gynecologists Women’s Health Care P, Committee on Health Care for Underserved W. Committee Opinion No. 569: oral health care during pregnancy and through the lifespan. Obstet Gynecol. 2013;122(2 Pt 1):417–422. doi: 10.1097/01.AOG.0000433007.16843.10. [DOI] [PubMed] [Google Scholar]
- 17.Pre-natal providers’ oral health knowledge doesn’t equal behavior. Center for Healthier Communities at Rady Children’s Hospital; Apr, 2010. [Accessed September 2016]. http://www.rchsd.org/documents/2014/02/pre-natal-providers-oral-health-knowledge-doesn't-equal-behavior.pdf. [Google Scholar]
- 18.May L, Suminski RR, Yeung AY, Linklater ER, Christensen C, Jahnke S. Pregnant patient knowledge of and obstetric provider advice on oral health. Journal of Dentistry and Oral Disorders & Therapy. 2014;2(1):6. [Google Scholar]
- 19.Vamos CA, Thompson EL, Avendano M, Daley EM, Quinonez RB, Boggess K. Oral health promotion interventions during pregnancy: a systematic review. Community Dent Oral Epidemiol. 2015;43(5):385–396. doi: 10.1111/cdoe.12167. [DOI] [PubMed] [Google Scholar]
- 20.Riedy CA, Weinstein P, Mancl L, et al. Dental attendance among low-income women and their children following a brief motivational counseling intervention: A community randomized trial. Soc Sci Med. 2015;144:9–18. doi: 10.1016/j.socscimed.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cibulka NJ, Forney S, Goodwin K, Lazaroff P, Sarabia R. Improving oral health in low-income pregnant women with a nurse practitioner-directed oral care program. J Am Acad Nurse Pract. 2011;23(5):249–257. doi: 10.1111/j.1745-7599.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 22.Reisine S, Douglass J, Aseltine R, Shanley E, Thompson C, Thibodeau E. Prenatal nutrition intervention to reduce mutans streptococci among low-income women. J Public Health Dent. 2012;72(1):75–81. doi: 10.1111/j.1752-7325.2011.00286.x. [DOI] [PubMed] [Google Scholar]
- 23.Skelton J, Mullins R, Langston LT, et al. CenteringPregnancySmiles: implementation of a small group prenatal care model with oral health. J Health Care Poor Underserved. 2009;20(2):545–553. doi: 10.1353/hpu.0.0138. [DOI] [PubMed] [Google Scholar]
- 24.Lin DL, Harrison R, Aleksejuniene J. Can a prenatal dental public health program make a difference? J Can Dent Assoc. 2011;77:b32. [PubMed] [Google Scholar]
- 25.Alliman J, Jolles D, Summers L. The Innovation Imperative: Scaling Freestanding Birth Centers, CenteringPregnancy, and Midwifery-Led Maternity Health Homes. J Midwifery Womens Health. 2015;60(3):244–249. doi: 10.1111/jmwh.12320. [DOI] [PubMed] [Google Scholar]
- 26.Caton JG, Polson AM. The interdental bleeding index: a simplified procedure for monitoring gingival health. Compend Contin Educ Dent. 1985;6(2):88, 90–82. [PubMed] [Google Scholar]
- 27.Chung LH, Gregorich SE, Armitage GC, Gonzalez-Vargas J, Adams SH. Sociodemographic disparities and behavioral factors in clinical oral health status during pregnancy. Community Dent Oral Epidemiol. 2014;42(2):151–159. doi: 10.1111/cdoe.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouth Healthy: Brushing Your Teeth. American Dental Association; [Accessed December 2016]. http://www.mouthhealthy.org/en/az-topics/b/brushing-your-teeth. [Google Scholar]
- 30.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta odontologica Scandinavica. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 31.Council IoMaNR. Improving access to oral health care for vulnerable and underserved populations. Washington, DC: The National Academies Press; 2011. [Google Scholar]